Abstract
Background
Leucocytes for individuals during pregnancy may form into different trajectory patterns. Since no studies have been conducted, we aim to examine the associations between leucocyte trajectory across pregnancy and offspring’s birth outcomes and growth during the first 2 years.
Methods
We conducted a retrospective study enrolled 1070 singleton pregnancies aged 21–46 years old between 2014 and 2018 in Huazhong University of Science and Technology Union Shenzhen Hospital, China. Leucocyte trajectories were modelled using growth mixture modelling and four trajectories were identified: moderate-increasing (n = 41), low-stable (n = 828), high-decreasing (n = 145) and low-increasing (n = 56).
Results
Relative to the low-stable group, logistic regression analysis after adjusting for covariates indicated that the odds ratios of preterm were 3.06 (95% confidence interval (CI): 1.43–6.23) for moderate-increasing, 0.78 (95% CI: 0.38–1.47) for high-decreasing and 0.68 (95% CI: 0.23–1.61) for the low-increasing group, respectively. By using generalized estimating equation analysis, we observed that infants in the moderate-increasing and low-increasing group had −0.35 and −0.21 (P < 0.01) lower head circumference z-score compared with the low-stable group, respectively. No significant association of leucocyte trajectory with other birth weight measures or anthropometric measure z-scores was found.
Conclusions
Changes in leucocytes across pregnancy affected the occurrence of preterm and offspring’s head circumference during the first 2 years of life.
Impact
-
Previous researches on the association of leucocytes with pregnancy outcomes mainly focused on leucocytes in a specific trimester.
-
No studies until now have been conducted to assess the influences of the leucocyte trajectories on the growth and development of infants.
-
Changes in leucocytes across pregnancy affected the occurrence of preterm and offspring’s head circumference during the first 2 years of life.
-
Our study will positively contribute to the dialogue regarding the treatment of pregnancies with different levels of inflammation in each trimester to minimize adverse pregnancy outcomes and optimize brain growth.
Similar content being viewed by others
Introduction
Maternal health during gestational development not only exerts long-lasting effects on the health consequences of the mother herself later in life but also has the capacity to contribute substantially to the offspring’s lifelong health through an altered in utero environment.1 Previous studies have suggested that systematic inflammation played an important role in the pathophysiology of common and serious pregnancy events.2,3 Leucocyte, a marker of subclinical inflammation that can be easily and inexpensively determined, has been included in the common perinatal screening tests instead of tumour necrosis factor-alpha and C-reactive protein in China. To investigate the impacts of leucocytes on birth outcomes and infants’ growth are worthier of clinical application and promotion.
There is increasing evidence suggesting that raising leucocytes can drive adverse birth events, including loss of pregnancy, preterm birth, intrauterine growth restriction and low birth weight (LBW).4,5,6 In addition, the promoting effect of leukocytosis on adverse pregnancy outcomes has been, respectively, verified in the first,5 second7 and third trimesters.6,7 However, pregnancy is a complex and dynamic progression of regulation of the immune system at mother–foetal interface (MFI).8 Invasion of environmental pathogens,9 change in weight,10 development of gestational diabetes mellitus11 and marked lifestyle transitions12 during pregnancy may alter immune status, resulting in the leucocyte concentration for individuals during pregnancy into different trajectory patterns. Previous researches on the association of leucocytes with pregnancy outcomes mainly focused on leucocytes in a specific trimester; it remains unknown whether the leucocyte trajectories in a different stage of pregnancy impact the risk of adverse birth outcomes. Also, no studies until now have been conducted to assess the influences of the leucocyte trajectories on the growth and development of infants.
Therefore, the aim of the current study was to examine the effects of leucocyte change trajectory across pregnancy on adverse pregnancy outcomes as well as offspring’s growth during the first 2 years of life in Chinese.
Materials and methods
Study population
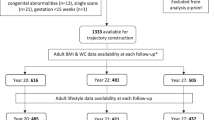
This is a retrospective cohort study of pregnant women with singleton attending for at least one routine hospital antenatal visit at each trimester delivered at Huazhong University of Science and Technology Union Shenzhen Hospital, between January 2016 and May 2018. A total of 3257 women who underwent their first routine hospital antenatal visit during 7–12 gestational weeks were enrolled. We excluded women who were missing leucocyte data in any pregnancy (n = 1388), had chronic infectious diseases such as hepatitis and liver cirrhosis (n = 226), renal diseases such as glomerulonephritis and hydronephrosis (n = 137), in vitro fertilization (n = 14), gestational diabetes mellitus or preexisting diabetes (n = 398), main pregnancy complications (intrahepatic cholestasis of pregnancy, placental abruption, placenta previa, pregnancy-induced hypertension etc., n = 22) and pregnancies complicated by venereal diseases such as syphilis and acquired immune deficiency syndrome (n = 2). After further excluding those without delivery records, 1092 participants were left for the analysis (Fig. 1). Ethics approval was obtained from the Ethics Committee of the Union Shenzhen Hospital of Huazhong University of Science and Technology (No. 2019072644).
Data collection and biochemical measurements
Data on maternal demographic characteristics, obstetric and medical history and maternal weight and height were obtained by a standardized questionnaire at the first antenatal visit. The questionnaire was reviewed by an obstetrician together with a nurse. All collecting fasting blood samples were processed within 1 h. Automated blood cell counts for leucocytes were performed on the Sysmex XN9000 (Sysmex Corporation, Kobe, Japan). The intra- and inter-day coefficients were 2.10% and 4.04%, respectively.
Pregnancy outcomes and anthropometric measurements
Measurements of the infants’ weight (kg, recorded with a precision of 0.1), height (cm, precision: 0.1 cm) and head circumference (cm, precision: 0.1 cm) were taken by trained personnel at birth and at 12 (±15 days) and 24 (±15 days) months using a standard stadiometer, digital panel indicator scale and measuring tape. Body mass index (BMI) was calculated as weight (kilogram)/height in metres square. Large-for-gestational-age (LGA) and small-for-gestational-age (SGA) neonates were defined as neonatal birth weight > 90th and <10th centiles for gestational age, respectively, according to Chinese sex-specific reference.13 LBW was classified as birth weight under 2500 g and macrosomia as a birth weight over 4000 g. Births before 37 weeks of gestation were classified as preterm.
Covariate
Covariates were selected a priori based on known associations with outcome. Factors included age, maternal education (junior high school or lower, senior high school and university or above), parental education (junior high school or lower, senior high school and university or above), foetal sex (male or female), parity (nulliparous or parous), folate supplementation during pregnancy (never taking or ever taking), alcohol consumption during pregnancy (ever drinking or never drinking), passive smoking from husband (yes or no), family history of diabetes (yes or no) and pre-pregnancy BMI calculated based on the self-reported pre-pregnancy weight in kilograms and height in centimetre at their first antenatal visit.
Statistical analysis
The growth mixture modelling was applied to explore the change trajectory of leucocytes during different pregnancy periods.14 First, we started by fitting a quartic polynomial to the models, increasing the number of groups up to five groups one by one. Model fit was assessed based on the Bayesian information criterion and chose the best-fitted model. Second, we compared the models with polynomial order (cubic, quadratic and linear) in order to determine the best shape of trajectory. The average posterior probability was used to verify the accuracy of the model, which should be up to 0.7. Besides, each group should include at least 1% of the population.15 A priori knowledge of the topic combined with an evaluation of the graphical shape of the trajectories was also considered. We coded distinct trajectories as a categorical variable and named them based on their visual appearance.
Mean values were calculated for leucocytes evaluated more than one time in the same trimester. Quantitative data were expressed as means ± standard deviation (SD) and categorical data were presented as n (%). Comparisons of proportions or means of baseline characteristics between groups were performed using the χ2 test or Fisher’s exact test or analysis of variance when appropriate. Odds ratios from all logistic regression models were computed with the low-stable group throughout pregnancy as the referent group after controlling for confounders.
As there were <15% of missing observations in growth and development during the first 2 years, missing values were filled by multiple imputations using the R package mice.16 To assess the effect of leucocyte trajectory on growth and development during the first 2 years. Weight-for-age, length-for-age, weight-for-length, BMI-for-age and head circumference-for-age z-scores were calculated according to the World Health Organization Child Growth Standards.17 Analysis of covariance (ANCOVA) was used for multivariable adjustment when comparing z-scores at birth between groups. The relationship between leucocyte and its trajectory and z-scores at 12 and 24 months of age was determined by generalized estimating equation analyses with an unstructured correlation matrix. A sensitivity analysis was performed without missing values to assess the influence of missing data on the analysis.
In order to explore more details, a cumulative average of leucocyte, leucocyte in the first, second and third trimester and SD of leucocyte were regarded as categorical variables (three tertiles) as well as continuous variables included in logistic regression. A restricted cubic spline regression model with five knots at 10, 25, 50, 75 and 90th percentiles independent variables were applied to detect non-linear association. All the statistical analyses were performed using the software R (version 4.0.0). Two-tailed P < 0.05 was considered to indicate statistical significance.
Results
Leucocyte trajectory during pregnancy
In the exploratory analysis, we modelled the leucocyte trajectory during the pregnancy period controlling for age and parity. Based on the statistical results, we determined that a four-group solution with linear term most ideally fit the data (Fig. 2 and Supplementary Table 1): 77.38% (n = 828) of participants kept a low leucocyte concentration during pregnancy (average leucocyte concentration range from 8.11 × 109/L in the first trimester to 8.80 × 109/L in the third trimester; referred as ‘low-stable pattern’); 13.55% (n = 145) of participants had elevated leucocyte concentration and then experienced a decrease (average leucocyte concentration range from 13.03 × 109/L in the first trimester to 11.58 × 109/L in the third trimester; referred as ‘high-decreasing pattern’); 5.23% (n = 56) of participants had initially moderate leucocyte concentration and then experienced an increase (average leucocyte concentration range from 8.07 × 109/L in the first trimester to 11.13 × 109/L in the third trimester; referred as ‘low-increasing pattern’) and 3.83% (n = 41) of participants had consistently elevated leucocyte concentration (average leucocyte concentration range from 10.06 × 109/L in the first trimester to 14.54 × 109/L in the third trimester; referred as ‘low-increasing pattern’).
Characteristics of the study population
Demographic and clinical data are shown in Table 1. Women in the low-increasing group were more likely to be older, better educated, nulliparous. Women in the moderate-increasing group were less educated and had a higher rate of nulliparous. There are no differences in demographic and clinical characteristics between participants included in the analysis and excluded from the analysis due to the insufficiency of repeated leucocyte measures (Supplementary Table 2).
Association of leucocyte trajectory and adverse birth outcomes
The ANCOVA with covariates reveal that change in leucocyte during pregnancy has a significant effect on head circumference z-score (P = 0.01), but not weight-for-age, height-for-age, weight-for-height, BMI-for-age z-scores at birth (Table 2). Table 3 shows the results of the logistic regression analysis for the association of leucocyte trajectory and the probability of developing adverse pregnancy outcomes. Relative to the low-stable group, the odds ratios of preterm were 3.06 (95% CI: 1.43–6.23) for moderate-increasing, 0.78 (95% CI: 0.38–1.47) for high-decreasing and 0.68 (95% CI: 0.23–1.61) for the low-increasing group after adjustment for measured covariates. However, no association was found between leucocyte trajectory and LBW, SGA, LGA and macrosomia (all P > 0.05).
Association of leucocyte and anthropometric measure z-score
Generalized estimating equation analysis of the relationship between leucocyte trajectory and anthropometric z-scores at birth, 12 and 24 months are shown in Table 4. Infants in the moderate-increasing group and high-decreasing group showed a 0.41 and 0.24 decrease in head circumference-for-age z-scores compared with the low-stable group, respectively. No association was found between the changes in anthropometric z-scores and leucocyte trajectory. Sensitivity analyses performed regarding the removing of missing data gave results similar to the main analysis (Supplementary Table 3).
The associations between leucocytes in the first, second and third trimester and anthropometric z-scores were shown (Supplementary Table 4). For every 1–109/L additional increase of leucocyte in the second and third trimester, the head circumference decreased by 3.65 × 10−2 and 4.53 × 10−2 on average after controlling for confounders during the follow-up period (P = 0.01 and P < 0.01, respectively).
Association of leucocyte level at each pregnancy stage and their average with adverse birth outcomes
High leucocyte level at each pregnancy stage and their average during pregnancy were significantly associated with increased probability of preterm in general, with the odds ratio (OR) for tertiles 3 vs tertiles 1 ranging from 1.44 to 1.56. When continuous values were used for estimation, per SD increasing of leucocyte leads to an 8–9% increase in preterm risk. Women in the top tertile group of leucocytes in the first trimester had a 78% higher risk of (OR = 1.78, 95% confidence interval (CI): 1.01–3.18) SGA or LBW, in comparison with the bottom tertile group. As for leucocytes in the third trimester, those in the top tertile experienced 43% (OR: 0.56, 95% CI: 0.37–0.83) lower LGA or macrosomia (Supplementary Fig. 1). No evidence of nonlinearity in the relationships between leucocyte and adverse pregnancy outcomes was provided (all P > 0.05 and Supplementary Fig. 2).
Discussion
To our knowledge, we firstly determined the change trajectory patterns of leucocytes during pregnancy with adverse pregnancy outcomes and offspring’s growth. Compared with the low-stable group, women who experienced moderate-increasing exhibited a higher risk of preterm. Offspring delivered by women undergone moderate-increasing and high-decreasing leucocyte trajectory exhibited higher head circumference z-score during the first 2 years in comparison with those born by women with low-stable leucocyte levels across pregnancy.
In the present study, 77.38% of pregnant women had a relatively low and stable level of leucocyte through pregnancy. Women in this group tended to be older, parous and well educated. For women in the low-increasing group, they were younger and more likely to be first-time mothers. Previous studies have shown that women in their first pregnancy may be at a greater chance to have excessive gestational weight gain than multiparas.18 Older and parous mothers may be more adherent to recommended lifestyle choices and thus tend to gain less weight than younger mothers, as strategies to mitigate inflammation level.19,20,21 The different trajectories of leucocytes during pregnancy may be at least partially due to parity and maternal age.
Although ample studies have shown that elevated inflammation during pregnancy leads to preterm birth,5,6,7 only a limited number of studies have been performed on changes of inflammation during pregnancy.22 The current study found that women in the moderate-increasing group but not the low-increasing group had an increased risk of preterm delivery compared with women in the low-stable group. Some studies have indicated that deleterious effects on preterm may be observed if inflammation level exceeds a certain threshold.23,24 Pitiphat et al. matching 117 pairs of preterm and term mothers found out that C-reactive protein levels of more than 8 mg/L in pregnancy were associated with preterm.23 A nested case–control study from the Pregnancy Exposures and Preeclampsia Prevention Study indicated that C-reactive protein ≥8 μg/mL conferred a 2.6- to 2.8-fold increased risk of preterm birth.24 The lack of association between the low-increasing group and preterm in the present study might be related to the lower possibility to meet the threshold value.
The high-decreasing leucocyte trajectory pattern was not associated with preterm delivery in the present study and showed a protective effect of preterm with the moderate-increasing group as reference (data not shown). A meta-analysis including four studies with 518 normal mothers and 506 infants suggested that decreasing maternal inflammation in pregnant women by oral probiotic administration did not reduce the incidence of preterm birth <37 weeks (relative risk: 0.92, 95% CI: 0.32–2.67).25 However, a limited number of studies may lead to selective bias. In vitro studies, anti-inflammatory agents including selenium, short-chain fatty acids and citrus flavone nobiletin suppressed inflammation-induced expression of proinflammatory cytokines and chemokines involved in active labour in human foetal membranes and myometrium, showing potential for the prevention of spontaneous preterm birth as well.26,27,28,29 Although previous studies have shown that elevated inflammation in early pregnancy was subsequently related to rising preterm,30,31 our findings suggested that early control of leukocytosis was favourable to prevent the occurrence of preterm.
Leukocytosis was considered to be a feature of normal pregnancy. The leucocyte counts were gradually and significantly increased from the first to the third trimester, and the change is subsequent to a progressive increase in granulocytes.32 Besides, the maternal immune system was transformed from a proinflammatory state in the first trimester guaranteeing the implant of the blastocyst to an anti-inflammatory state in the second and third trimester allowing tolerance of the semi-allogenic foetus and its rapid growth.33 Specific leucocyte populations mediated the transition from the pro- to the anti-inflammatory state. T lymphocytes took part through a shift from a type 1 T-helper lymphocyte (Th1) response, oriented toward cell-mediated immunity, toward a Th2 response, which favoured humoral immunity by stimulating B cells to increase the production of maternal antibodies and T regulatory cells increased that contribute to the maintenance of immunological tolerance to the foetus by actively suppressing self-reactive lymphocytes.34,35
The MFI may play a vital role in the onset of obstetrical complications including preterm delivery. The protective functions of placental membranes, modulation of a natural killer cell, macrophage and Th cell functions in the maternal component of the MFI by altered expression of unique human leucocyte antigen system class I and regulation of several immunomodulators including prostaglandin E2, interleukin-10 (IL-10) and IL-4 were involved in the development of the embryos.34,36 However, a cellular immunological imbalance causing MFI injury may lead to placental inflammation.8 Certainly, some degree of placental inflammation can cause a decrease in foetal perfusion and foetal hypoxia, resulting in obstetrical complications including preeclampsia.37 Intrauterine inflammation-induced oxidative stress increased the level of hypoxia-inducible factor-1, which was an oxygen-sensitive transcription factor and can bridge hypoxia.38 The mechanistic pathway of how leucocyte exposure may lead to preterm birth is still controversial. Some evidence has shown that inflammatory mediators were involved in uterine activation of contraction-associated proteins, such as oxytocin receptor, connexin 43, prostaglandin-endoperoxide synthase 2 and the prostaglandin F receptor PTGFR, production of uterine stimulants such as prostaglandins from foetal membranes and myometrium involved in the degradation of the foetal membranes and remodelling of the myometrium.39,40
Maternal infection and autoimmune diseases promoted an inflammatory response, inducing a sharp increase in leucocyte count.41,42 Highly increased risk of preterm and other complications was also reported in pregnant women with infection and autoimmune diseases.43,44 Thus, the inclusion of pregnancies with infection and autoimmune diseases may lead to an overestimation of obstetrical complications risk in high-increasing and low-increasing groups. Our data did not confirm any association between leucocyte trajectory and LBW, SGA, LGA and macrosomia. Up to date, there only existed one paper that referred to the association of inflammation change during pregnancy with birth weight. In contrast with our results, a study among 2356 Japanese pregnancies who had full-term singleton delivery reported that the ratio of leucocyte in the third trimester to that in the first trimester was positively associated with SGA birth (OR: 3.02, 95% CI: 1.54–5.92).45 Nevertheless, the ratio of leucocytes in the third trimester to the first trimester ignoring the initial inflammation status in early pregnancy could not comprehensively reflect the dynamic change in inflammation during pregnancy. More researches are needed.
Interestingly, the significant associations of leucocytes with foetal and child growth were mostly found in the second and third trimesters instead of the first trimester. In line with our finding, Daniela and co-workers reported that the predictive performance of preeclampsia was improved only with the values of high-sensitivity C-reactive protein and IL-6 in the second trimester of pregnancy instead of those in the first trimester.46 Similarly, Giollabhui et al. followed 737 pregnant women and their offspring till late childhood and found that the strongest tracking coefficients between foetal growth characteristics and adverse birth outcomes have been presented in late pregnancy among three trimesters.47 It is suggested that the first trimester of pregnancy is a pro-inflammatory phase in order to secure the adequate repair of the uterine epithelium and the removal of cellular debris as an adaption to the presence of the foetus. Differently, the predominant immunological feature was transferred into the induction of an anti-inflammatory state in the second phase of pregnancy and rapid foetal growth and development occurred in the same period.48 Thus, elevated inflammation in mid to late pregnancy may exert a profound effect on the offspring. The evidences of significant association of inflammation in the first trimester were also provided in several studies;30,49,50 however, a one-time measure of inflammation at baseline may underestimate the true effect of inflammation on pregnancy outcomes and offspring. Anti-inflammatory agents25,26,27 and marked lifestyle transitions12 in mid-late pregnancy may lead to a decrease of inflammation in women who had rising inflammation in the early pregnancy. In our study, 78% of women with initial elevated inflammation were showing a downward trend.
A link between maternal inflammation during pregnancy and the development of the brain in the offspring has been supported by several studies.51,52,53 A repeated-measurement analysis from the LIFECODES birth cohort study has shown the inverse association between the oxidative stress marker 8-isoprostane and head circumference during the pregnancy (β = −0.13, 95% CI: −0.24, −0.02).51 A study with 429 pregnant women in Philippines demonstrated that C-reactive protein was inversely related to offspring body weight (−0.047 ± 0.017 kg/log-mg/L), length (−0.259 ± 0.092 cm/log-mg/L) and head circumference with 2 weeks of birth (−0.102 ± 0.068 cm/log-mg/L).52 Weber et al. reported that elevated protein carbonyls (a marker for oxidative stress) in mothers was associated with a lower head circumference from the results of 200 newborns.53 In addition, long-term injury of inflammation might lead to irreparable damage of functional development of infant’s brain such as autism,54 bipolar disorder55 and schizophrenia.56 Not only the moderate-increasing group but also the low-increasing group was associated with head circumference z-score compared with a low-stable group in our study. It is also in line with the fact that early gestation has been shown to be an important period for brain development, with rapid neuronal multiplication of the forebrain observed at 10–18 weeks, immediately followed by multiplication of glial cells.57
Although the specific guidelines varied in different countries, a routine blood test at each trimester of pregnancy was generally recommended.58 According to the recommendation of the American College of Obstetrics and Gynecologists and Centers for Disease Control and Prevention,59,60 routine blood tests should be undertaken at the first prenatal care visit. Pregnant women with leukocytosis were encouraged to reduce weight gain and undertake a dietary intervention. Sometimes anti-inflammation drugs were additionally prescribed to optimize leucocyte control. The findings of our study may support the use of non-pharmacological interventions or drug therapy or both, shortening follow-up intervals and increasing follow-up times and timely adjustments of treatment plans according to the trajectory of leucocyte in pregnancies with leukocytosis to minimize the risk of preterm delivery or altered foetal growth.
Our study has several strengths with its longitudinal, retrospective design, change trajectory analysis and well-measured covariates. There were also a few notable limitations in this study. First, some confounders were not available such as physical activity and dietary intake, although we have controlled a large number of covariates. Second, given the fact that about 50% of the initial population was excluded because of missing data on leucocytes. To address this issue, we compared the characteristics of women who were excluded because of missing data with those included in the current study and overall found most of them to be similar. Third, the short trajectory period (the pregnancy) was focused on the present study. A longer period contains preconception and more measures of leucocyte were required for further validation of our results. Fourth, the generalizability of the study findings may be limited due to the recruitment of the study population (100% Chinese) and the selection of the study districts. Fifth, there are anthropometric measures about 12.89–14.06% missing data in our study during the follow-up. However, analyses after multiple imputations of the missing values yielded similar findings. Finally, the different intervals of measures may affect the modelling of trajectory patterns, although we based on at least three trimesters’ values without missing data to improve the accuracy of modelling.
In conclusion, the moderate-increasing trajectory of leucocytes was significantly associated with a higher risk of preterm birth and head circumference during the first 2 years. Elevated cumulative average, first-, second- and third-trimester levels were also significantly associated with a higher probability of preterm as well as head circumference during the first 2 years.
References
Neiger, R. Long-term effects of pregnancy complications on maternal health: a review. J. Clin. Med. 6, 76 (2017).
Challis, J. R. et al. Inflammation and pregnancy. Reprod. Sci. 16, 206–215 (2009).
Kalagiri, R. R. et al. Inflammation in complicated pregnancy and its outcome. Am. J. Perinatol. 33, 1337–1356 (2016).
Sabatier, F. et al. Neutrophil activation in preeclampsia and isolated intrauterine growth restriction. Am. J. Obstet. Gynecol. 183, 1558–1563 (2000).
Tzur, T., Weintraub, A., Sergienko, R. & Sheiner, E. Can leukocyte count during the first trimester of pregnancy predict later gestational complications? Archiv. Gynecol. Obstet. 287, 421–427 (2012).
Hsu, W. Y. et al. Low body weight gain, low white blood cell count and high serum ferritin as markers of poor nutrition and increased risk for preterm delivery. Asia Pac. J. Clin. Nutr. 22, 90–99 (2013).
Sun, T. et al. Elevated first-trimester neutrophil count is closely associated with the development of maternal gestational diabetes mellitus and adverse pregnancy outcomes. Diabetes 69, 1401–1410 (2020).
Yang, F., Zheng, Q. & Jin, L. Dynamic function and composition changes of immune cells during normal and pathological pregnancy at the maternal-fetal interface. Front. Immunol. 10, 2317 (2019).
Harjunmaa, U. et al. Periapical infection may affect birth outcomes via systemic inflammation. Oral Dis. 24, 847–855 (2018).
Catalano, P. M. & Shankar, K. Obesity and pregnancy: mechanisms of short term and long term adverse consequences for mother and child. BMJ 356, j1 (2017).
Pantham, P., Aye, I. L. & Powell, T. L. Inflammation in maternal obesity and gestational diabetes mellitus. Placenta 36, 709–715 (2015).
Renault, K. M. et al. Impact of lifestyle intervention for obese women during pregnancy on maternal metabolic and inflammatory markers. Int. J. Obes. 41, 598–605 (2017).
Dai, L. et al. Birth weight reference percentiles for Chinese. PLoS ONE 9, e104779 (2014).
Ram, N. & Grimm, K. J. Growth mixture modeling: a method for identifying differences in longitudinal change among unobserved groups. Int. J. Behav. Dev. 33, 565–576 (2009).
Berlin, K. S., Parra, G. R. & Williams, N. A. An introduction to latent variable mixture modeling (part 2): longitudinal latent class growth analysis and growth mixture models. J. Pediatr. Psychol. 39, 188–203 (2014).
van Buuren, S. & Groothuis-Oudshoorn, K. Mice: multivariate imputation by chained equations in R. J. Stat. Softw. 45, 67 (2011).
de Onis, M. The WHO Child Growth Standards 254–269 (S Karger AG, 2008).
Tabet, M., Harper, L. M., Flick, L. H. & Chang, J. J. Gestational weight gain in the first two pregnancies and perinatal outcomes in the second pregnancy. Paediatr. Perinat. Epidemiol. 31, 304–313 (2017).
Gaillard, R. et al. Risk factors and outcomes of maternal obesity and excessive weight gain during pregnancy. Obesity 21, 1046–1055 (2013).
Rodrigues, P. L., de Oliveira, L. C., Brito Ados, S. & Kac, G. Determinant factors of insufficient and excessive gestational weight gain and maternal-child adverse outcomes. Nutrition 26, 617–623 (2010).
Paulino, D. S., Surita, F. G., Peres, G. B., do Nascimento, S. L. & Morais, S. S. Association between parity, pre-pregnancy body mass index and gestational weight gain. J. Matern. Fetal Neonatal Med. 29, 880–884 (2016).
Harper, M. et al. Change in mononuclear leukocyte responsiveness in midpregnancy and subsequent preterm birth. Obstet. Gynecol. 121, 805–811 (2013).
Pitiphat, W. et al. Plasma C-reactive protein in early pregnancy and preterm delivery. Am. J. Epidemiol. 162, 1108–1113 (2005).
Catov, J. M., Bodnar, L. M., Ness, R. B., Barron, S. J. & Roberts, J. M. Inflammation and dyslipidemia related to risk of spontaneous preterm birth. Am. J. Epidemiol. 166, 1312–1319 (2007).
Grev, J., Berg, M. & Soll, R. Maternal probiotic supplementation for prevention of morbidity and mortality in preterm infants. Cochrane Database Syst. Rev. 12, Cd012519 (2018).
Kalansuriya, D. M., Lim, R. & Lappas, M. In vitro selenium supplementation suppresses key mediators involved in myometrial activation and rupture of fetal membranes. Metallomics 12, 935–951 (2020).
Moylan, H. E. C., Nguyen-Ngo, C., Lim, R. & Lappas, M. The short-chain fatty acids butyrate and propionate protect against inflammation-induced activation of mediators involved in active labor: implications for preterm birth. Mol. Hum. Reprod. 26, 452–468 (2020).
Morwood, C. J. & Lappas, M. The citrus flavone nobiletin reduces pro-inflammatory and pro-labour mediators in fetal membranes and myometrium: implications for preterm birth. PLoS ONE 9, e108390 (2014).
Beksac, M. S., Tanacan, A., Ozten, G. & Cakar, A. N. Low-dose low-molecular-weight heparin prophylaxis against obstetrical complications in pregnancies with metabolic and immunological disorder-associated placental inflammation. J. Matern. Fetal Neonatal Med. 33, 1–8 (2020).
Al-Husban, N., Al-Atrash, H., Alhayek, N., Al-Soud, K. & Alhusban, M. Platelet and white blood cell (WBC) counts in the first trimester and pregnancy outcome: prospective controlled study. J. Fetal Med. 6, 89–94 (2019).
Zhu, H. & Yang, M. J. Maternal plasma concentrations of macrophage migration inhibitory factor at first trimester as a predictive biomarker of preterm delivery in Chinese women. Clin. Chim. Acta 483, 286–290 (2018).
Lurie, S., Rahamim, E., Piper, I., Golan, A. & Sadan, O. Total and differential leukocyte counts percentiles in normal pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 136, 16–19 (2008).
Cinicola, B. et al. The protective role of maternal immunization in early life. Front. Pediatr. 9, 638871 (2021).
Erlebacher, A. Immunology of the maternal-fetal interface. Annu. Rev. Immunol. 31, 387–411 (2013).
Zhang, Y. H. & Sun, H. X. Immune checkpoint molecules in pregnancy: focus on regulatory T cells. Eur. J. Immunol. 50, 160–169 (2020).
Tilburgs, T., Scherjon, S. A. & Claas, F. H. Major histocompatibility complex (Mhc)-mediated immune regulation of decidual leukocytes at the fetal-maternal interface. J. Reprod. Immunol. 85, 58–62 (2010).
Gurbuz, R. H. et al. Impaired placentation and early pregnancy loss in patients with Mthfr polymorphisms and type-1 diabetes mellitus. Fetal Pediatr. Pathol. 38, 376–386 (2019).
Cotechini, T. & Graham, C. H. Aberrant maternal inflammation as a cause of pregnancy complications: a potential therapeutic target? Placenta 36, 960–966 (2015).
Xu, C. et al. Pgf2α regulates the expression of uterine activation proteins via multiple signaling pathways. Reproduction 149, 139–146 (2014).
Xu, C. et al. Effects of Pgf 2α on the expression of uterine activation proteins in pregnant human myometrial cells from upper and lower segment. J. Clin. Endocrinol. Metab. 98, 2975–2983 (2013).
Poblete, A. et al. Fetal and maternal white cells and B- and T-lymphocyte subpopulations in pregnant women with recent infection. Fetal Diagn. Ther. 16, 378–383 (2001).
Han, B. K. et al. Neutrophil and lymphocyte counts are associated with different immunopathological mechanisms in systemic lupus erythematosus. Lupus Sci. Med. 7, e000382 (2020).
Tanacan, A. et al. Impact of extractable nuclear antigen, anti-double stranded DNA, antiphospholipid antibody, and anticardiolipin antibody positivity on obstetrical complications and pregnancy outcomes. Hum. Antibodies 27, 135–141 (2019).
Kolstad, K. D. et al. Preterm birth phenotypes in women with autoimmune rheumatic diseases: a population-based cohort study. BJOG 127, 70–78 (2020).
Harita, N. et al. Increment of absolute neutrophil count in the third trimester and increased risk of small-for-gestational-age birth: Hirakata Risk Associated with Pregnancy Assessment Research (Hirapar). Eur. J. Obstet. Gynecol. Reprod. Biol. 164, 30–34 (2012).
Oancea, M. et al. Evaluation of inflammatory markers in pregnant women at risk, for the prediction of preeclampsia. Acta Med. Marisiensis 60, 94–98 (2014).
Mac Giollabhui, N. et al. Maternal inflammation during pregnancy and offspring psychiatric symptoms in childhood: timing and sex matter. J. Psychiatr. Res. 111, 96–103 (2019).
Mor, G., Cardenas, I., Abrahams, V. & Guller, S. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann. NY Acad. Sci. 1221, 80–87 (2011).
Yang, Y. et al. Relationship between dietary inflammatory index, Hs-Crp level in the second trimester and neonatal birth weight: a cohort study. J. Clin. Biochem Nutr. 66, 163–167 (2020).
Nachman, R. M. et al. Intrauterine inflammation and maternal exposure to ambient Pm2.5 during preconception and specific periods of pregnancy: the Boston Birth Cohort. Environ. Health Perspect. 124, 1608–1615 (2016).
Ferguson, K. K. et al. Associations between repeated ultrasound measures of fetal growth and biomarkers of maternal oxidative stress and inflammation in pregnancy. Am. J. Reprod. Immunol. 80, e13017–e13017 (2018).
Kuzawa, C. W., Fried, R. L., Borja, J. B. & McDade, T. W. Maternal pregnancy C-reactive protein predicts offspring birth size and body composition in Metropolitan Cebu, Philippines. J. Dev. Orig. Health Dis. 8, 674–681 (2017).
Weber, D. et al. Oxidative stress markers and micronutrients in maternal and cord blood in relation to neonatal outcome. Eur. J. Clin. Nutr. 68, 215–222 (2014).
Brown, A. S. et al. Elevated maternal C-reactive protein and autism in a National Birth Cohort. Mol. Psychiatry 19, 259–264 (2014).
Canetta, S. E. et al. Serological documentation of maternal influenza exposure and bipolar disorder in adult offspring. Am. J. Psychiatry 171, 557–563 (2014).
Fineberg, A. M. & Ellman, L. M. Inflammatory cytokines and neurological and neurocognitive alterations in the course of schizophrenia. Biol. Psychiatry 73, 951–966 (2013).
Stiles, J. & Jernigan, T. L. The basics of brain development. Neuropsychol. Rev. 20, 327–348 (2010).
Tran, K. & McCormack, S. Screening and Treatment of Obstetric Anemia: A Review of Clinical Effectiveness, Cost-effectiveness, and Guidelines. CADTH Rapid Response Reports (Canadian Agency for Drugs and Technologies in Health, 2019).
American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 95: anemia in pregnancy. Obstet. Gynecol. 112, 201–207 (2008).
Centers for Disease Control and Prevention. Recommendations to prevent and control iron deficiency in the United States.MMWR Recomm. Rep. 47, 1–29 (1998).
Funding
This work was supported by the Shenzhen Science and Technology Innovation Committee (grant number JCYJ20190809102203602); the Guangdong Basic and Applied Basic Project (grant number 2019A1515110456) and the Shenzhen Nanshan District Science and Technology Project (grant number 2019007).
Author information
Authors and Affiliations
Contributions
H.C. drafted the manuscript and Z.Z. performed the data analysis. Y.L., Y.W., R.S. and G.D. attained the data. Y.Z. and X.L. interpreted the data. L.L. designed the study and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Patient consent
Consent was not required since this is a retrospective cohort study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Chen, H., Zhang, Z., Zhou, Y. et al. Maternal leucocyte trajectory across pregnancy associated with offspring’s growth. Pediatr Res 92, 862–870 (2022). https://doi.org/10.1038/s41390-021-01827-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01827-6