Abstract
Background
Hypoxic–ischemic encephalopathy (HIE) is a major contributor to death and disability worldwide. Remote ischemic postconditioning (RIPC) may offer neuroprotection but has only been tested in preclinical models. Various preclinical models with different assessments of outcomes complicate interpretation. The objective of this systematic review was to determine the neuroprotective effect of RIPC in animal models of HIE.
Methods
The protocol was preregistered at The International Prospective Register of Systematic Reviews (PROSPERO) (CRD42020205944). Literature was searched in PubMed, Embase, and Web of Science (April 2020). A formal meta-analysis was impossible due to heterogeneity and a descriptive synthesis was performed.
Results
Thirty-two papers were screened, and five papers were included in the analysis. These included three piglet studies and two rat studies. A broad range of outcome measures was assessed, with inconsistent results. RIPC improved brain lactate/N-acetylaspartate ratios in two piglet studies, suggesting a limited metabolic effect, while most other outcomes assessed were equally likely to improve or not.
Conclusions
There is a lack of evidence to evaluate the neuroprotective effect of RIPC in HIE. Additional studies should aim to standardize methodology and outcome acquisition focusing on clinically relevant outcomes. Future studies should address the optimal timing and duration of RIPC and the combination with therapeutic hypothermia.
Impact
-
This systematic review summarizes five preclinical studies that reported inconsistent effects of RIPC as a neuroprotective intervention after hypoxia–ischemia.
-
The heterogeneity of hypoxia–ischemia animal models employed, mode of postconditioning, and diverse outcomes assessed at varying times means the key message is that no clear conclusions on effect can be drawn.
-
This review highlights the need for future studies to be designed with standardized methodology and common clinically relevant outcomes in models with documented translatability to the human condition.
Similar content being viewed by others
Introduction
Hypoxic–ischemic encephalopathy (HIE) is a major contributor to death and disability in neonates worldwide.1 Remote ischemic postconditioning (RIPC), achieved by short periods of nonlethal ischemia to a limb after a hypoxic–ischemic (HI) insult, has been investigated as a potential novel neuroprotectant in animal models of HIE.2 To date, there has been no systematic appraisal of the published data on RIPC in HIE to guide the direction and efforts of future studies. Key design issues involve selecting the appropriate model, how to apply RIPC, and the clinical relevance of the assessed outcomes. Knowledge gaps and limitations need to be identified when weighing the potential for translation to clinical studies and treatment of human newborns. RIPC has been proposed as a tissue-protective strategy in a variety of adult conditions including myocardial infarction, cardiac arrest, and stroke.3,4 However, the developing brain may respond differently to injury compared to the adult brain, and neuroprotective treatment may have different effects, as seen with therapeutic hypothermia (TH). Thus, conceivable improvements in the treatment of human neonates have been reached by the translation of preclinical results from experiments in young mammals rather than the extrapolation of results from studies of adult humans. A review of preclinical animal studies can improve transparency regarding the translation from preclinical to clinical trials.5 Thus, the purpose of this systematic review was to evaluate the neuroprotective effect of RIPC in animal models of neonatal HIE.
Materials and methods
Review protocol
This systematic review was conducted in accordance with the Collaborative Approach to Meta-Analysis and Review of Animal Data from Experimental Studies (CAMARADES) and SYstematic Review Center for Laboratory animal Experimentation (SYRCLE).6,7 The protocol was registered in The International Prospective Register of Systematic Reviews (PROSPERO) published on 5 October 2020 (registration number: CRD42020205944).8 We also adopted the preferred reporting items for systematic review and meta-analysis (PRISMA) guidelines.9 In accordance with the PRISMA statement, this systematic review was designed based on the PICO criteria (population, intervention, control, and outcomes) as follows:
-
Types of population: newborn animals exposed to a hypoxic and/or ischemic insult in an experimental setting.
-
Types of intervention: RIPC.
-
Types of comparators: no RIPC.
-
Types of outcome: any measure of neuropathology or neurological impairment.
-
Types of study design: experimental controlled trials.
Search strategy
We searched PubMed, Embase, and Web of Science online databases using the search terms: (hypoxic ischemic encephalopathy OR perinatal asphyxia OR hypoxic ischemic brain injury) AND (postconditioning) AND (newborn OR neonate). The reference lists of included studies were screened for possible additional eligible studies. Only in vivo studies of the newborn (or near to full-term newborn human-equivalent) animal models were included. Studies were allowed any means for the establishment of the insult (e.g., carotid ligation, endotracheal tube clamping, clamping of the umbilical cord, or reduced fraction of inspired oxygen). Intervention (exposed) animals were animals treated with RIPC. Controls (unexposed) were animals subjected to supportive care only, i.e., no neuroprotective intervention. Furthermore, studies were included regardless of the timing, duration, number of cycles, and technique used to achieve RIPC.
Studies were excluded if they did not fulfill the defined PICO criteria. Studies that achieved postconditioning by other means than remote ischemia were excluded. The literature search and screening of publications were performed by two reviewers (T.C.K.A. and K.J.K.). In case of discrepancies, consensus was reached by discussion with a third reviewer (B.S.K.).
Data extraction
Data extraction was performed by one reviewer (T.C.K.A.) and checked for inconsistencies by a second reviewer (K.J.K.). We extracted information on the author, year of publication, animal species, number of animals, randomization or matching procedures, insult characteristics, details on the RIPC procedure (timing after insult, technique, duration, number of cycles), and animal temperature during the experiment, medication including anesthetic drugs provided during the experiment, and detailed information on outcome measurements.
Risk of bias and quality assessment
The risk of bias and quality of the included studies was assessed by two reviewers (T.C.K.A. and K.J.K.). In case of discrepancies, consensus was reached by discussion with a third reviewer (B.S.K.). The quality of the studies was assessed using a modified CAMARADES study quality checklist.10 The modified checklist contained the following: (1) publication in a peer-reviewed journal, (2) randomization to intervention or control, (3) blinded assessment of outcome, (4) statement of control of temperature, (5) sample size calculation, (6) statement of compliance with animal welfare regulations, (7) statement regarding possible conflict of interest, (8) use of an established and suitable animal model, (9) incomplete outcome data, and (10) information on the health status of the animals at baseline. Assessment of the risk of bias was performed using the SYRCLES´s risk of bias tool.11 Publication bias was assessed in a qualitative manner (no funnel plot) due to the few studies identified.
Data analysis
We performed a systematic review including a descriptive summary of the results due to the anticipated heterogeneity between studies that would limit our possibility to conduct a meta-analysis.
Results
Study selection
We found 32 records in PubMed, 32 in Embase, and 11 in Web of Science, resulting in 32 unique records. No further studies were identified after screening the reference list of included studies. After we applied the exclusion criteria and duplicates were removed, five studies were eligible for full-text assessment and included in the qualitative synthesis. Reasons for exclusion were “not original research” (e.g., review or case report), “other postconditioning” (e.g., postconditioning with hypoxia or sevoflurane), and “other reason” (e.g., exposure/intervention incompatible with the PICO criteria or additional neuroprotective treatments used). The study selection process is illustrated in the PRISMA flow chart (Fig. 1).
Study characteristics
All studies were performed in laboratory settings by use of established animal models for HIE. Two were rat studies and three were piglet studies.12,13,14,15,16 The HI insult was established by a combination of cerebral ischemia by carotid artery occlusion and generalized hypoxia by reduced inspired oxygen fraction in four studies.12,13,15,16 In one study, the HI insult was induced by stepwise generalized hypoxia targeting a prespecified level and duration of hypotension.14 In all five studies RIPC was applied by occlusion of blood flow to both hind limbs. One study applied plastic strips,14 two studies used rubber bands,12,13 and two studies used a dedicated device.15,16 RIPC was applied in four cycles in all five studies. One study used 5 min of ischemia and 5 min of reperfusion,14 while four studies used 10 min of ischemia and 10 min of reperfusion.12,13,15,16 Temperature was reported in all studies except one. All reported methods of anesthesia. Study characteristics of the five studies are summarized in Table 1.
Quality of included studies and risk of bias
The median quality score for the five studies was 8 (range 6–10) according to the modified CAMARADES checklist. A summary of the quality scores is presented in Table 2. All studies were published in peer-reviewed journals, used an established animal model, reported possible conflicts of interest, included a statement of compliance with animal welfare regulations, practiced randomization to intervention or control, and blinding of outcome assessment. Four studies reported temperature. Only two of five studies reported sample size calculation and only one study reported the general health status of the animals prior to intervention.
Risk of bias items according to the SYRCLE’s risk of bias tool was difficult to assess for several of the studies due to lack of information. If unclear, assessment is marked with “?” in the summary presented in Table 3. In two of five studies, we were only able to assess the risk of bias for less than half of the different domains. None of the studies reported random selection of animals for outcome assessment, but all studies reported blinded outcome assessment. Four of five studies reported on attrition and animal mortality or morbidity in the follow-up period from insult to assessment of outcome. We were unable to assess the risk of publication bias using the Funnel plot. All studies reported a positive result on some of the acquired outcomes. Accordingly, publication bias is likely.
Results of individual studies
A summary of the different outcomes, time of acquisition, number of animals, and results of included studies are presented in Table 4.
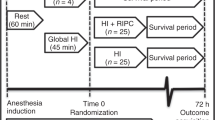
Kyng et al. evaluated the neuroprotective effect of RIPC at 72 h after randomization of siblings of same sex to a HI insult by a gradual decrease of inspired oxygen fraction aiming for a target mean arterial blood pressure in piglets. RIPC was applied 1 h after HI in four cycles of 5 min of ischemia and reperfusion. The outcome was assessed by magnetic resonance imaging (MRI), magnetic resonance spectroscopy (MRS), neuropathology, and neurobehavioral score.14 They found a lower whole-brain lactate/N-acetylaspartate (Lac/NAA) ratio and in the basal ganglia in piglets that received RIPC than in untreated piglets. No difference was found in white matter or the thalamus. The level of cerebral edema, measured by diffusion-weighted imaging, was similar in the two groups as were brain histology and neurobehavioral assessment of the animals.
Rocha-Ferreira et al. evaluated the neuroprotective effect of RIPC indicated by reduced nitrosative stress at 48 h after HI in a piglet model of HIE.16 HI was induced by carotid artery occlusion combined with a decrease of inspired oxygen fraction. RIPC was applied immediately after HI in four cycles of 10 min of ischemia and reperfusion. By use of immunohistochemistry, they found a reduced amount of nitrotyrosine deposits, a product of peroxynitrite, a potent oxidizing and nitrating agent, in the brain of RIPC-treated animals. Consistent with this, they also found reduced expression of inducible nitric oxide synthase (iNOS) and increased expression of endothelial NOS (eNOS) in RIPC-treated animals. No difference was found in neuronal NOS (nNOS). Furthermore, immunohistochemical methods revealed increased microglial and astrocyte activity in RIPC-treated animals.
Ezzati et al. measured the effects of RIPC at 24 and 48 h in a piglet model of HIE.15 HI was induced by carotid artery occlusion combined with a decrease of inspired oxygen fraction. RIPC was applied immediately after HI in four cycles of 10 min of ischemia and reperfusion. Outcomes were MRS, amplitude-integrated electroencephalography/electroencephalography (aEEG/EEG), neuropathology, and gene expression analysis of cerebral tissue. MRS performed 48 h after the insult showed a lower Lac/NAA ratio in white matter and higher whole-brain nucleotide triphosphate/exchangeable phosphate pool in animals treated by RIPC compared to untreated animals. There was no difference in Lac/NAA ratio in the thalamus. Neuropathological analysis showed less terminal deoxynucleotidyl transferase dUTP nick-end labeling-positive cells in the corpus callosum, internal capsule, and periventricular white matter in RIPC-treated animals. Animals exposed to RIPC also showed an increased number of oligodendrocytes in the corpus callosum, and periventricular white matter as well as a lower number of microglia cells in the corpus callosum. Gene expression analysis identified 74 genes with a different response in animals treated with RIPC; 63 genes were downregulated and 11 upregulated. Further analysis of white matter showed reduced gene expression of ABCC9, CART, RGS8, and SLC4 in the RIPC group. aEEG/EEG analysis showed no difference.
Drunalini et al. was the only study that investigated the effect of delayed RIPC, and of RIPC applied at multiple timepoints.12 In 10-day-old rat pups HI was induced by right carotid artery occlusion combined with a decrease of inspired oxygen fraction. RIPC was applied at 24 h in one group and repeatedly at 24, 48, and 72 h in another in four cycles of 10 min of ischemia and reperfusion. Outcomes were neurobehavioral tests, brain weight, gross and microscopic brain tissue morphology, and organ weight. They found a more favorable result of the neurobehavioral test, foot fault test, in the animals that received RIPC at multiple timepoints compared to untreated controls. They found no difference between the two groups that received RIPC compared to the untreated group with regards to other neurobehavioral tests, organ weight, or brain volume loss.
Zhou et al. evaluated the proposed neuroprotective mechanisms of RIPC by the opioid receptor/Akt pathway in 10-day-old rat pups.13 The study defined several groups of animals that received various potential neuroprotective treatments. For this systematic review, only animals exposed to a HI insult that received RIPC and no treatment were extracted. HI was induced by carotid artery occlusion combined with a decrease of inspired oxygen fraction. RIPC was applied immediately after HI in four cycles of 10 min of ischemia and reperfusion. They found better performance in three different neurobehavioral tests in animals treated with RIPC. They also found a higher pAkt density and lower Bax density in cerebral tissue from animals treated with RIPC compared to animals without treatment suggestive of Akt blockage of the Bax-mediated proapoptotic pathway. RIPC-treated animals had small infarct volume but no difference in brain weight.
Discussion
To our knowledge, this is the first systematic review on the potential neuroprotective effect of RIPC in animal models developed to reflect HIE by induction of a standardized HI insult. We identified five studies performed in both small and large animal models with a variety of outcome measurements. Four of five studies included outcome data and one study evaluated only on the potential mechanism of action of RIPC. Due to the differences in studies with respect to animal species, the HI insult, RIPC application, and outcome measurements we were unable to provide a meaningful common quantitative measure of the effect of RIPC in HIE by meta-analysis. Instead, study results were assessed and interpreted by the use of qualitative, structured methods. Whole-brain Lac/NAA ratio was lower in animals treated with RIPC than in controls in both piglet studies using this measure. None of the rat studies reported on this outcome. Results from studies of neuropathology ranged from no difference to fewer TUNEL-positive cells with RIPC treatment. Rats with HIE treated with RIPC performed better than with no RIPC on some short-term behavioral tests, while piglets’ test performance was independent of whether RIPC was provided. Immunohistochemistry and gene expression analysis indicated activation of several protective mechanisms with RIPC. There was great inconsistency in results between studies and the included studies reported several outcome measures for which no effect was found. Four of five included studies applied a localized insult through carotid ligation in combination with hypoxia. Compared to the global hypoxic insult with multiorgan injury in neonatal HIE, carotid ligation risks more similarities with neonatal stroke and may further complicate the translation of results. Whether improvements in specific biomarkers of brain injury and proxy outcomes translate to an overall neuroprotective effect in human neonates warrants further investigation. Several different outcomes analyzed in studies without prespecified primary outcomes increase the risk of bias in interpretation.
MRI and immunohistochemistry
In human observational studies, Lac/NAA ratio has been shown to be an accurate early biomarker of neurodevelopmental outcome in clinical trials of neonatal HIE.17,18,19 In the piglet studies by Kyng et al. and Ezzati et al., RIPC resulted in lower brain Lac/NAA ratios measured by MRS at 48 and 72 h after the HI insult.14,15 The timing of outcome assessment must be taken into account when interpreting results. In the primary phase of injury, lactate production is predominantly caused by anaerobe metabolism.20 At 48 and 72 h after the insult, gas exchange and circulation have been re-established. At this time point in the secondary phase of injury, lactate production most likely results from mitochondrial failure and secondary cell death.21 There was inconsistency in the two studies included in this review where Kyng et al. found a difference in deep gray matter and whole brain, but not in white matter, while Ezzati et al. found a difference in white matter but not deep gray matter.
Zhou et al. investigated the effect of RIPC on the P13K/Akt pathway and found that RIPC-treated animals had increased expression of pAkt at 24 and 48 h after HI.13 They also found reduced Bax expression in RIPC-treated animals, suggestive of Akt-dependent block of the Bax-dependent proapoptotic pathway.22 Rocha-Ferreira et al. found reduced levels of iNOS and reduced amount of nitrotyrosine deposits 48 h after HI in RIPC-treated piglets.15 They also found increased levels of eNOS. Contrary to the two other NOS isoforms, nNOS and iNOS, eNOS activation has been proposed to contribute to neuroprotection through vasodilation.23
Two of the studies included in this review investigated the effect of RIPC on neuromodulation.15,16 Ezzati et al. found reduced expression of Iba1-positive cells in the corpus callosum of RIPC-treated piglets.15 In contrast, Rocha-Ferreira et al. found a higher level of microglial activation of RICP-treated piglets.16 However, the role of microglial activation in HIE is complex as microglia may have pro- or anti-inflammatory properties, depending on polarization.24
Short-term neurobehavioral outcomes
Three of the included studies evaluated the effect of RIPC on short-term neurobehavioral outcomes.12,13,14 Kyng et al. utilized a standardized neurological score for functional assessment of piglets and found similar performance in piglets treated with and without RIPC when evaluated at 24, 48, and 72 h after the HI insult.14 Zhou et al. performed functional assessment 4 weeks after the insult, with modified grip-traction test, forelimb placement test, and back pressure test.13 They found that RIPC-treated rats performed better than untreated controls in all three tests.13 Drunalini et al. performed sensory–motor function tests 5 weeks after HI.12 They found that rats that received RIPC performed better in the foot fault test but not on the wire hang, t-maze, water maze, or beam balance test.12 Consequently, the limited neuroprotective effect of RIPC suggested by MRS and neuropathology data do no correspond to results by neurobehavioral testing in the secondary phase of injury and the effect on neurobehavioral test at later timepoints corresponding to the tertiary phase of injury are inconsistent.25
Limitations
The studies included had a median quality score of 8 (range 6–10) evaluated by the CAMARADES checklist. Only one study reported on the health status of the animals. Inclusion of impaired or sick animals may influence results, in particular, if not equally distributed in the two exposure groups.26 Three of five studies provided no sample size calculations. Sample size calculation is important to reduce the number of animals included in order to comply with high ethical standards and the 3R principle,27 to optimize the possibility of detecting a true effect, and to avoid overestimating an association by chance.28
All studies but one reported on animal temperature control. However, only two studies reported consecutive data on temperature during the experiment.14,15 This is essential as TH is the current gold standard treatment for HIE.29 In a review on temperature control from 2017 by Galinsky et al., 31% of the studies on neuroprotective strategies failed to report on temperature.30 If animals are not kept within the normal range of body temperature both hyper- and hypothermia may influence the outcome and if not evenly distributed between the two intervention groups bias may result. When animals are kept in general anesthesia during the entire experiment, body temperature is controlled by the caregivers, which also underlines the importance of blinding of treatment allocation. In longer experiments where animals are kept in cages, the random allocation for housing is key as cage size, material, placement, bedding, and number of animals placed in the cage may affect thermoregulation and stress level.31
A limitation of this review is the small number of studies with very different outcome measures, and therefore no possibility to conduct a meta-analysis. Furthermore, RIPC was evaluated in two different animal species using different modes of inducing HI. Of note, all but one study used hypoxia in combination with carotid artery ligation, which may not model clinical HIE. We were unable to evaluate the full risk of bias in some of the included studies due to missing information. In particular information on the risk of bias items related to the internal validity of the study was missing, i.e., the extent to which the design and conduct of the experiment eliminated the possibility of bias.26 “Blinding (performance)” and “Random outcome assessment” were the two domains where information was absent in several of the studies. If not properly blinded, handling of the animals by caregivers and technical personnel could contribute to performance bias especially when there are longer observation times and the animals are critically ill and need continuous and intensive care. Lack of random allocation to outcome assessment would, if present, contribute to detection bias. An example is the time point chosen for outcome assessment, as the circadian rhythm in most animals will influence several biological processes.32 All five studies were graded as “high risk of bias” in the domain “Other sources of bias.” The reason was a risk of bias from the use of anesthetic drugs, since all studies used drugs that may have neuroprotective properties. However, this would only create a bias if neuroprotection by drugs abolished the potential effect from RIPC and thus create a bias towards no neuroprotective effect of RIPC, i.e., the null hypothesis.33
To ensure reproducibility and transparency, it is advisable for future studies to report in accordance with the updated ARRIVE 2.0 guidelines and to report sufficient information for complete assessment with the SYRCLES’s risk of bias tool.34 Only three of the five included studies reported their findings in accordance with the ARRIVE guidelines.35
Knowledge gaps and future perspectives
The studies included in this review investigated various outcomes acquired at different timepoints, some of which improved after RIPC, and some not. Thus, there is a lack of consistency and significant knowledge gaps remain. As TH is currently the gold standard treatment for newborns with HIE, the combined effect of RIPC and TH calls for an investigation. So far, only one study in piglets has investigated this. No added neuroprotective effect of RIPC was revealed by aEEG, MRI, and MRS.36 The HI insult was mild and may therefore not be in keeping with clinical findings in TH. Outcome measurements were only acquired within the first 24 h after the HI insult, potentially missing the neuroprotective effect in the secondary phase of injury. Thus, further experiments are needed to study RIPC combined with TH after a more severe HI insult. Studies included in this review applied RIPC in four cycles with 5 or 10 min of ischemia and a similar duration of reperfusion. Only one study investigated the effect of RIPC applied several hours after the insult and RIPC applied at multiple timepoints (Table 1). Experimental dosing of the conditioning treatment is important. Initial animal studies on preconditioning suggested a very steep dose–response-like curve, achieving maximal response once a certain threshold is passed.37 Studies of myocardial infarction in rats have indicated that the effect of RIPC increases with repetition over days.38 Studies of RIPC in humans have also shown a dose dependence in the number of cycles, with a threshold of >2 cycles of 5 min to achieve an effect.3 The timing, duration, and number of cycles of RIPC thus need further investigation. No adverse effects have been noted in clinical trials of RIPC for other conditions in human adults. There were no control (sham) animals included in this review and thus potential adverse effects have not been evaluated. The evidence base for translation to clinical trials would benefit from a consensus on design, standards, and key outcomes as applied in the preclinical evaluation of novel neuroprotective drugs for acute ischemic stroke.39 We therefore suggest, in addition to mechanistically explorative outcomes, to emphasize clinically relevant biomarkers such as MRS and neurobehavioral outcomes bridging animal and human studies.
Conclusion
The findings in this review were inconsistent across studies with respect to both methodology and outcomes, and not all biomarkers analyzed improved after RIPC. Whether this translates to neuroprotection after HI insults require further investigation. Thus, additional studies exploring the optimal timing and duration of RIPC, and the potential effect in addition to TH are warranted. This review highlights the need for common clinically relevant outcomes in standardized models with documented translatability to the human condition in the design of future studies.
References
Douglas-Escobar, M. & Weiss, M. D. Hypoxic-ischemic encephalopathy: a review for the clinician. JAMA Pediatr. 169, 397–403 (2015).
Adstamongkonkul, D. & Hess, D. C. Ischemic conditioning and neonatal hypoxic ischemic encephalopathy: a literature review. Cond. Med. 1, 9–16 (2017).
Loukogeorgakis, S. P. et al. Transient limb ischemia induces remote preconditioning and remote postconditioning in humans by a KATP channel-dependent mechanism. Circulation 116, 1386–1395 (2007).
Zhao, J. J. et al. Remote ischemic postconditioning for ischemic stroke: a systematic review and meta-analysis of randomized controlled trials. Chin. Med. J. 131, 956–965 (2018).
Ritskes-Hoitinga, M. et al. Systematic reviews of preclinical animal studies can make significant contributions to health care and more transparent translational medicine. Cochrane Database Syst. Rev. ED000078 (2014). https://doi.org/10.1002/14651858.ED000078.
CAMARADES. Collaborative Approach to Meta Analysis and Review of Animal Data from Experimental Studies. http://www.camarades.info/ (2013).
P-269 SYRCLE. Systematic Review Centre for Laboratory animal Experimentation 269. https://www.syrcle.network (2012).
Booth, A. The pros and pros of registration on PROSPERO. BJOG 119, 904–905 (2012).
Shamseer, L. et al. Preferred reporting items for systematic review and meta-analysis protocols (prisma-p) 2015: elaboration and explanation. BMJ 349, g7647 (2015).
Macleod, M. R., O’Collins, T., Howells, D. W. & Donnan, G. A. Pooling of animal experimental data reveals influence of study design and publication bias. Stroke 35, 1203–1208 (2004).
Hooijmans, C. R. et al. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 14, 43 (2014).
Drunalini Perera, P. N. et al. Delayed remote ischemic postconditioning improves long term sensory motor deficits in a neonatal hypoxic ischemic rat model. PLoS ONE 9, e90258 (2014).
Zhou, Y. et al. Remote limb ischemic postconditioning protects against neonatal hypoxic-ischemic brain injury in rat pups by the opioid receptor/akt pathway. Stroke 42, 439–444 (2011).
Kyng, K. J. et al. Short-term outcomes of remote ischemic postconditioning 1 h after perinatal hypoxia–ischemia in term piglets. Pediatr. Res. 89, 150–156 (2021).
Ezzati, M. et al. Immediate remote ischemic postconditioning after hypoxia ischemia in piglets protects cerebral white matter but not grey matter. J. Cereb. Blood Flow. Metab. 36, 1396–1411 (2016).
Rocha-Ferreira, E. et al. Immediate remote ischemic postconditioning reduces brain nitrotyrosine formation in a Piglet Asphyxia Model. Oxid. Med. Cell Longev. 2016, 5763743 (2016).
Lally, P. J. et al. Magnetic resonance spectroscopy assessment of brain injury after moderate hypothermia in neonatal encephalopathy: a prospective multicentre cohort study. Lancet Neurol. 18, 35–45 (2019).
Peden, C. J. et al. Proton spectroscopy of the neonatal brain following hypoxic-ischaemic injury. Dev. Med. Child Neurol. 35, 502–510 (1993).
Cheong, J. L. Y. et al. Proton MR spectroscopy in neonates with perinatal cerebral hypoxic-ischemic injury: metabolite peak-area ratios, relaxation times, and absolute concentrations. Am. J. Neuroradiol. 27, 1546–1554 (2006).
Park, R. Lactic acidosis. West J. Med. 133, 418–424 (1980).
Hagberg, H., Mallard, C., Rousset, C. I. & Thornton, C. Mitochondria: hub of injury responses in the developing brain. Lancet Neurol. 13, 217–232 (2014).
Gardai, S. J. et al. Phosphorylation of Bax ser184 by Akt regulates its activity and apoptosis in neutrophils. J. Biol. Chem. 279, 21085–21095 (2004).
Leker, R. R. et al. Expression of endothelial nitric oxide synthase in the ischemic penumbra: relationship to expression of neuronal nitric oxide synthase and vascular endothelial growth factor. Brain Res. 909, 1–7 (2001).
Erkenstam, N. H. et al. Temporal characterization of microglia/macrophage phenotypes in a mouse model of neonatal hypoxic-ischemic brain injury. Front. Cell Neurosci. 10, 286 (2016).
Davidson, J. O. et al. Perinatal brain injury: mechanisms and therapeutic approaches. Front. Biosci. 23, 2204–2226 (2018).
Bart van der Worp, H. et al. Can animal models of disease reliably inform human studies? PLoS Med. 7, 1–8 (2010).
Steinmeyer, K. et al. Cloning and functional expression of rat CLC-5, a chloride channel related to kidney disease. J. Biol. Chem. 270, 31172–31177 (1995).
Button, K. S. et al. Power failure: Why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 14, 365–376 (2013).
Jacobs, S. E. et al. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst. Rev. 2013, CD003311 (2013).
Galinsky, R. et al. In the era of therapeutic hypothermia, how well do studies of perinatal neuroprotection control temperature? Dev. Neurosci. 39, 7–22 (2017).
Clough, G. Environmental effects on animals used in biomedical research. Biol. Rev. 57, 487–523 (1982).
Marrino, P., Gavish, D., Shafrir, E. & Eisenberg, S. Diurnal variations of plasma lipids, tissue and plasma lipoprotein lipase, and VLDL secretion rates in the rat. A model for studies of VLDL metabolism. Biochim. Biophys. Acta 920, 277–284 (1987).
Chen, G., Kamat, P. K., Ahmad, A. S. & Doré, S. Distinctive effect of anesthetics on the effect of limb remote ischemic postconditioning following ischemic stroke. PLoS ONE 15, e0227624 (2020).
du Sert, N. P. et al. The arrive guidelines 2.0: updated guidelines for reporting animal research. PLoS Biol. 18, e3000410 (2020).
Kilkenny, C. et al. Improving bioscience research reporting: the arrive guidelines for reporting animal research. PLoS Biol. 8, 8–9 (2010).
Andelius, T. C. K. et al. No Added neuroprotective effect of remote ischemic postconditioning and therapeutic hypothermia after mild hypoxia-ischemia in a piglet model. Front. Pediatr. 8, 299 (2020).
Yellon, D. M. & Downey, J. M. Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol. Rev. 83, 1113–1151 (2003).
Wei, M. et al. Repeated remote ischemic postconditioning protects against adverse left ventricular remodeling and improves survival in a rat model of myocardial infarction. Circ. Res. 108, 1220–1225 (2011).
Fisher, M. Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke 30, 2752–2758 (1999).
Acknowledgements
This research was funded by Ludvig and Sara Elsass Foundation (grant number 20-3-0296) and The Health Research Foundation of Central Denmark Region.
Author information
Authors and Affiliations
Contributions
Conceptualization, T.C.K.A., B.S.K., and K.J.K.; writing the original draft, T.C.K.A., T.B.H., B.S.K., and K.J.K.; writing-review and editing, T.C.K.A., T.B.H., B.S.K., and K.J.K. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Andelius, T.C.K., Henriksen, T.B., Kousholt, B.S. et al. Remote ischemic postconditioning for neuroprotection after newborn hypoxia–ischemia: systematic review of preclinical studies. Pediatr Res 91, 1654–1661 (2022). https://doi.org/10.1038/s41390-021-01656-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01656-7