Abstract
Background
The aim of this study was to develop reference renal saturation (rSrO2) curves in premature infants, depict how they differ from cerebral saturation (rScO2) curves, and evaluate the effect of blood pressure on these values using near-infrared spectroscopy (NIRS).
Methods
This is a prospective cohort study of 57 inborn infants <12 h and <30 weeks gestation. rScO2, rSrO2, fractional tissue oxygen extraction (FTOE), and mean arterial blood pressure (MAP) were continuously monitored every 30 s for 96 h. Quantile regression was used to establish nomograms, and mean saturation values were evaluated for different MAP ranges.
Results
Median rSrO2 at the start of monitoring was ~10% higher than rScO2. rSrO2 showed a significant decline over time while rScO2 peaked at 26 h. FTOE demonstrated a similar but inverse trend to their saturation counterparts. rScO2 declined as MAP increased, while rSrO2 showed a peak and decline as MAP increased.
Conclusions
We provide rSrO2 reference curves for the first 4 days of life, which differ in their trajectory from rScO2 and from what has previously been reported for rSrO2 in the full-term population. In addition, we observed a peak and decline in renal saturation with increasing MAP, suggesting a renovascular response to blood pressure changes.
Impact
-
This article depicts reference renal saturation curves during the perinatal transition in preterm infants.
-
We show how renal saturation compares to cerebral saturation trends over time.
-
We describe a peak and decline in renal saturation with increasing MAP, suggesting a renovascular response to blood pressure changes.
Similar content being viewed by others
Introduction
Hemodynamic assessment and management are particularly challenging in extremely preterm infants. Currently, there is no consensus and high variability among centers in defining normal blood pressure in this population.1,2 The blood pressure threshold that would ensure adequate tissue substrate delivery to prevent ischemic injury is unknown. In addition, blood pressure in this population has been shown to be a poor surrogate for assessing cardiac output.3,4 Due to these challenges, the use of non-invasive monitoring to assess organ status is increasing.
Near-infrared spectroscopy (NIRS) is a tool used to non-invasively measure regional oxygen saturation. Given the vulnerability of the premature infant to cranial injury during periods of hemodynamic instability, the majority of research incorporating NIRS has focused on cerebral monitoring. However, focusing solely on cerebral monitoring may leave infants vulnerable to silent ischemia in the mesenteric and splanchnic circulations during periods of compensated shock.5,6,7
The renal circulation in premature infants is more sensitive to episodes of desaturation than the cerebral circulation with resulting increase in oxygen extraction in the renal tissue as measured by NIRS.8 Recent studies have shown low renal saturations in the first day of life to be a predictor of acute kidney injury in premature infants.9,10 Acute kidney injury in this population has been shown to increase morbidity and mortality. Renal saturation is also more strongly positively correlated with rising hemoglobin values and a hemodynamically significant patent ductus arteriosus than cerebral saturation.11,12,13,14 In full-term post-operative cardiac neonates, the use of two-site NIRS monitoring (cerebral and renal) is a better surrogate marker for both mixed venous oxygen saturation and cardiac index than cerebral saturation alone.15,16
The combination of simultaneous cerebral and renal monitoring may prove beneficial in evaluating hemodynamic status in the premature population. However, data establishing normal values for renal saturation over time in premature infants are limited and are a major barrier to using this information clinically in this population. The objective of this study was to provide reference curves for cerebral and renal NIRS measurements in infants <30 weeks gestation in the first 4 days of life. We also aimed to evaluate how these measurements relate to blood pressure with the hypothesis that renal saturation will be more responsive to fluctuations in blood pressure than cerebral saturations.
Methods
Population and data collection
This was a prospective cohort study of newborns 240–296 weeks gestation admitted to the University of Maryland Medical Center Neonatal Intensive Care Unit (NICU) who had continuous intra-arterial blood pressure monitoring within the first 12 h of life. Subjects were excluded if they had known congenital heart disease, genetic syndromes, multiple anomalies, or if parents declined study participation. Patients were removed from analysis if they had <12 h of continuous monitoring or died within the monitoring period. This study was approved by the University of Maryland School of Medicine Institutional Review Board, and informed parental consent was obtained per IRB guidelines. Obstetric and neonatal demographic and clinical data were obtained from the medical record for each patient.
Continuous data monitoring and processing
Cerebral and renal saturations were evaluated with the NIRS device (INVOS 5100C Regional Saturation Monitor, Covidien, Mansfield, MA) for 96 h or until the intra-arterial line was removed, whichever came first. Neonatal sensors were placed in a uniform fashion over the forehead (cerebral) and posterior flank between T10 and L1 (renal) by a limited number of trained providers. Any movement or replacement in the sensors was noted. The skin under the sensors was checked twice daily to monitor for skin irritation or breakdown. NIRS saturations (cerebral, rScO2 and renal, rSrO2), SpO2 (Nellcor, Medtronic, Minneapolis, MN) and mean arterial blood pressure (MAP) via an indwelling umbilical arterial or peripheral arterial catheter were continuously recorded and time-synchronized every 30 s using a data aggregation device (Vital Sync, Covidien, Mansfield, MA). Fractional tissue oxygen extraction (FTOE) (SpO2-rSO2)/SpO2 was calculated for each data point for both cerebral (FTOE-C) and renal (FTOE-R) saturations. Clinicians were blinded to NIRS data by using the monitor in a research mode that displayed only signal strength, not numbers. The data were retrospectively reviewed and artifacts in MAP and rScO2 such as those due to blood sampling and movement were removed manually prior to analysis. Epochs that contained measurements with significant hypoxemia, SpO2 < 80%, were removed from analysis to avoid inclusion of events affecting the stability of organ tissue metabolic rate similar to previous methodology.17
Statistical analysis
Statistical analysis was performed using SAS 9.3 software (SAS Institute, Inc., Cary, NC).
Univariate analysis of baseline characteristics was conducted. Continuous data were presented as mean (standard deviation) or median (range) depending on the distribution. Categorical data were presented as numbers (%).
Quantile regression modeling (a regression method that can provide a more complete picture of the overall distribution of data)18 was used to construct reference curves over time for rScO2, rSrO2, FTOE-C, and FTOE-R. To allow flexibility in the estimated curves, we modeled these quantities as functions of multiple different functions of time.18 We used this method to estimate the 5th, 10th, 25th, 50th, 75th, 90th, and 95th percentiles of the distributions.
The study period was divided into 60 min epochs starting from the time of birth. Based on previously published data,19 it was decided, a priori, to include factors that may affect regional saturations including birth weight (BW), gestational age, small for gestational age status defined as BW <10%ile, and vasopressor exposure in the model. To assess the relationship of regional saturation over blood pressure ranges, MAP and rSO2 values were time synchronized and averaged in 60 min epochs. We then divided MAPs into range bins, and for each bin, the mean rSO2 and SE were estimated from a mixed effect model to account for repeated measures. Comparisons between both time points and MAP ranges were completed using linear mixed-effect modeling to account for repeated measures, within-patient clustering and controlled for potential covariables.
Results
Patient population
During the study period from June 2013 through January 2016, 62 subjects were enrolled in the study. Five subjects were removed secondary to death within 96 h of monitoring or <12 h of continuous monitoring, leaving 57 subjects for analysis. Twenty-six percent of subjects were enrolled by 6 h of life, and <1% of the data were removed in processing. Study characteristics are shown in Table 1.
Trends over time
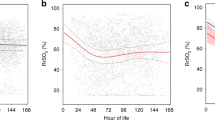
Table 2 presents model estimates and 95% confidence intervals for the 50th quantile for all parameters. A model-based sample reference curve for a 26-week, 850 g AGA infant not exposed to vasopressors is shown for rSO2 and FTOE over time in Fig. 1a–d. Cerebral saturations were noted to peak at 26 h with a statistically significant increase of 4.7% from 12 to 26 h (p < 0.0001). For the 50th quantile, each week increase in gestation increases rScO2 by 2% (p < 0.0001) and vasopressor exposure increases rScO2 by 1% (p = 0.0004). In the lower quantiles, SGA status was noted to decrease the saturation by up to 4% (p < 0.001). FTOE-C demonstrated an inverse distribution with a decrease of −0.06 from 12 to 26 h. For the 50th quantile, every week increase of GA decreases FTOE-C by 0.02 (p < 0.0001) and vasopressor exposure decreases FTOE-C by 0.02 (p < 0.0001). SGA status increased estimates at the higher quantiles up to 0.03 (p < 0.003).
Model estimated quantiles of regional saturations and FTOE for a 26-week, 850 g, AGA infant not exposed to vasopressors (a rScO2, b FTOE-C, c rSrO2, and d FTOE-R) by the hour of life. The 50th percentile is represented by a solid black line, the 25th and 75th percentile by the dashed line, the 10th and 90th percentile by the dash-dot line, and the 5th and 95th percentile are represented by the dotted line.
Compared to cerebral, renal distribution had a wider 95% confidence interval at 96 hours of life. The 50th quantile for renal saturations was approximately 10% higher than cerebral at the start of monitoring but was similar to cerebral measurements by 96 hours of life. In addition, we show that while the cerebral saturation peaks at 26 h of life, renal saturation shows a steady decline of 15% from 12 to 96 h (p < 0.0001). Similarly, FTOE-R steadily increases by 0.19 from 12 to 96 h (p < 0.0001). For the 50% quantile, each gram of BW increases the rSrO2 estimate by 0.004% (p = 0.01), SGA status increases the rSrO2 estimate by 4.5% (p < 0.0001), and vasopressor exposure decreases the estimate by 1.4% (p = 0.01). The effect of GA on the estimate was significant only at the lower quantiles with a decrease of up to 2% for every week of gestation gained (p < 0.03). For FTOE-R, only SGA status and vasopressor exposure had a significant effect on the estimate in the 50th quantile with SGA status decreasing the estimate by 0.05 (p < 0.0001) and vasopressor exposure increasing the estimate by 0.02 (p < 0.0001).
rSO2, FTOE, and blood pressure
Figure 2 depicts regional saturation and FTOE trends over blood pressure ranges using mixed-effect modeling to account for repeated measures. All comparisons between blood pressure bins control for gestational age and vasopressor exposure. rScO2 decreases by 5% (p < 0.0001) from a MAP range of <25 mmHg to a MAP of >50 mmHg. Inversely, we note a significant increase in FTOE-C of 0.05 (p < 0.0001) from the lowest to the highest MAP bin. For rSrO2, there is a significant increase of 4% from the <25 mmHg to the 25–29 mmHg bin (p < 0.0001), a subsequent significant decline of 13.5% from the 25–29 mmHg bin to the 45–49 mmHg, and then an increase of 3.8% as the map increases from the 45–49 mmHg bin to the highest MAP bin (p = 0.03). Inversely, FTOE-R decreases by 0.05 from the <25 mmHg to the 25–29 mmHg bin (p < 0.0001) then subsequently increases by 0.1 from the 25–29 mmHg bin to the > 50 mmHg bin (p < 0.0001).
Mean ± SE of regional saturation(cerebral – solid black, renal solid grey) and FTOE (cerebral dashed black, renal dashed grey) by MAP bin as estimated by mixed-effect modeling. ǂDenotes p < 0.0001 for rSrO2 and FTOE-R as compared to the <25 mmHg bin. #Denotes a p < 0.0001 as compared to the 25–29 mmHg bin for rSrO2 and FTOE-R. *Denotes a p < 0.0001 as compared to the <25 mmHg bin for all points. **Denotes a p = 0.03 as compared to the 45–49 mmHg bin for rSrO2 only. All p values are adjusted for gestational age and vasopressor exposure.
Discussion
To our knowledge, this is the largest cohort of preterm infants <30 weeks GA to provide reference curves of renal saturation over time in the first 96 h of life. We demonstrated that the relationship between cerebral and somatic saturation is different in preterm infants than what has been reported in adults and older children. As compared to values in an older population where somatic values are higher than cerebral values,11 we found that in this population, while rSrO2 was initially higher than rScO2, by day of life 4, the values were similar. In addition, the median value of rSrO2 at 24 h of life of ~80% is also lower than what has been reported for full-term infants where the value is closer to 90%.20,21 This may be a reflection of decreased renal perfusion, increased metabolic demand, or a combination of both. We also note that, unlike cerebral metrics, gestational ages does not have a significant effect on the 50th quantile estimates of either renal saturations or FTOE-R, which is similar to previous findings.11
The preterm infant is undergoing a dramatic transition from intrauterine to extrauterine life in the first 4 days of life. It cannot be assumed that the metabolic activity in the cerebral and somatic tissue will behave in the same manner. In fact, we noted a steady decline in renal saturation with a concomitant increase in FTOE-R over time. This may be explained by the diuretic phase of the preterm infant suggesting an increase in renal metabolic activity during this time period.22,23 In addition, the percentage of cardiac output the fetal kidneys receive is just a fraction of the adult amount and increases dramatically over the first week after birth.24 A study in 14 preterm infants 29–33 weeks gestation noted decreased renal saturation over time; however, the slope was not as steep and may be explained by the more mature population.11 Similar to what we observed, Richter et al.25 demonstrated an increase in FTOE-R from day 1 to 5, which coincides with an increased glomerular filtration rate of nearly eightfold noted in the first week of life.24
We noted a significant effect of growth restriction on renal saturations with an increase of 4% in the median value of rSrO2 and a decrease of 0.05 in the FTOE-R, similar to a small cohort previously published.26 We speculate that the impact of growth restriction may be secondary to delayed renal maturation and lower function leading to decreased oxygen extraction or alternatively, could be secondary, in part, to increased renal blood flow. The latter explanation is supported by previous work that demonstrated that growth-restricted infants maintain similar renal blood flow to their appropriate-sized counterparts despite their smaller size.26 In addition, growth-restricted infants often show accelerated erythropoiesis resulting in elevated levels of hemoglobin which may also contribute to elevated saturations.27 We also noted that size was a more important covariable than gestation in predicting the renal saturation trajectories. It may be that smaller infants have a lower nephron mass which may be exacerbated in situations of growth restriction.28 In contrast to other studies,19,29 we did not note a significant effect of SGA status on cerebral saturations. We potentially attribute this difference to different modeling methods and accounting for BW within our model.
Although somatic data are still limited in the preterm population, there is accumulating information on cerebral saturation alone. In agreement with a large single-center study of nearly 1000 neonates <32 weeks that was conducted to establish normal values in this population,19 we noted similar results with cerebral perfusion related to gestational age and increasing with time to a peak, then slowly declining. These normative values served as the target range for intervention in the SafeBoosC Phase II randomized clinical trial.30 In this trial, the addition of cerebral NIRS targeted therapy reduced the burden of cerebral hyperoxia and hypoxia. This improved cerebral oxygenation stability showed a trend towards reduction in secondary outcomes of severe brain injury and mortality. We speculate that focusing on cerebral saturation alone may have limited the ability to identify the period of compensated shock prior to the development of cerebral hypoxia, and incorporating renal monitoring may better identify infants at risk for severe IVH and death.
The addition of renal monitoring to cerebral monitoring may allow the provider to better recognize compensated shock. Though this study was designed to detect longitudinal trends in regional perfusion, we noted that the rSrO2 showed higher variability than rScO2. This variability is likely a result of tissue circulation vulnerable to fluctuations of blood flow, pressure, and oxygenation.31,32 This increased variability increases the utility of utilizing renal saturations as a trend monitor with often the renal saturation showing a more stark change than cerebral saturation.33 We also show that as blood pressure rises, renal saturation increases to a peak in the 25–29 mmHg bin then falls to a nadir in the 45–49 mmHg MAP bin, where the peak may indicate the blood pressure with optimal renal function. Cerebral saturation, however, shows a steady decline as MAP increases. We speculate that this decrease in regional saturation in response to elevated blood pressure may be due to vascular reactivity. Heightened renal vascular resistance response may be secondary to higher levels of angiotensin II, upregulation of alpha receptors, or a combination of factors.24 A previous study34 used the point of maximal cerebrovascular activity to determine optimal blood pressure in preterm infants. Similar to what we note with renal saturations, the reactivity described by da Costa et al34 is U shaped and worsens on either side of the optimal blood pressure. Further studies will be needed to determine if the renal saturation alone or the cerebral to renal tissue oxygen ratio can be used in a similar manner.
This study is not without limitations. As this was a single-center study, there may be some limitations to generalizing this data given that treatment strategies such as targeted oxygen saturation, ventilation strategies, and blood pressure therapy may differ between institutions. Also, as a referral center, there may have higher rates of maternal pathologies such as pre-eclampsia that may affect the neonate postnatally. Though we controlled for multiple co-morbidities, there may be factors affecting renal saturation that were not accounted for. Though the sensors were placed by a limited number of providers in a uniform fashion to decrease positional variability, the kidney is small, and minor positional differences may affect the accuracy of measurement. There was a very high level of variability noted in the renal saturation measures which should be approached with some caution when drawing conclusions. It has also been shown in animal models that renal saturations may underestimate signals, especially during times of acute hypoxia.35
In conclusion, this study provides reference values for renal saturation in premature infants in the first 4 days of life. Similar to cerebral saturation, renal saturation is affected by postnatal age but is less influenced by gestational age. We also depict the relationship between blood pressure and regional saturation with a peak and subsequent decline as blood pressure increases. Future studies should focus on the incorporation of two-site NIRS monitoring to determine whether real-time indices can be identified to augment clinical management.
References
Dempsey, E. M. & Barrington, K. J. Treating hypotension in the preterm infant: when and with what: a critical and systematic review. J. Perinatol. 27, 469–478 (2007).
Al-Aweel, I. et al. Variations in prevalence of hypotension, hypertension, and vasopressor use in NICUs. J. Perinatol. 21, 272–278 (2001).
Kluckow, M. & Evans, N. Relationship between blood pressure and cardiac output in preterm infants requiring mechanical ventilation. J. Pediatr. 129, 506–512 (1996).
Kluckow, M. & Evans, N. Superior vena cava flow in newborn infants: a novel marker of systemic blood flow. Arch. Dis. Child. Fetal Neonatal Ed. 82, F182–F187 (2000).
Giannini, E. et al. Previous hepatitis B virus infection is associated with worse disease stage and occult hepatitis B virus infection has low prevalence and pathogenicity in hepatitis C virus-positive patients. Liver Int. 23, 12–18 (2003).
Kvarstein, G., Mirtaheri, P. & Tonnessen, T. I. Detection of organ ischemia during hemorrhagic shock. Acta Anaesthesiol. Scand. 47, 675–686 (2003).
Reilly, P. M., Wilkins, K. B., Fuh, K. C., Haglund, U. & Bulkley, G. B. The mesenteric hemodynamic response to circulatory shock: an overview. Shock 15, 329–343 (2001).
Petrova, A. & Mehta, R. Near-infrared spectroscopy in the detection of regional tissue oxygenation during hypoxic events in preterm infants undergoing critical care. Pediatr. Crit. Care Med. 7, 449–454 (2006).
Dorum, B. A., Ozkan, H., Cetinkaya, M. & Koksal, N. Regional oxygen saturation and acute kidney injury in premature infants. Pediatr. Int. 63, 290–294 (2021).
Bonsante, F. et al. Low renal oxygen saturation at near-infrared spectroscopy on the first day of life is associated with developing acute kidney injury in very preterm infants. Neonatology 115, 198–204 (2019).
McNeill, S., Gatenby, J. C., McElroy, S. & Engelhardt, B. Normal cerebral, renal and abdominal regional oxygen saturations using near-infrared spectroscopy in preterm infants. J. Perinatol. 31, 51–57 (2011).
Chock, V. Y., Rose, L. A., Mante, J. V. & Punn, R. Near-infrared spectroscopy for detection of a significant patent ductus arteriosus. Pediatr. Res. 80, 675–680 (2016).
Seidel, D. et al. Changes in regional tissue oxygenation saturation and desaturations after red blood cell transfusion in preterm infants. J. Perinatol. 33, 282–287 (2013).
Aktas, S. et al. Effects of blood transfusion on regional tissue oxygenation in preterm newborns are dependent on the degree of anaemia. J. Paediatr. Child Health 55, 1209–1213 (2019).
Hoffman, G. M., Ghanayem, N. S. & Tweddell, J. S. Noninvasive assessment of cardiac output. Semin. Thorac. Cardiovasc. Surg. Pediatr. Card. Surg. Annu. 12–21 (2005).
Gil-Anton, J. et al. Combined cerebral and renal near-infrared spectroscopy after congenital heart surgery. Pediatr. Cardiol. 36, 1173–1178 (2015).
Alderliesten, T. et al. Cerebral oxygenation, extraction, and autoregulation in very preterm infants who develop peri-intraventricular hemorrhage. J. Pediatr. 162, 698.e2–704.e2 (2013).
Chen, C. An introduction to quantile regression and the quantreg procedure. https://Support.Sas.Com/Resources/Papers/Proceedings/Proceedings/Sugi30/213-30.Pdf (2002).
Alderliesten, T. et al. Reference values of regional cerebral oxygen saturation during the first 3 days of life in preterm neonates. Pediatr. Res. 79, 55–64 (2016).
Bernal, N. P., Hoffman, G. M., Ghanayem, N. S. & Arca, M. J. Cerebral and somatic near-infrared spectroscopy in normal newborns. J. Pediatr. Surg. 45, 1306–1310 (2010).
Bailey, S. M., Hendricks-Munoz, K. D. & Mally, P. Cerebral, renal, and splanchnic tissue oxygen saturation values in healthy term newborns. Am. J. Perinatol. 31, 339–344 (2014).
Bhatia, J. Fluid and electrolyte management in the very low birth weight neonate. J. Perinatol. 26(Suppl 1), S19–S21 (2006).
Modi, N. Management of fluid balance in the very immature neonate. Arch. Dis. Child. Fetal Neonatal Ed. 89, F108–F111 (2004).
Nada, A., Bonachea, E. M. & Askenazi, D. J. Acute kidney injury in the fetus and neonate. Semin. Fetal Neonatal Med. 22, 90–97 (2017).
Richter, A. E. et al. The effect of maternal antihypertensive drugs on the cerebral, renal and splanchnic tissue oxygen extraction of preterm neonates. Neonatology 110, 163–171 (2016).
Terstappen, F. et al. Elevated renal tissue oxygenation in premature fetal growth restricted neonates: an observational study. PLoS ONE 13, e0204268 (2018).
Baschat, A. A. et al. Hematologic profile of neonates with growth restriction is associated with rate and degree of prenatal Doppler deterioration. Ultrasound Obstet. Gynecol. 41, 66–72 (2013).
Zohdi, V. et al. Low birth weight due to intrauterine growth restriction and/or preterm birth: effects on nephron number and long-term renal health. Int. J. Nephrol. 2012, 136942 (2012).
Baik-Schneditz, N. et al. Effect of intrauterine growth restriction on cerebral regional oxygen saturation in preterm and term neonates during immediate postnatal transition. Neonatology 117, 324–330 (2020).
Hyttel-Sorensen, S. et al. Cerebral near infrared spectroscopy oximetry in extremely preterm infants: phase II randomised clinical trial. BMJ 350, g7635 (2015).
Rhee, C. J. et al. Renovascular reactivity measured by near-infrared spectroscopy. J. Appl. Physiol. 113, 307–314 (2012).
Morales, D. L. et al. Restoration of renal function in shock by perfusion of the renal artery with venous blood: a counterintuitive approach. Crit. Care Med. 30, 1297–1300 (2002).
Chock, V. Y., Variane, G. F. T., Netto, A. & Van Meurs, K. P. NIRS improves hemodynamic monitoring and detection of risk for cerebral injury: cases in the neonatal intensive care nursery. J. Matern. Fetal Neonatal Med. 33, 1802–1810 (2020).
da Costa, C. S. et al. Monitoring of cerebrovascular reactivity for determination of optimal blood pressure in preterm infants. J. Pediatr. 167, 86–91 (2015).
Pichler, G., Cheung, P. Y., Tze-Fun, L., Li, E. S. & Schmolzer, G. M. Is renal tissue oxygen desaturation during severe hypoxia underestimated? An observational study in term newborn piglets. Nephrology 20, 107–109 (2015).
Acknowledgements
We wish to thank Elise Janofsky and Ashley Bathgate for their assistance in subject enrollment and data collection. We also wish to thank the families of the children in this study.
Funding
This study is funded by the Mentored Population and Clinical Research Program of the American Heart Association, Award #14CRP18140003 to S.B.H., L.S.M., and R.M.V.
Author information
Authors and Affiliations
Contributions
S.B.H. was responsible for the concept and design, original draft, collection, analysis and interpretation of data, and drafting and revising the manuscript. R.M.V. and L.S.M. were responsible for the concept and design, analysis and interpretation of data, and revising the manuscript. All authors have approved the manuscript as submitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by the University of Maryland Medical Center Institutional Review Board, and informed parental consent was obtained per the IRB guidelines.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hoffman, S.B., Magder, L.S. & Viscardi, R.M. Renal versus cerebral saturation trajectories: the perinatal transition in preterm neonates. Pediatr Res 92, 1437–1442 (2022). https://doi.org/10.1038/s41390-022-01984-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-01984-2
This article is cited by
-
The relationship between trajectories of renal oxygen saturation and acute kidney injury: a prospective cohort study with a secondary analysis
Aging Clinical and Experimental Research (2024)
-
Current state of renal NIRS monitoring in the NICU: results from a CHNC Survey
Journal of Perinatology (2023)
-
Renal oxygenation measured by near-infrared spectroscopy in preterm neonates in the first week
Pediatric Research (2022)