Abstract
Background
The corpus callosum (CC) is suggested as an indirect biomarker of white matter volume, which is often affected in preterm birth. However, diagnosing mild white matter injury is challenging.
Methods
We studied 124 children born preterm (mean age: 8.4 ± 1.1 years), using MRI to assess CC measurements and cognitive/motor outcomes based on the Wechsler Intelligence Scale for Children-V (WPPSI-V) and Movement Assessment Battery for Children-2 (MABC-2).
Results
Children with normal outcomes exhibited greater height (10.2 ± 2.1 mm vs. 9.4 ± 2.3 mm; p = 0.01) and fractional anisotropy at splenium (895[680–1000] vs 860.5[342–1000]) and total CC length (69.1 ± 4.8 mm vs. 67.3 ± 5.1 mm; p = 0.02) compared to those with adverse outcomes. All measured CC areas were smaller in the adverse outcome group. Models incorporating posterior CC measurements demonstrated the highest specificity (83.3% Sp, AUC: 0.65) for predicting neurological outcomes. CC length and splenium height were the only linear measurements associated with manual dexterity and total MABC-2 score while both the latter and genu were related with Full-Scale Intelligence Quotient.
Conclusions
CC biometry in children born very preterm at school-age is associated with outcomes and exhibits a specific subregion alteration pattern. The posterior CC may serve as an important neurodevelopmental biomarker in very preterm infants.
Impact
-
The corpus callosum has the potential to serve as a reliable and easily measurable biomarker of white matter integrity in very preterm children.
-
Estimating diffuse white matter injury in preterm infants using conventional MRI sequences is not always conclusive.
-
The biometry of the posterior part of the corpus callosum is associated with cognitive and certain motor outcomes at school age in children born very preterm.
-
Length and splenium measurements seem to serve as reliable biomarkers for assessing neurological outcomes in this population.
Similar content being viewed by others
Introduction
Corpus callosum (CC) is the largest telencephalic commissural tract and biggest white matter structure in the human central nervous system. It is present in placental mammals and constitutes the highest level of neocortical interhemispheric connection.1 Bundles of axonal fibers that cross midline forming the CC are paramount to integrate lateralized sensory-motor tasks.2 Taking into account the extensive connections of very diverse cortical areas (sensory, motor, integration areas…), it has been shown that the biometry and microarchitecture of the CC is related to cognitive and motor functions. As the most important human commissural tract, CC measurements on midsagittal plane could serve as surrogate markers to estimate total cerebral white matter volume in children with white matter diseases.3,4,5,6
Preterm birth is a major worldwide health problem.7 In the past three decades survival of preterm infants has increased and, even though moderate to severe brain injury has been reduced, the risk of neurodevelopmental impairment remains high for those preterm infant born before 32 weeks of gestation.8,9,10 White matter injury (WMI) is the most prevalent brain injury in very preterm infants.11,12 It is considered as a spectrum of neuropathological injuries including: focal cystic necrosis, punctate and focal microscopic necrosis and diffuse non-necrotic lesions.13 Up to 50% of very preterm infants (VPT) show some degree of WMI on magnetic resonance imaging (MRI)14,15 with diffuse WMI being the most prevalent form.16 MRI is the most sensitive tool for WMI detection in infants,17,18,19 however conventional MRI sequences at term equivalent age (TEA) are not able to detect mild forms of diffuse WMI. Moreover, some postmortem studies have shown that up to 82% of periventricular leukomalacia have only microscopic necrotic foci.20
CC measurements have been widely used in WMI scoring classifications. Whether by MRI or ultrasound, most of them include CC measurements in their scales (mostly thinning of CC).17,21,22 The pattern of CC development throughout childhood has been proven to be different in those born preterm from their peers at term, related to gestational age (GA) at birth.23,24,25,26 However, there is still no consensus on what the best approach to CC biometry as a biomarker for long-term neurodevelopmental outcomes is, and many classifications and subdivisions of the CC have been proposed according to its anatomy and function.27 Many of these subdivisions are not easily replicable, are time-consuming and require segmentation post-processing algorithms not available in all hospitals. Complementing conventional sequences, diffusor tensor imaging (DTI) provides a new insight into the microstructural characteristic of brain structures. DTI is based on measuring spatial diffusion of water molecules along the white matter tracts. Quantitative parameters like fractional anisotropy (FA), which is considered a biomarker related to myelination, allow the study of the differential maturational process that occur in children who were preterm compared to those who were born at TEA.28,29,30,31 Previous studies using DTI have linked lower CC thickness in preterm infants with abnormal bundles.31
Our aim is to study the CC biometry and FA in school-age children (aged 6–11) who were VPT, and their association with neurodevelopmental motor and cognitive outcomes.
Methods
Participants
This is a prospective observational cohort study including school-age children who were born at a GA equal to or less than 32 weeks and/or with a birth weight equal to or less than 1500 g, admitted to the Neonatal Intensive Care Unit (NICU) in Puerta del Mar University Hospital, Cadiz, Spain. We included a retrospective analysis of the MRI performed at TEA. This study was reviewed and approved by the local Research and Ethics Committee and with parental or legal guardian signed informed consent.
Exclusion criteria were chromosomal or genetic identified anomalies, proven metabolic or malignant disorders, congenital neurological malformation, and congenital infections.
Those children born preterm included, with a mean age of 8.3 years old, were assessed from January 2020 to December 2022. We performed MRI, motor and neuropsychological assessments. Perinatal, neonatal clinical course and neuroimaging data were collected retrospectively.
Socioeconomical status (SES) was measured using maternal level of education at the time of recruitment32,33 which was then categorized into three groups according to the number of years of maternal education: primary or secondary school, undergraduate degree, or postgraduate degree (low, medium and high, respectively). Perinatal and postnatal variables were prospectively collected. We considered moderate to severe bronchopulmonary dysplasia if there was need for supplemental oxygen and/or positive pressure at 36 weeks postmenstrual age; significant patent ductus arteriosus if requiring surgical or pharmacological closure; late onset sepsis in the presence of systemic signs of infection and isolation of a bacterial pathogen in blood culture after the first 72 h of life; confirmed necrotizing enterocolitis (Bell Stage II or higher); and severe retinopathy of prematurity (stage 3 or higher).
Magnetic resonance imaging
We performed an MRI at elementary school age (6–11 years) on the included subjects, and we retrieved, when available, the TEA-MRI that was performed as part of the standard clinical practice at the time.
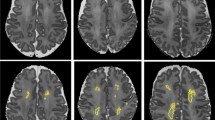
MRI scans were performed using the Magnetom Symphony 1.5 T scanner (Siemens Health Care, Erlangen, Germany) located in the radiology unit. MRI scans included T1-weighted volumetric images (Multiplanar Reconstruction (MPR)) acquired using the 1.5 Tesla scanner (slice thickness 1.0 mm; echo-time 3.53 ms; flip angle 15°; field of view 192 × 256 mm2), axial spin echo T2-weighted images and diffusion tensor imaging (DTI). Every MRI was studied by an experienced radiologist who classified the findings as abnormal if there was evidence of brain injury or normal/mild abnormalities. Neuroimaging measures were performed using Carestream (© 2019 Carestream Health, Version 12.1.5.5151) and CC biometry was assessed using MPR T1-weighted sequences. DTI was acquired with a multirepetition, single-shot echo planar sequence with 120 gradient directions (TR, 3100; TE, 98; flip angle 90; no gap), and diffusion weighting of 1000 s/mm2 (b value) and an image without diffusion weighting, resulting in an in-plane resolution of 1.3 mm. FA ranges from 0 (indicating isotropic diffusion of water molecules) to 1 (anisotropic), and it rises with the development of white matter microstructure.34 Mean FA values were obtained from 2 manually selected CC regions of interest (genu and splenium of the CC) on one selected axial plane of the color FA maps (Fig. 2).
MRI at TEA was evaluated using the scale published by ref. 21 This classification assesses development and injury of cortical and deep gray matter, white matter, and cerebellum. The overall score obtained classifies MRI findings as: normal (0–3 points) or abnormal: mild (4–7 points), moderate (8–11 points) and severe (≥12 points).
Corpus callosum measurements
We measured, according to ref. 35, five linear parameters in a T1-weighted midline sagittal section: CC length as the distance between the most anterior aspect of the genu and the most posterior aspect of the splenium. Thickness measurements of the CC at different levels (genu, body, isthmus and splenium) were taken tracing a line from the bottom to the top, perpendicular to the curvilinear axis of CC (Fig. 2). All these linear measurements were expressed in millimeters.
We assessed the total midsagittal area of the CC and performed a subdivision of this total area into five sections according to the studies by Witelson,36 Duara,37 Hofer,38 Delacoste,39 Shin40 and Westerhausen.41 In order to do this, CC length was divided in 5 sections and then tracing upward perpendicular lines. Anterior and posterior areas count as one-fifth of total CC area, respectively. The central area is the part in between both anterior and posterior, three-fifths of the total CC area. Areas were expressed in square millimeters.
Neurodevelopmental outcomes
Cognitive function was evaluated using the Wechsler Intelligence Scale for Children—Fifth Edition (WISC-V).42 This scale provides tests and composite scores (indices) reflecting intellectual functioning in five specific domains (working memory, verbal comprehension, visuospatial, processing speed, and fluid reasoning) and Full-Scale Intelligence Quotient (FSIQ), a standardized composite score with a mean of 100 and a standard deviation (SD) of 15. A cut-off value of 85 was considered to further classify participants in groups of normal versus borderline/stablished intellectual disability.
Motor abilities were evaluated using the Movement Assessment Battery for Children, Second Edition (MABC-2)43 and divided in 3 domains: manual dexterity, aiming and catching, and balance. The sum of the scores of three domains gives a total MABC-2 punctuation and percentile score. A total MABC-2 percentile score of 15 or over is considered normal. A percentile score of under 15 is considered a risk of movement disability and under 5 is considered diagnostic for developmental coordination disorder.42
Children with cerebral palsy with a Gross Motor Functional Classification System level II to III that were not able to complete all the subtests of motor testing would be considered to score under 5 on the MABC-2 total score and have an imputed percentile value of 0.1, according to similar studies.44
We considered a global adverse outcome in those children who scored under the 15th centile in MABC-2 total percentile score and/or had a FSIQ score of less than 85.
Statistical analysis
Quantitative variables were described using the median (Md) or mean value and interquartile range [IQR] or SD, according to their distribution. Bivariate analysis was performed using Pearsonʼs chi-squared test or Fisherʼs exact test for categorical data, and Studentʼs t-test or Mann–Whitney U test for continuous variables.
Multivariable linear and logistic regression models were used on perinatal variables. We performed multivariable regression and logistic models to study the relationship between CC measurements and neurodevelopmental outcome, including perinatal variables (GA, birth weight and sex), and age at scan. Variables were selected based on the theoretical background and results of bivariate analysis.
Moreover, to allow for non-hierarchical models, we also selected the best logistic model by using the method of all possible equations, which identified the best subset for logistic regression based on all the possible combinations of independent variables (linear measurements of CC and areas, adjusting by birth weight, GA and sex). For each subset area under the curve (AUC), Akaike information criterion (AIC), Schwarz Bayesian Criterion (BIC), sensitivity and specificity were studied as goodness-of-fit measures. Internal validation of the model was performed with cross-validation on the specified models in order to evaluate the model’s ability to fit out-of-sample data. Standardized beta coefficients were estimated to compare the effect size of the independent variables.
Statistical analysis was conducted using Stata 17.0 (Stata Statistical Software: Release 17. College Station, TX: StataCorp LP). A result was considered statistically significant at p < 0.05.
Results
Clinical characteristics and long-term outcome
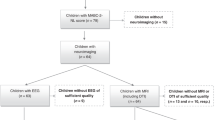
During the study period we contacted 197 eligible subjects. We excluded thirty-nine children (19.8%): 26 declined to participate, 10 were lost to follow-up and 3 were excluded for medical conditions. Our final study population included 158 children between 6 and 11 years of age. The inclusion process and final sample size are summarized in Fig. 1. We performed an MRI on 137 (85.6%) of the included subjects, with 120 (87.6%) of them having mild abnormalities or normal conventional MRI. Moderate/severe abnormal MRI findings and/or cranial ultrasound conditions are described in Table 1s (supplementary material). Of those with an MRI, 121 (88.3%) completed motor evaluation and cognitive outcome was assessed on 124 (90.5%). The median value of total MABC-2 score was 7 [IQR 1–15], corresponding to the 16th percentile [IQR 0.1–95] and the mean FSIQ value was 93.1 (SD 12.7). A detailed description of the scores obtained in all the subtests of MABC-2 and WISC-V is included in Table 2s in the supplementary material.
Those with an adverse outcome (n = 58 (47.9%)) compared to those with a normal outcome (n = 63 (52.1%)) were born with lower birth weight (1165 grams [IQR 630–2345] vs 1325 grams [IQR 550–2120]; p = 0.01). No other clinical or demographic characteristics of the studied participants differed significantly between groups (Table 1).
School-age corpus callosum measurements related to perinatal characteristics
GA was associated with CC length (β = −0.794; p = 0.01) and isthmus thickness (β = 0.123; p = 0.01) and birth weight was related to total and segmented areas and CC length (Table 2). We found no association of sex and maternal level of education to school age CC measurements.
Corpus callosum measurements at school age and global outcome
At school age, two linear CC measurements were different between groups of normal and adverse outcomes: height at splenium (10.2 mm (SD 2.1) vs. 9.4 mm (SD 2.3); respectively p = 0.02) and total CC length (69.1 mm (SD = 4.8) and 67.3 mm (SD 5.1), p = 0.02). Some of the CC areas were smaller among those with adverse outcomes with a total area of 513.5 mm2 (SD 79) vs 481.7 mm2 (SD 101), p = 0.03 and central area of 191.7 mm2 (SD 34.6) vs 178.8 mm2 (SD 47.4), p = 0.04. When excluding those patients with moderate/severe abnormalities in cUS or MRI (those included in Table 1s) we found no major changes to our results except for measurements of total and central areas of the CC, which would not be associated to adverse outcome in the absence of moderate/severe brain injury (Table 3s).
We observed higher FA values for the splenium in those with normal outcomes compared to those with adverse outcomes (895 [IQR 680–1000] vs 860.5 [343–1000]) (Table 1). FA at splenium and height at splenium showed similar results when accounting for GA, birth weight and age at MRI (Table 4s in supplementary material). Age at MRI (mean 8.3 years (SD 1.1)) was not different between outcome groups (p = 0.66).
When comparing different subsets obtained, those including measurements of the posterior part of CC (height at splenium: β coefficient = −0.07; p = 0.55 and FA at splenium: β = −0.01; p = 0.03; model p = 0.02) showed higher specificity (83.3%, AUC: 0.65) related to school-age global outcome.
Corpus callosum subdivision and subscales of motor and cognitive function
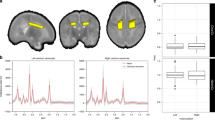
Each cognitive and motor subtest of WISC-V and MABC-2 and their relationship with CC measurements were studied (Fig. 3 and Table 3). Accounting for birth weight, GA, sex, and age at MRI we found that CC length and height at splenium were related to manual dexterity (β = 1.04, p = 0.01; and β = 2.23, p = 0.05, respectively) and with most of the cognitive subscales. Height at genu was also related to some of the cognitive subtests. Length of the CC and height at splenium were the only linear measurements related to the total MABC-2 score (β = 1.10, p = 0.05; and β = 2.61, p = 0.03, respectively). Both parameters were also related to FSIQ (β = 0.81, p = 0.01; and β = 1.94, p = 0.01).
CC posterior area showed a relationship with all dimensions of cognitive function in WISC-V (Fig. 4 and Table 3).
We found no relationship between FA in the genu or in the splenium with any of the motor or cognitive subscales.
Corpus callosum at term equivalent age and long-term outcome
We retrospectively reviewed the available clinical MRI at TEA of 84 patients from this cohort. The clinical, neuroimaging, demographic and long-term outcome data of this subgroup are detailed in Table 5s. Accounting for GA and birth weight, only length of CC was related with all the long term motor outcomes (Fig. 1s in supplementary material). Height at genu showed relation with manual dexterity and with FSIQ. Total area and posterior area of the CC at TEA were also related with manual dexterity at school age (Fig. 1s).
Discussion
In this study of children born very preterm and assessed at elementary school age, we found smaller CC measurements related to adverse outcomes with total length of CC and splenium region size related to both cognitive and motor function. Our study showed a significant relationship of the posterior region of the CC with manual dexterity and all cognitive domains in school-age VPT. This finding could be related with impaired white matter development after preterm birth affecting tracts crossing this region of the CC that links extensive areas of sensory and motor integration, being fundamental in tasks such as bimanual function and multiple aspects of cognition.
The CC is an easily measurable structure reflecting brain connections, brain volume and white matter volume.4,5,6,23,45 In VPT, CC altered biometry should not be considered as an isolated disruption in its development as a consequence of preterm birth. However, this anatomical and microstructural alteration of the CC could be part of the spectrum of encephalopathy of prematurity, which includes global WMI. While easy to visualize on the midsagittal plane and easy to measure, no discernable anatomical boundaries divide CC segments. For this reason, there are many proposed subdivision schemes.27 Based on models which used DTI and connectivity maps,27,38 and considering the well-known relative importance of anterior and posterior regions, we performed a five-subdivisions model (Fig. 2) similar to Delacoste,39 Shin40 and according to Westerhausen.41 This approach is intended to be easily reproducible in clinical practice and to show results congruent with CC connectivity. In this age range, CC biometry remains relatively stable,35,46 even in VPT children.26,47
a length; b total area; c linear measurements of genu (purple), body (green), isthmus (light blue) and splenium (red) (performed according to ref. 33. d anterior and posterior subdivision areas. e Region of interest FA measurement at the genu (blue dot) and splenium (white dot).
Altered patterns of total white matter volume and thinning of CC are common findings in preterm infants48,49,50,51,52 with a global reduction of the CC size in children who were born preterm, mostly in the splenium.24,30,53 Our findings are in line with previous studies, with gestational age and weight at birth being strongly related to CC length and areas at elementary school age (Table 1).23,24,50 CC biometry in these subjects is related with adverse late motor,3,51 and neuropsychological outcomes,23,24,50 and the splenium is the specific region that correlates the most with cognition.23,54,55 Our sample size has allowed us to depict different outcomes of VPT infants relating to the size of the CC, with previous studies mostly having focused on the comparison with healthy term-born children. While smaller CC has been previously demonstrated, the impact of preterm birth on different areas and related to the outcome has not been previously addressed. Our results show that the posterior part of the CC, measured as height at splenium, posterior area, and FA at splenium, is related to adverse prognosis highlighting the importance of this region in motor and cognitive pathways. The splenium of the CC is involved in transference of axons that are related to language, visuospatial integration, complex cognitive functions, behavior and consciousness.46 Accordingly, Luders et al.56 related the posterior part of the CC and some regions of the anterior part of the CC with total brain volume and FSIQ.56 Regional brain volume studies in children born preterm demonstrated reduced white and gray matter volumes in sensorimotor and parieto-occipital regions in preterm compared with full-term infants.41 Our findings may underline the relative importance of the splenium in cognitive function and all its subsets in a high-risk neurological population.
Considering the approach based on measuring areas of CC on the mid-sagittal plane, all areas were related to overall prognosis. However, this relationship did not hold when adjusting for GA, sex, birth weight and age at MRI. Although linear measurements of the CC have customarily been used in WMI classification,17,21,22 an area-based approach could provide data with higher predictive value and/or more complex information. Correlation between CC segmental areas and cognitive processes in elementary school-age children have been demonstrated in previous studies.57 However, there are not many similar studies in children who were preterm.23,24,50,58 more studies are needed to assess the usefulness of measures of CC areas at full-term age as long-term prognostic predictors.
During early life and at school age, children who were born VPT have lower FA values when compared to those born at term.30,59,60,61 Furthermore, children born VPT display a lower FA in CC attributable to white matter development disruption in the context of WMI.28 FA is also reduced in non-preterm children with thickened corpus callosum due to other conditions.31 Our results are in line with previous studies that found an inverse relation between FA assessed by DTI, and motor and cognitive impairment, with splenium being one of the most important regions.55,62
The association between CC abnormalities and fine motor and bimanual coordination is well-known.27,63,64 In our study, length of CC and height at splenium in school age were related to manual dexterity. On the same way, in the subgroup of patients who had an MRI at TEA, total and posterior areas and length of CC were consistently related with manual dexterity. Similar results, highlighting the importance of the CC in general, and splenium in particular, were obtained both in healthy adolescents65 and children with cerebral palsy.66 Further research, is warranted to unravel what measurements of the corpus callosum in VPT in the neonatal period may serve as long term predictor of fine motor and bimanual coordination.
Many of the CC measurements were related to WISC-V subtests, with total length, height at splenium, total area and posterior area impacting the most on cognitive outcome. However, contradictory findings have been reported relating to regional division and cognitive function. Hutchinson et al.67 found smaller posterior areas of the CC in those subjects with higher FSIQ. Nevertheless, this study was based on healthy young adults (mean age 19.2 years old) whereas an accelerated growth pattern of the CC has been described in those who were VPT.47,68 Except for this single study, our results are in line with other studies where CC subregions were found to be directly related to FSIQ and WISC-V subscales.24,47,54,55,69 It should be noted that parameters measuring the entire CC (length and total area) and posterior region (height at splenium and posterior area) at school age are broadly related to intellectual function. We also found that genu at TEA and at school-age was related to FSIQ, but not with long-term motor performance. Interestingly, the relationship of genu with FSIQ was demonstrated at TEA and at school age (Figs. 3 and 4).
Interestingly, despite being suggested as a valid measure in some neuroimaging studies17,21,22 during the neonatal period, we found that the height of the corpus callosum measured at the body and central areas of the CC at elementary school age, were not statistically significant related to any of the cognitive or motor outcomes. We also found no relationship between height at body and an overall adverse prognosis.
While this is one of the studies with a larger sample size of CC biometry in school-age children who were VPT, it has certain limitations that should be addressed. We did not perform a comparison with term-born children as we did not have a control group. However, our aim was to study the differences in CC growth among VPT and our large sample size has allowed us to successfully address this objective. Another limitation of our study is the method of acquiring FA, as other methods, such as track-based spatial statistics have proven to be superior to region of interest (ROI) measurement.70 However, while other ROI in different areas are more troublesome, ROI in the CC has a high interrater agreement.71
CC biometry in school-age children who were VPT is related with both cognitive and motor outcomes. Length and splenium measurements appear to be good biomarkers of neurological outcome in this population. Future studies are needed to demonstrate the usefulness of these measures in the neonatal period as the most reliable, within the study of the CC as a white matter biomarker.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Raybaud, C. The corpus callosum, the other great forebrain commissures, and the septum pellucidum: anatomy, development, and malformation. Neuroradiology 52, 447–477 (2010).
Suarez, R., Gobius, I. & Richards, L. J. Evolution and development of interhemispheric connections in the vertebrate forebrain. Front. Hum. Neurosci. 8, 497 (2014).
Panigrahy, A., Barnes, P. D., Robertson, R. L., Sleeper, L. A. & Sayre, J. W. Quantitative analysis of the corpus callosum in children with cerebral palsy and developmental delay: correlation with cerebral white matter volume. Pediatr. Radio. 35, 1199–1207 (2005).
Andronikou, S. et al. Corpus callosum thickness on mid-sagittal MRI as a marker of brain volume: a pilot study in children with HIV-related brain disease and controls. Pediatr. Radio. 45, 1016–1025 (2015).
Andronikou, S. et al. Corpus callosum thickness in children: an MR pattern-recognition approach on the midsagittal image. Pediatr. Radio. 45, 258–272 (2015).
Balevich, E. C. et al. Corpus callosum size and diffusion tensor anisotropy in adolescents and adults with schizophrenia. Psychiatry Res. 231, 244–251 (2015).
Purisch, S. E. & Gyamfi-Bannerman, C. Epidemiology of preterm birth. Semin. Perinatol. 41, 387–391 (2017).
Moore, T. et al. Neurological and developmental outcome in extremely preterm children born in England in 1995 and 2006: the EPICure studies. BMJ 345, e7961 (2012).
Pierrat, V. et al. Neurodevelopmental outcome at 2 years for preterm children born at 22 to 34 weeks’ gestation in France in 2011: EPIPAGE-2 cohort study. BMJ 358, j3448 (2017).
Pierrat, V. et al. Neurodevelopmental outcomes at age 5 among children born preterm: EPIPAGE-2 cohort study. BMJ 373, n741 (2021).
Schneider, J. & Miller, S. P. Preterm brain injury: white matter injury. Handb. Clin. Neurol. 162, 155–172 (2019).
Volpe, J. J. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 8, 110–124 (2009).
Back, S. A. White matter injury in the preterm infant: pathology and mechanisms. Acta Neuropathol. 134, 331–349 (2017).
Volpe, J. J. Cerebral white matter injury of the premature infant-more common than you think. Pediatrics 112, 176–180 (2003).
Dyet, L. E. et al. Natural history of brain lesions in extremely preterm infants studied with serial magnetic resonance imaging from birth and neurodevelopmental assessment. Pediatrics 118, 536–548 (2006).
Buser, J. R. et al. Arrested preoligodendrocyte maturation contributes to myelination failure in premature infants. Ann. Neurol. 71, 93–109 (2012).
Woodward, L. J., Anderson, P. J., Austin, N. C., Howard, K. & Inder, T. E. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N. Engl. J. Med. 355, 685–694 (2006).
Inder, T. E., Anderson, N. J., Spencer, C., Wells, S. & Volpe, J. J. White matter injury in the premature infant: a comparison between serial cranial sonographic and MR findings at term. AJNR Am. J. Neuroradiol. 24, 805–809 (2003).
Maalouf, E. F. et al. Comparison of findings on cranial ultrasound and magnetic resonance imaging in preterm infants. Pediatrics 107, 719–727 (2001).
Pierson, C. R. et al. Gray matter injury associated with periventricular leukomalacia in the premature infant. Acta Neuropathol. 114, 619–631 (2007).
Kidokoro, H., Neil, J. J., Inder, T. E. & New, M. R. imaging assessment tool to define brain abnormalities in very preterm infants at term. AJNR Am. J. Neuroradiol. 34, 2208–2214 (2013).
Agut, T. et al. Preterm white matter injury: ultrasound diagnosis and classification. Pediatr. Res. 87, 37–49 (2020).
Narberhaus, A. et al. Gestational age at preterm birth in relation to corpus callosum and general cognitive outcome in adolescents. J. Child Neurol. 22, 761–765 (2007).
Caldu, X. et al. Corpus callosum size and neuropsychologic impairment in adolescents who were born preterm. J. Child Neurol. 21, 406–410 (2006).
Anderson, N. G., Laurent, I., Cook, N., Woodward, L. & Inder, T. E. Growth rate of corpus callosum in very premature infants. AJNR Am. J. Neuroradiol. 26, 2685–2690 (2005).
Siffredi, V. et al. Corpus callosum structural characteristics in very preterm children and adolescents: developmental trajectory and relationship to cognitive functioning. Dev. Cogn. Neurosci. 60, 101211 (2023).
Gooijers, J. & Swinnen, S. P. Interactions between brain structure and behavior: the corpus callosum and bimanual coordination. Neurosci. Biobehav. Rev. 43, 1–19 (2014).
Malavolti, A. M. et al. Association between corpus callosum development on magnetic resonance imaging and diffusion tensor imaging, and neurodevelopmental outcome in neonates born very preterm. Dev. Med. Child Neurol. 59, 433–440 (2017).
van Pul, C. et al. Quantitative fiber tracking in the corpus callosum and internal capsule reveals microstructural abnormalities in preterm infants at term-equivalent age. AJNR Am. J. Neuroradiol. 33, 678–684 (2012).
Nagy, Z. et al. Preterm children have disturbances of white matter at 11 years of age as shown by diffusion tensor imaging. Pediatr. Res. 54, 672–679 (2003).
Merlini, L., Anooshiravani, M., Kanavaki, A. & Hanquinet, S. Microstructural changes in thickened corpus callosum in children: contribution of magnetic resonance diffusion tensor imaging. Pediatr. Radio. 45, 896–901 (2015).
Cirino, P. T. et al. Measuring socioeconomic status: reliability and preliminary validity for different approaches. Assessment 9, 145–155 (2002).
Asztalos, E. V. et al. Association between primary caregiver education and cognitive and language development of preterm neonates. Am. J. Perinatol. 34, 364–371 (2017).
Drobyshevsky, A. et al. Developmental changes in diffusion anisotropy coincide with immature oligodendrocyte progression and maturation of compound action potential. J. Neurosci. Off. J. Soc. Neurosci. 25, 5988–5997 (2005).
Garel, C. et al. Biometry of the corpus callosum in children: MR imaging reference data. AJNR Am. J. Neuroradiol. 32, 1436–1443 (2011).
Witelson, S. F. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain 112, 799–835 (1989).
Duara, R. et al. Neuroanatomic differences between dyslexic and normal readers on magnetic resonance imaging scans. Arch. Neurol. 48, 410–416 (1991).
Hofer, S. & Frahm, J. Topography of the human corpus callosum revisited-comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage 32, 989–994 (2006).
de Lacoste, M. C., Kirkpatrick, J. B. & Ross, E. D. Topography of the human corpus callosum. J. Neuropathol. Exp. Neurol. 44, 578–591 (1985).
Shin, Y. W. et al. Sex differences in the human corpus callosum: diffusion tensor imaging study. Neuroreport 16, 795–798 (2005).
Westerhausen, R. et al. Effects of handedness and gender on macro- and microstructure of the corpus callosum and its subregions: a combined high-resolution and diffusion-tensor MRI study. Brain Res. Cogn. 21, 418–426 (2004).
Wechsler, D. WISC-V: Technical and Interpretive Manual. 1-268. PsychCorp (2013).
Henderson S, S. D., Barnett A. Movement Assessment Battery for Children-Second Edition, (Movement ABC-2) (The Psychological Corporation, 2007).
Cayam-Rand, D. et al. Interaction between preterm white matter injury and childhood thalamic growth. Ann. Neurol. 90, 584–594 (2021).
Sheikhi, S., Saboory, E. & Farjah, G. H. Correlation of nerve fibers in corpus callosum and number of neurons in cerebral cortex: an innovative mathematical model. Int J. Neurosci. 128, 995–1002 (2018).
Blaauw, J. & Meiners, L. C. The splenium of the corpus callosum: embryology, anatomy, function and imaging with pathophysiological hypothesis. Neuroradiology 62, 563–585 (2020).
Allin, M. et al. Growth of the corpus callosum in adolescents born preterm. Arch. Pediatr. Adolesc. Med. 161, 1183–1189 (2007).
Peterson, B. S. et al. Regional brain volumes and their later neurodevelopmental correlates in term and preterm infants. Pediatrics 111, 939–948 (2003).
Skranes, J. et al. Clinical findings and white matter abnormalities seen on diffusion tensor imaging in adolescents with very low birth weight. Brain 130, 654–666 (2007).
Nosarti, C. et al. Corpus callosum size and very preterm birth: relationship to neuropsychological outcome. Brain 127, 2080–2089 (2004).
Moses, P. et al. Regional size reduction in the human corpus callosum following pre- and perinatal brain injury. Cereb. Cortex 10, 1200–1210 (2000).
Rademaker, K. J. et al. Larger corpus callosum size with better motor performance in prematurely born children. Semin. Perinatol. 28, 279–287 (2004).
Nosarti, C. et al. Preterm birth and structural brain alterations in early adulthood. Neuroimage Clin. 6, 180–191 (2014).
Peterson, B. S. et al. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA 284, 1939–1947 (2000).
Fryer, S. L. et al. Microstructural integrity of the corpus callosum linked with neuropsychological performance in adolescents. Brain Cogn. 67, 225–233 (2008).
Luders, E. et al. Positive correlations between corpus callosum thickness and intelligence. Neuroimage 37, 1457–1464 (2007).
Moreno, M. B., Concha, L., Gonzalez-Santos, L., Ortiz, J. J. & Barrios, F. A. Correlation between corpus callosum sub-segmental area and cognitive processes in school-age children. PLoS One 9, e104549 (2014).
Cuzzilla, R. et al. Relationships between early postnatal cranial ultrasonography linear measures and neurodevelopment at 2 years in infants born at <30 weeks’ gestational age without major brain injury. Arch. Dis. Child. Fetal Neonatal Ed. 108, 511–516 (2023).
Huppi, P. S. et al. Microstructural development of human newborn cerebral white matter assessed in vivo by diffusion tensor magnetic resonance imaging. Pediatr. Res. 44, 584–590 (1998).
O’Gorman, R. L. et al. Tract-based spatial statistics to assess the neuroprotective effect of early erythropoietin on white matter development in preterm infants. Brain 138, 388–397 (2015).
Thompson, D. K. et al. Accelerated corpus callosum development in prematurity predicts improved outcome. Hum. Brain Mapp. 36, 3733–3748 (2015).
Cahill-Rowley, K. et al. Prediction of gait impairment in toddlers born preterm from near-term brain microstructure assessed with DTI, using exhaustive feature selection and cross-validation. Front Hum. Neurosci. 13, 305 (2019).
Mercuri, E. et al. Evaluation of the corpus callosum in clumsy children born prematurely: a functional and morphological study. Neuropediatrics 27, 317–322 (1996).
Rudisch, J., Butler, J., Izadi, H., Birtles, D. & Green, D. Developmental characteristics of disparate bimanual movement skills in typically developing children. J. Mot. Behav. 50, 8–16 (2018).
Muetzel, R. L. et al. The development of corpus callosum microstructure and associations with bimanual task performance in healthy adolescents. Neuroimage 39, 1918–1925 (2008).
Hung, Y. C., Robert, M. T., Friel, K. M. & Gordon, A. M. Relationship between integrity of the corpus callosum and bimanual coordination in children with unilateral spastic cerebral palsy. Front Hum. Neurosci. 13, 334 (2019).
Hutchinson, A. D. et al. Relationship between intelligence and the size and composition of the corpus callosum. Exp. Brain Res. 192, 455–464 (2009).
Karolis, V. R. et al. Volumetric grey matter alterations in adolescents and adults born very preterm suggest accelerated brain maturation. Neuroimage 163, 379–389 (2017).
Young, J. M. et al. White matter microstructural differences identified using multi-shell diffusion imaging in six-year-old children born very preterm. Neuroimage Clin. 23, 101855 (2019).
Lebel, C., Treit, S. & Beaulieu, C. A review of diffusion MRI of typical white matter development from early childhood to young adulthood. NMR Biomed. 32, e3778 (2019).
Ozturk, A. et al. Regional differences in diffusion tensor imaging measurements: assessment of intrarater and interrater variability. AJNR Am. J. Neuroradiol. 29, 1124–1127 (2008).
Funding
This study was funded by the Cádiz integrated territorial initiative for biomedical research, European Regional Development Fund (ERDF) 2014-2020 (ITI-0019-2019), the Department of Health and Families, Andalusian Regional Government, 2020; grant number PI-0016-2020 and the Department of Economic Transformation, Industry, Knowledge, and Universities. Andalusian Regional Government. Project co-funded by 80% by the European Union, within the framework of the ERDF Andalusia 2014-2020 Operational Program; grant number P20-00915. Manuel Lubián-Gutiérrez has a “Rio Hortega” research training contract (CM22/00100) from the Ministry of Science, Innovation and Universities (Instituto de Salud Carlos III), Spanish Government.
Author information
Authors and Affiliations
Contributions
M.L.-G., I.B.-F., and S.P.L.-L. have played a fundamental role in the conception and design of the work, in the analysis and interpretation of the study data, in the editing of the paper and in the approval of its final version. A.Z.-O. has actively contributed to the data acquisition, interpretation/measurement of MRI images, and has been involved in the approval of the final version of the paper. Y.M.-A., N.J.-L. and Y.S.-S. have actively contributed to the data acquisition, psychological assessment, and has been involved in the approval of the final version of the document.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Informed consent was obtained from all participants included in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lubián-Gutiérrez, M., Benavente-Fernández, I., Marín-Almagro, Y. et al. Corpus callosum long-term biometry in very preterm children related to cognitive and motor outcomes. Pediatr Res (2024). https://doi.org/10.1038/s41390-023-02994-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-023-02994-4