Abstract
Background
A very-low-birth-weight (VLBW) preterm infants is associated with an increased risk of impaired neurodevelopmental outcomes. In this study, we investigated how neonatal brain metabolite concentrations changed with postmenstrual age and examined the relationship between changes in concentration (slopes) and neurodevelopmental level at 3–4 years.
Methods
We retrospectively examined 108 VLBW preterm infants who had brain single-voxel magnetic resonance spectroscopy at 34–42 weeks’ postmenstrual age. Neurodevelopment was assessed using a developmental test, and subjects were classified into four groups: developmental quotient <70, 70–84, 85–100, and >100. One-way analyses of covariance and multiple-comparison post hoc tests were used to compare slopes.
Results
We observed correlations between postmenstrual age and the concentrations of N-acetylaspartate and N-acetylaspartylglutamate (tNAA) (p < 0.001); creatine and phosphocreatine (p < 0.001); glutamate and glutamine (p < 0.001); and myo-inositol (p = 0.049) in the deep gray matter; and tNAA (p < 0.001) in the centrum semiovale. A significant interaction was noted among the tNAA slopes of the four groups in the deep gray matter (p = 0.022), and we found a significant difference between the <70 and 85–100 groups (post hoc, p = 0.024).
Conclusions
In VLBW preterm infants, the slopes of tNAA concentrations (adjusted for postmenstrual age) were associated with lower developmental quotients at 3–4 years.
Impact
-
In very-low-birth-weight preterm-born infants, a slower increase in tNAA brain concentration at term-equivalent age was associated with poorer developmental outcomes at 3–4 years.
-
The increase in tNAA concentration in very-low-birth-weight infants was slower in poorer developmental outcomes, and changes in tNAA concentration appeared to be more critical than changes in tCho for predicting developmental delays.
-
While tNAA/tCho ratios were previously used to examine the correlation with neurodevelopment at 1–2 years, we used brain metabolite concentrations.
Similar content being viewed by others
Introduction
A very low birth weight (VLBW) in preterm infants is associated with an increased risk of impaired neurodevelopmental outcomes, and early identification and intervention may effectively improve developmental outcomes.1,2,3,4,5,6 The incidence of major brain injuries has decreased with advances in neonatal care for preterm infants. However, some neonates who do not show significant brain injuries still exhibit later developmental disabilities.4,7,8,9 Thus comprehensive assessment of the prognosis of neonates must consider additional information.
Magnetic resonance spectroscopy (MRS) is a non-invasive technique for assessing cerebral metabolite levels. In predicting neurodevelopmental outcomes for LBW preterm infants, MRS has been used to measure the ratios of brain metabolites.10,11,12,13,14,15,16,17 However, studies have reported a number of different patterns, with some reporting correlations between the ratios of N-acetylaspartate and N-acetylaspartylglutamate (total N-acetylaspartate (tNAA)) to glycerophosphocholine (including choline-containing compounds) and phosphocholine (tCho), i.e., tNAA/tCho, and subsequent neurodevelopment.11,13,15,16 Since these ratios are affected by alterations in the levels of any of the above-mentioned metabolites, it is difficult to determine which metabolic disturbances cause neurological impairments, and slight pathological changes at the metabolite level may be overlooked. To address this in the present study, we retrospectively investigated neonatal brain metabolite concentrations in preterm-born infants with VLBW. Specifically, we examined whether the rates of change (slopes) in metabolite concentrations differed with postmenstrual age in subjects classified by neurodevelopmental level at 3–4 years. Regarding metabolite ratios, which are more clinically accessible values, we also investigated these changes with tCho as the denominator.
Materials and methods
Ethics statement
Our retrospective study was approved by the Institutional Ethical Review Board of our hospital, and the requirement to obtain written informed consent was waived.
Subjects
Our data were collected from a series of preterm patients admitted to the neonatal intensive care unit of our hospital between April 2013 and April 2016. The original cohort included 148 infants born at 23–35 gestational weeks with a birth weight of <1500 g, who were examined using brain proton magnetic resonance imaging (MRI) and MRS at a postmenstrual age of 34–42 weeks. Infants with major congenital malformations, chromosomal anomalies, severe hypoxic–ischemic encephalopathy, severe intraventricular hemorrhage, and/or cystic periventricular leukomalacia were not included in the dataset (n = 14). In addition, some infants did not undergo the follow-up neurodevelopmental examination at 3–4 years (n = 26). After these exclusions, data from 108 subjects were included in the present analysis (Fig. 1).
Brain MRI and MRS examinations
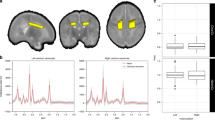
Each subject underwent one brain proton MRI and MRS examination during the neonatal period. All MRI and MRS scans were performed using a clinical 3-T magnetic resonance system (Magnetom Verio; Siemens Healthineers, Erlangen, Germany). Radio frequency signal transmission and reception were performed using a whole-body coil (bore diameter: 70 cm) and a 32-channel head coil (inner diameter: 22 cm), respectively. The excitation frequency was set to 2.38 ppm (123.20 MHz). During the MR examination, the neonates were placed in a horizontal supine position, wrapped in vacuum-type immobilization bags (CFI Medical Solutions, Fenton, MI), and heart rate and transcutaneous oxygen saturation were monitored continuously using a pulse oximeter (Nonin, Plymouth, MN). All MRS data included in the present study were collected as part of a routine brain MR examination conducted for clinical diagnostic purposes. Single-voxel MRS data were obtained using the point-resolved spectroscopic localization sequence18 with a water presaturation pulse. The echo time/repetition time (TE/TR) was 30/5000 ms, with 8–16 excitations. Volumes of interest (VOIs) occupied 2.0–7.6 mL and 2.4–7.4 mL volumes of the deep gray matter and the centrum semiovale, respectively (Fig. 2). The locations of VOIs were identified using transverse fast spin echo T2-weighted imaging (TE/TR, 119–123/5000 ms; field of view, 105–136 × 150 mm2; matrix size, 180–232 × 256; slice thickness, 2 mm; 44–56 slices without gaps) or 3D MP-RAGE T1-weighted imaging (TE/TR/inversion time, 2.14/1570/800 ms; field of view, 150 × 120 mm2; matrix size, 192 × 192; slice thickness, 1 mm). Spectra for the same VOIs were also obtained without the water presaturation pulse; these were used to correct eddy current-induced phase shifts and quantify metabolite concentrations. The total time required for the single-proton MRS examination of two VOIs was approximately 10 min.
a A neonate with a severely delayed outcome at a postmenstrual age of 40 weeks. b A neonate with a normal outcome at a postmenstrual age of 39 weeks. Thin lines show the original spectra, while bold lines represent the fitted lines produced by LCModel. The volumes of interest are overlaid on T2-weighted images. Glx glutamate and glutamine, Lac lactate, mIns myo-inositol, tCho glycerophosphocholine (including choline-containing compounds) and phosphocholine, tCr creatine and phosphocreatine, tNAA total N-acetylaspartate (N-acetylaspartate and N-acetylaspartylglutamate).
MR data quantification
MRS data processing, including signal quantification, was performed using the LCModel software (version 6.2–1G; http://s-provencher.com/lcmodel.shtml).19 Spectra with signal-to-noise ratios ≤4 were excluded from the present study. The signal-to-noise ratio, which is displayed in the LCModel output files, was defined as the ratio of the maximum spectral peak minus the baseline, divided by twice the root mean square residual.19 In the present study, the absolute concentrations of five major metabolites were measured: N-acetylaspartate and N-acetylaspartylglutamate (tNAA), creatine and phosphocreatine (tCr), glycerophosphocholine (including choline-containing compounds) and phosphocholine (tCho), glutamate and glutamine (Glx), and myo-inositol (mIns). To calculate the concentration of each metabolite, the water concentrations of the deep gray matter and centrum semiovale were set to 48.9 and 51.7 M, respectively,20 and the reduction of each metabolite and water peak according to the T1 and T2 values was also considered. T1 and T2 for the water in the deep gray matter and centrum semiovale were set to 1956 and 121 ms and 2566 and 206 ms, respectively. The T1 and T2 values of the metabolites were as follows21,22: the T1 values for tNAA, tCr, tCho, Glx, and mIns were 1310, 1660, 1180, 1180, and 1180 ms, respectively; the T2 values for tNAA, tCr, tCho, Glx, and mIns were 369, 199, 384, 199, and 199 ms, respectively. Supplemental 1 (online) includes details regarding MR spectroscopic signal quantification.
Metabolite ratios were obtained using the values displayed in the LCModel output files: tNAA/tCho, tCr/tCho, Glx/tCho, and mIns/tCho.
Neurodevelopmental assessment
Neurodevelopmental outcomes were assessed using the Kyoto Scale of Psychological Development, a psychological developmental test, which is reported to closely correlate with the Bayley-III scale.23 The latest version of the Kyoto Scale of Psychological Development was standardized using data from 2677 Japanese children in 2001, and the developmental quotient (DQ) score was 100.6 ± 13.4 (mean ± 1 standard deviations (SD)).24 Psychologists who were blinded regarding the perinatal details of the subjects and the proton MRI and MRS data administered the test. A total of 102 children completed the test at 3 years of age, and 6 children who missed the 3-year follow-up appointment underwent the test at approximately 4 years of age. Thus 108 children (median age, 3.0 years; interquartile range, 3.0–3.1 years) provided follow-up data. Scores 1–2 SDs below standard norms were considered to indicate mild developmental delays, while moderate-to-severe developmental delays were indicated by scores of at least 2 SDs below the standard norms: (1) normal (DQ > 100); (2) borderline (DQ = 85–100); (3) mild delay (DQ = 70–84); and (4) moderate-to-severe delay (DQ < 70).3 Subjects with a Gross Motor Function Classification System (GMFCS) grade of 3–5, blindness without any fixation or following, profound hearing deficits, and/or epilepsy were defined as having a moderate-to-severe delay.
Statistical analysis
Fisher’s exact test and Kruskal–Wallis test were used to compare the clinical features of the subjects. We used one-way analyses of covariance (ANCOVA) with postmenstrual age as a covariate and multiple-comparison post hoc tests to compare the rates of change (slopes) in metabolite concentrations and metabolite ratios with respect to age among the four groups classified by developmental outcomes.
All statistical analyses were performed using MATLAB (MathWorks, Natick, MA) or IBM SPSS Statistics 24 (IBM, Chicago, IL). p Values < 0.050 were considered to be significant.
Results
The clinical characteristics of the subjects are shown in Table 1. Twelve out of 108 subjects (11%) were defined as normal, 44 (41%) as borderline, 32 (30%) as showing a mild delay, and the remaining 20 (19%) as showing a mild-to-severe delay (GMFCS score >2, one child; blindness, no children; profound hearing deficit, one child; epilepsy, no children). The child with a GMFCS score >2 and the child with deafness had DQ scores of 45 and 66, respectively. Significant differences were observed among the four developmental outcome groups in terms of sepsis (p = 0.01) and chronic lung disease (p = 0.02).
In total, 212 MR spectra (107 deep gray matter and 105 centrum semiovale) were obtained from 108 subjects. MRS scans at the centrum semiovale level could not be performed in two patients due to a change in their clinical condition. In addition, two spectra, one each in the deep gray matter and centrum semiovale, were excluded from the study because they had a low signal-to-noise ratio of 4. The MR spectral signal-to-noise ratio was 12 (9–13) [median (interquartile range)], and the metabolite Cramér–Rao lower bounds, which are displayed as the percent SD in the LCModel fits, were as follows [median (interquartile range)]: tNAA, 7% (6–8%); tCr, 4% (4–5%); tCho, 4% (4–4%); Glx, 15% (13–17%); and mIns, 5% (5–6%). Among the overall metabolite concentrations, there were significant increases with postmenstrual age in tNAA (p < 0.001), tCr (p < 0.001), and Glx (p < 0.001) in the deep gray matter and tNAA (p < 0.001) in the centrum semiovale. In contrast, a significant decrease was observed with postmenstrual age in mIns in the deep gray matter (p = 0.049; Fig. 3). The metabolite ratios for which significant changes were observed are as follows: tNAA/tCho (p < 0.001), tCr/tCho (p < 0.001), Glx/tCho (p = 0.001), and mIns/tCho (p = 0.025) in the deep gray matter and tNAA/tCho (p < 0.001) in the centrum semiovale (Supplemental Fig. 1).
Lines in the plot indicate the correlation between metabolite concentrations according to postmenstrual age, as calculated via a one-way analysis of covariance (p < 0.05). CS centrum semiovale, dGM deep gray matter, Glx glutamate and glutamine, mIns myo-inositol, tCho glycerophosphocholine (including choline-containing compounds) and phosphocholine, tCr creatine and phosphocreatine, tNAA total N-acetylaspartate (N-acetylaspartate and N-acetylaspartylglutamate).
A significant interaction was observed among the tNAA slopes from the four developmental groups in the deep gray matter (postmenstrual age × group, p = 0.022 by ANCOVA; Fig. 4a), indicating that a slower rate of increase in tNAA concentrations was associated with a lower DQ score. Post hoc multiple comparisons revealed a significant difference in slopes from groups with DQ scores <70 and those with slopes from 85 to 100 (p = 0.024, Fig. 4b). While the slopes of the tNAA/tCho ratio in the deep gray matter approached significance for all four groups (p = 0.090), none of the slopes for the other metabolite concentrations or ratios showed significant interactions. Specifically, for the postmenstrual age × group interaction, the p values for tCr, tCho, Glx, and mIns in the deep gray matter were 0.647, 0.370, 0.595, and 0.378, respectively; those for tNAA, tCr, tCho, Glx, and mIns in the centrum semiovale were 0.847, 0.538, 0.969, 0.506, and 0.730, respectively; those for Glx/tCho, and mIns/tCho in the deep gray matter were 0.616, 0.831, and 0.844, respectively; and those for tNAA/tCho, Glx/tCho, and mIns/tCho in the centrum semiovale were 0.593, 0.178, 0.593, and 0.852, respectively.
a Scatterplots of change in tNAA concentrations. b Slopes with comparison intervals from the data in a. Asterisk (*) represents significant difference (p < 0.050) revealed by: a a one-way analysis of covariance; and b post hoc multiple comparison. DQ developmental quotient, tNAA total N-acetylaspartate (N-acetylaspartate and N-acetylaspartylglutamate).
Discussion
In this retrospective study, we quantified the concentrations of brain metabolites in 108 preterm infants with VLBW who had no major brain injuries during the neonatal period. We found that overall changes in metabolite concentration were similar to those reported in previous studies.25,26,27,28 Our key results are as follows. First, children with lower DQ scores had slower rates of increase in tNAA concentrations in the deep gray matter (Fig. 4, p = 0.022). Second, compared with the tNAA/tCho ratio (p = 0.090), the rate of increase in tNAA concentration was more strongly correlated with neurodevelopmental status. Third, the metabolites included in the ratios that significantly changed with age (Supplemental Fig. 1) also changed in terms of concentration (Fig. 3). This indicates that metabolite ratios with tCho as the denominator may be most appropriate for evaluating changes in metabolite levels during the neonatal period, especially as it is difficult to obtain absolute metabolite concentrations at this time. Although previous studies have shown that the tNAA/tCho ratio is correlated with subsequent neurodevelopment,11,13,15,16 deducing which metabolites contribute significantly to changes in tNAA/tCho has been a difficult task. In the present study, changes in tNAA concentrations appeared to be more closely tied to developmental delays than changes in tCho concentrations.
Our finding regarding a relationship between the slope of the tNAA concentration (p = 0.022) and a lower developmental quotient is similar to recent findings reported by Chau et al., who found a relationship between slower increases in tNAA/tCho ratios in the basal nuclei and increasing severity of motor outcomes at a corrected age of 18 months (p = 0.02).13 However, we only observed a slight change in tNAA/tCho ratios (p = 0.090) in the present study. A possible explanation for this difference is that only one MRS examination was conducted per subject in the present study, and the sample size (n = 108) was smaller. In contrast, the study by Chau et al. included data from two MRS examinations per subject and had a larger sample population (n = 157). Therefore, repeated MRS examinations and a large sample size appear to be necessary for the detection of these differences. Furthermore, differences in the data acquisition methods (single-voxel MRS vs. multi-voxel MRS) and the age at neurodevelopmental assessment (3–4 years vs. corrected age of 18 months) may have influenced the obtained results.
Regarding the significant interaction found between the tNAA slope and DQ in the deep grey matter but not in the centrum semiovale, previous studies have also reported that both white matter and deep gray matter abnormalities were strongly associated with prognosis in preterm infants.8,13,29,30,31 For instance, Chau et al. reported a strong association between abnormal microstructure and metabolism in the basal nuclei, with adverse outcomes in preterm newborn.13 Further, Anderson et al. reported that structural abnormalities in the deep gray matter were particularly predictive of adverse neurodevelopmental outcomes.31 Regarding changes in the level of NAA, which is produced in neural mitochondria, a reduction in NAA is a well-known marker of neuronal or axonal loss in many neurological disorders. Further, the degree of NAA depletion is proportional to the degree of brain injury.27,32,33,34 Combining our data with previous findings, it is possible that changes due to microstructural damage may be more pronounced in deep gray matter compared with white matter, even if structural MRI reveals no changes. Alternatively, our selected VOI may have better reflected abnormalities in the deep gray matter. Indeed, as the white matter examined in the present study was from only one region, i.e., centrum semiovale, the MRS may not have captured abnormalities in the white matter region.
Our data showed that sepsis and chronic lung disease occurred more frequently in neonates with a poor developmental outcome at 3–4 years of age (Table 1). Previous studies have reported that neonatal sepsis and/or chronic lung disease could impair neurodevelopmental outcomes.35,36 Hüppi et al. demonstrated that developmental disruption and plasticity in the lungs and brain was closely associated with events inducing inflammation, oxidative stress, and endocrine disruption.37 These events could disturb NAA production in neuronal mitochondria,32,33 leading to reductions in tNAA concentrations in subjects with low DQ scores.
Limitations
Subjects
Although repeated MRS measurements are preferable to obtain rates of change (slopes) in metabolite concentrations, each subject in the present study underwent MRS examination only once during the neonatal period. Furthermore, the sample size (n = 108) was relatively small, particularly in the group defined as normal (DQ > 100, n = 12). This may be reflected in the slopes for this group, as the dataset had the broadest distribution (Fig. 4b). Further, neurodevelopment was assessed at 3–4 years of age, which is relatively young and may thus have suboptimal reliability for predicting neurodevelopment.7 A more long-term assessment, such as the Wechsler Intelligence Scale for Children (which is generally administered to children 6–16 years of age), may have yielded more detailed information regarding developmental deficits. Prospective studies with repeated MRS measurements, a larger sample size during the neonatal period, and neurodevelopmental evaluations at older ages are needed.
Water concentration and relaxation times
Although we set the water concentrations as fixed values when estimating the metabolite concentrations, water concentrations in the brain are known to decrease with postmenstrual age, as are the T1 and T2 values for water.20,38,39 Williams et al. reported a strong correlation between R1 and R2, and a linear relationship between R1 and water concentration in the neonatal brain.20 This suggests that estimated metabolite concentrations at a younger postmenstrual age may need to be age-corrected. Taking this into account, the age-related changes in metabolite concentration (slopes) that we obtained may have been more moderate. However, after age correction, we still observed a significant difference in the tNAA in the deep gray matter among the four groups (ANCOVA, p = 0.042; post hoc, p = 0.046). Supplemental 1 (online) includes the detailed calculation method.
Chemical shift displacements
In the PRESS sequence used in this study, the slice selection gradients led to chemical shift displacements of the metabolites and water peaks,40 i.e., each metabolite and water peak was spatially displaced against the excitation frequency (2.38 ppm). For a VOI of 20 × 20 × 20 mm3, tNAA peak displacement was approximately 0.3, 1.0, and 1.0 mm in the x, y, and z directions, respectively, while the water peak displacement was approximately 1.8, 6.2, and 6.2 mm in the x, y, and z directions, respectively. Given these displacements, cerebrospinal fluid outside of the VOI might have been included in infants with lower cerebral volumes.
Conclusions
The slopes of the increases in tNAA concentrations in the deep gray matter in VLBW preterm infants during the neonatal period were associated with neurodevelopmental level at 3–4 years of age. The slopes of change in tNAA concentrations were more strongly related to developmental outcomes than those of tNAA/tCho ratios, suggesting that evaluations of separate changes in tNAA are preferable for predicting longer developmental delays. Further studies are needed to clarify the relationship between neonatal metabolite functions and neurodevelopmental outcomes in infants with VLBW.
Data availability
Data are available from the corresponding author upon reasonable request.
References
Larroque, B. et al. Neurodevelopmental disabilities and special care of 5-year-old children born before 33 weeks of gestation (the EPIPAGE study): a longitudinal cohort study. Lancet 371, 813–820 (2008).
de Kieviet, J. F. et al. Brain development of very preterm and very low-birthweight children in childhood and adolescence: a meta-analysis. Dev. Med. Child Neurol. 54, 313–323 (2012).
Pascal, A. et al. Neurodevelopmental outcome in very preterm and very-low-birthweight infants born over the past decade: a meta-analytic review. Dev. Med. Child Neurol. 60, 342–355 (2018).
Wilson-Costello, D. E. & Payne, A. H. in Fanaroff and Martin’s Neonatal-Perinatal Medicine (eds Martin, R. J., Fanaroff, A. A. & Walsh, M. C.) 1091–1109 (Elsevier, 2019).
Anderson, P. J., Cheong, J. L. & Thompson, D. K. The predictive validity of neonatal MRI for neurodevelopmental outcome in very preterm children. Semin. Perinatol. 39, 147–158 (2015).
Johnston, M. V. Plasticity in the developing brain: implications for rehabilitation. Dev. Disabil. Res. Rev. 15, 94–101 (2009).
Hintz, S. R. & Parikh, N. A. in Fanaroff and Martin’s Neonatal-Perinatal Medicine (eds Martin, R. J., Fanaroff, A. A. & Walsh, M. C.) 1110–1122 (Elsevier, 2019).
Kidokoro, H. et al. Brain injury and altered brain growth in preterm infants: predictors and prognosis. Pediatrics 134, e444–e453 (2014).
Volpe, J. J. The encephalopathy of prematurity–brain injury and impaired brain development inextricably intertwined. Semin. Pediatr. Neurol. 16, 167–178 (2009).
Augustine, E. M. et al. Can magnetic resonance spectroscopy predict neurodevelopmental outcome in very low birth weight preterm infants? J. Perinatol. 28, 611–618 (2008).
Van Kooij, B. J. et al. Cerebellar volume and proton magnetic resonance spectroscopy at term, and neurodevelopment at 2 years of age in preterm infants. Dev. Med. Child Neurol. 54, 260–266 (2012).
Gadin, E. et al. Volumetric MRI and MRS and early motor development of infants born preterm. Pediatr. Phys. Ther. 24, 38–44 (2012).
Chau, V. et al. Abnormal brain maturation in preterm neonates associated with adverse developmental outcomes. Neurology 81, 2082–2089 (2013).
Bapat, R., Narayana, P. A., Zhou, Y. & Parikh, N. A. Magnetic resonance spectroscopy at term-equivalent age in extremely preterm infants: association with cognitive and language development. Pediatr. Neurol. 51, 53–59 (2014).
Kendall, G. S. et al. White matter NAA/Cho and Cho/Cr ratios at MR spectroscopy are predictive of motor outcome in preterm infants. Radiology 271, 230–238 (2014).
Hyodo, R. et al. Magnetic resonance spectroscopy in preterm infants: association with neurodevelopmental outcomes. Arch. Dis. Child. Fetal Neonatal Ed. 103, F238–F244 (2018).
Taylor, M. J. et al. Magnetic resonance spectroscopy in very preterm-born children at 4 years of age: developmental course from birth and outcomes. Neuroradiology 60, 1063–1073 (2018).
Bottomley, P. A. Spatial localization in NMR-spectroscopy in vivo. Ann. NY Acad. Sci. 508, 333–348 (1987).
Provencher, S. W. Estimation of metabolite concentrations from localized in-vivo proton NMR-spectra. Magn. Reson. Med. 30, 672–679 (1993).
Williams, L. A. et al. Neonatal brain: regional variability of in vivo MR imaging relaxation rates at 3.0 T-initial experience. Radiology 235, 595–603 (2005).
Mlynarik, V., Gruber, S. & Moser, E. Proton T1 and T2 relaxation times of human brain metabolites at 3 Tesla. NMR Biomed. 14, 325–331 (2001).
Ganji, S. K. et al. T2 measurement of J-coupled metabolites in the human brain at 3T. NMR Biomed. 25, 523–529 (2012).
Kono, Y. et al. Developmental assessment of VLBW infants at 18 months of age: a comparison study between KSPD and Bayley III. Brain Dev. 38, 377–385 (2016).
Ikusawa, M., Matsushita, Y. & Nakase, A. Shinpan K Shiki Hattatsu Kensahou 2001 [The Kyoto Scale of Psychological Development Test 2001] (Choyodo Printing Co. [in Japanese], 2002).
Tomiyasu, M. et al. Neonatal brain metabolite concentrations: an in vivo magnetic resonance spectroscopy study with a clinical MR system at 3 Tesla. PLoS ONE 8, e82746 (2013).
Kreis, R. et al. Brain metabolite composition during early human brain development as measured by quantitative in vivo 1H magnetic resonance spectroscopy. Magn. Reson. Med. 48, 949–958 (2002).
Panigrahy, A., Nelson, M. D. Jr & Bluml, S. Magnetic resonance spectroscopy in pediatric neuroradiology: clinical and research applications. Pediatr. Radiol. 40, 3–30 (2010).
Pouwels, P. J. et al. Regional age dependence of human brain metabolites from infancy to adulthood as detected by quantitative localized proton MRS. Pediatr. Res. 46, 474–485 (1999).
Abernethy, L. J., Cooke, R. W. & Foulder-Hughes, L. Caudate and hippocampal volumes, intelligence, and motor impairment in 7-year-old children who were born preterm. Pediatr. Res. 55, 884–893 (2004).
Setanen, S. et al. Prediction of neuromotor outcome in infants born preterm at 11 years of age using volumetric neonatal magnetic resonance imaging and neurological examinations. Dev. Med. Child Neurol. 58, 721–727 (2016).
Anderson, P. J. et al. Associations of newborn brain magnetic resonance imaging with long-term neurodevelopmental impairments in very preterm children. J. Pediatr. 187, 58–65 e51 (2017).
Moffett, J. R. et al. N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog. Neurobiol. 81, 89–131 (2007).
Benarroch, E. E. N-acetylaspartate and N-acetylaspartylglutamate: neurobiology and clinical significance. Neurology 70, 1353–1357 (2008).
Govindaraju, V., Young, K. & Maudsley, A. A. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 13, 129–153 (2000).
Pek, J. H. et al. Neurocognitive impairment after neonatal sepsis: protocol for a systematic review and meta-analysis. BMJ Open 10, e038816 (2020).
Bohm, B. & Katz-Salamon, M. Cognitive development at 5.5 years of children with chronic lung disease of prematurity. Arch. Dis. Child. Fetal Neonatal Ed. 88, F101–F105 (2003).
Hüppi, P., Sizonenko, S. & Amato, M. Lung disease and brain development. Biol. Neonate 89, 284–297 (2006).
Counsell, S. J. et al. T2 relaxation values in the developing preterm brain. AJNR Am. J. Neuroradiol. 24, 1654–1660 (2003).
Schneider, J. et al. Evolution of T1 relaxation, ADC, and fractional anisotropy during early brain maturation: a serial imaging study on preterm infants. AJNR Am. J. Neuroradiol. 37, 155–162 (2016).
Smith, A. S. et al. Intracranial chemical-shift artifacts on MR images of the brain: observations and relation to sampling bandwidth. AJR Am. J. Roentgenol. 154, 1275–1283 (1990).
Acknowledgements
The authors thank Mr. M. Sato, Mr. K. Kusagiri, Mr. Y. Muramoto, and Ms. A. Kitagawa for their help during the acquisition of MRI and MRS data and Ms. K. Sato, Ms. A. Suzuki, and Ms. E. Mitsui for their assistance with data analyses. This study was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI [Grant Numbers 15K09943 and 19K08213] and the Kanagawa Municipal Hospital Pediatric Research Fund.
Author information
Authors and Affiliations
Contributions
M.T., J.S., K.T., and N.A. were involved in the conception and design of the study and the acquisition of MRI and MRS data. M.T. performed statistical analyses and drafted the manuscript. M.T., J.S., H.K., M.E., and T.O. were involved in the analysis and interpretation of data. All authors revised the manuscript critically for important intellectual content, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Patient consent
Our retrospective study was approved by the Institutional Ethical Review Board of our hospital [Human Research Ethics Committee of the Kanagawa Children’s Medical Center (approval number 1907-7)]. The requirement to obtain written informed consent was waived because the proton MRS data used in this study were obtained during routine clinical MR examinations of the brain and provided additional information for radiological diagnosis.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tomiyasu, M., Shibasaki, J., Kawaguchi, H. et al. Altered brain metabolite concentration and delayed neurodevelopment in preterm neonates. Pediatr Res 91, 197–203 (2022). https://doi.org/10.1038/s41390-021-01398-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01398-6