Abstract
Background
Small for gestational age (SGA) infants have an increased risk for neonatal mortality and morbidities. However, few studies have examined the risk of large for gestational age (LGA) on these factors. We compared the risk of mortality and morbidities in LGA premature infants with those of appropriate for gestational age (AGA) infants.
Methods
Premature infants who were born between 2003 and 2012 at <26 weeks of gestational age were included. Relative risks of mortality and morbidities were evaluated between LGA and AGA infants.
Results
From 6898 extremely premature infants, 357 (5.2%), 5530 (80.2%), and 1011 (14.7%) were LGA, AGA, and SGA, respectively. A total of 5887 infants (5530 AGA and 357 LGA) were examined after excluding infants with congenital anomalies, unknown sex, and deficient data. The risk of mortality in LGA and AGA infants did not differ (relative risk (95% confidence interval) 1.04 (0.83–1.32)). Compared to AGA infants, LGA infants did not increase the risk of morbidities, including intraventricular hemorrhage, cystic periventricular leukomalacia, treated retinopathy of prematurity, necrotizing enterocolitis, and bronchopulmonary dysplasia.
Conclusions
This study demonstrates that being born LGA does not correlate with an increased risk of mortality and morbidities in extremely premature infants.
Impact
-
It is currently unknown if being large for gestational age is a risk for neonatal morbidity.
-
A total of 6898 preterm infants born <26 weeks gestational age were included in the study.
-
It was found that being large for gestational age was not related to increased risk of mortality and morbidities.
Similar content being viewed by others
Introduction
Infants who are born small for gestational age (SGA) are often associated with increased mortality.1 SGA infants are also predisposed to hypoglycemia,2 hypothermia,3 polycythemia,4 and thrombocytopenia5 compared to infants born appropriate for gestational age (AGA) after birth. Furthermore, in premature infants, SGA infants were at increased risks of mortality and morbidities, including respiratory distress syndrome, necrotizing enterocolitis (NEC), late-onset sepsis (LOS), treated retinopathy of prematurity (ROP), and bronchopulmonary dysplasia (BPD).6
In contrast, the term large for gestational age (LGA) is meant to convey a concern of excessive growth. Babies born LGA are usually defined by weight, determined as >90th percentile at birth according to gestational age (GA) and sex. Due to genetic factors or increased supply of nutrients, excessive fetal growth can occur. Obese mothers and mothers with pre-gestational diabetes mellitus or gestational diabetes mellitus (GDM) could be the cause of being born with LGA. Early excessive fetal growth resulting from Beckwith–Wiedemann syndrome and other genetic disorders could result in LGA. Infants who are born with LGA may have an increased risk for short-term outcomes, such as shoulder dystocia,7 neonatal hypoglycemia,8 and longer hospital stay.9 Concerning long-term outcomes, a recent systematic review and meta-analysis highlighted that high birth weight (BW) is independently associated with increased overweight risk during childhood and adulthood.10 Additionally, epidemiological studies have shown a strong association between being born LGA and later adverse metabolic and cardiovascular outcomes.11,12,13 However, little is known of outcomes regarding LGA extremely premature babies.
Therefore, we hypothesize that being born LGA could increase the risk of mortality and morbidities in extremely premature infants. The aim of this study was to evaluate short‐term mortality and morbidities, such as intraventricular hemorrhage (IVH), periventricular leukomalacia (PVL), ROP, NEC, and BPD, for infants born LGA at extremely preterm using the nationwide database of the Neonatal Research Network of Japan (NRNJ).
Methods
Study design and participants
This retrospective observational study cohort included all extremely premature infants born at <26 weeks of GA and admitted to a neonatal intensive care unit (NICU) registered in the NRNJ from January 01, 2003 to December 31, 2012. During the study period, about 60% of the participating NICUs were Level III and 40% were Level II units. The NRNJ database covered almost 70% of all nationally delivered preterm infants with a BW ≤1500 g in 2012.14 Data collection was approved by the research ethics committee at each participating site. Neonates with SGA, congenital malformations, any missing data, and transferred from other hospitals were excluded. Anonymously collected information about infants was unlinked from individual data.
Data collection
Perinatal records were included for maternal age, maternal diabetes, infant sex, GA, BW, and Apgar score. SGA, AGA, and LGA infants were defined as lower than the 10th percentile, between the 10th and 90th percentile, and more than the 90th percentile for BW, respectively, based on GA and sex in accordance with Japanese neonatal anthropometric charts for gestational age at birth.15 GA was determined in the following order: (1) early prenatal ultrasound, (2) the best estimation of the last menstrual period, and (3) physical examination at birth.
The following outcomes were recorded for each group: mortality, IVH, cystic PVL, treated ROP, NEC, BPD, early-onset sepsis (EOS), LOS, and patent ductus arteriosus (PDA). Death was defined as that occurring during the hospitalization period and not after discharge. BPD was defined as requirement of supplemental oxygen at 36 completed weeks postmenstrual age (36 weeks and 0 days to 36 weeks and 6 days inclusive). NEC was defined according to the Bell et al. criteria and included all stages of NEC (stages 1–3).16 IVH was defined according to the Papile et al. criteria using head ultrasound, computed tomography (CT), or magnetic resonance imaging (MRI) and included all grades of IVH (grades 1–4).17 Cystic PVL was also defined using head ultrasound, CT, or MRI. ROP was considered treated if the worst stage of ROP was at III or greater according to the criteria proposed by the Ministry of Health, Labor, and Welfare of Japan, which was equivalent to stage III or greater in the International Classification of ROP, and if treatment was required. PDA was defined based on the presence of circulatory failure by using echocardiography and clinical findings. EOS and LOS were defined as sepsis that occurs within and after 7 days of birth, respectively.
Outcomes
The primary outcome was defined as death before discharge. Secondary outcomes included ten major morbidities, including IVH, cystic PVL, treated ROP, NEC, BPD, EOS, LOS, and PDA, which were compared between LGA and AGA extremely premature infants who survived and were discharged from hospital. The risks of mortality and morbidities stratified by GA were also compared between LGA and AGA infants. Data were compared in LGA infants stratified by GA, GDM, and ponderal index (PI). PI was obtained from weight and length (weight in g × 100/length in cm3).18
Statistical analysis
Demographic data were assessed with medians and interquartile ranges (IQRs) or frequency (%) where appropriate. Statistical analysis was performed using Mann–Whitney U test for comparison of medians and the chi-squared test for comparison of proportions using the EZR software (Saitama Medical Center, Jichi Medical University, Saitama, Japan).19 A p value <0.05 was considered to be significant.
Results
Subject characteristics
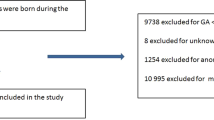
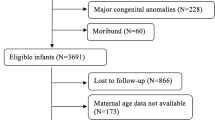
In this study, a total of 7985 extremely premature infants who were born at less than 26 weeks of GA and who were admitted to a NICU participating in the NRNJ from January 01, 2003 to December 31, 2012 were enrolled in the study. Of these, 1087 infants were excluded due to congenital abnormality (n = 369), any missing data (n = 486), and transfer from another hospital (n = 232). The remaining subjects (n = 6898) were divided into three groups: 1011 (14.7%) of SGA, 5530 (80.2%) of AGA, and 357 (5.2%) of LGA infants. Because this study aimed to evaluate AGA and LGA infants, SGA infants were excluded from further analysis (Fig. 1).
Characteristics of LGA and AGA extremely premature infants are listed in Table 1. LGA infants had significantly lower GA (median (IQR), 24.2 (23.2–25.0) vs. 24.3 (23.5–25.2) weeks, p < 0.01), heavier BW (813 (700–904) vs. 668 (585–752) g, p < 0.01), older maternal age (32 (28–36) vs. 31 (27–35) years, p < 0.01), and less multiple births (10 vs. 17%, p < 0.01) compared to AGA infants. There was no significant difference between LGA and AGA infants in terms of Apgar score at 1 and 5 min, the rates of maternal DM, and sex.
Infant mortality and morbidities
Table 2 shows mortality and morbidities in LGA and AGA extremely premature infants. Overall, it was found that mortality did not differ between LGA and AGA infants (18 vs. 17%, relative risk (RR) (95% confidence interval (CI)) 1.04 (0.83–1.32), p = 0.72). The rates of delivery room deaths in LGA and AGA infants were 0.3 and 0.7%, respectively (RR (95% CI) 0.43 (0.06–3.13), p = 0.61). In infants who survived their NICU stay (294 LGA and 4596 AGA), there were no statistical differences in incidences of IVH (36 vs. 32%, RR (95% CI) 1.13 (0.96–1.33), p = 0.16), cystic PVL (5% vs. 4%, RR (95% CI) 1.16 (0.70–1.94), p = 0.67), treated ROP (50 vs. 46%, RR (95% CI) 1.10 (0.97–1.23), p = 0.18), NEC (3 vs. 3%, RR (95% CI) 1.23 (0.65–2.32), p = 0.65), BPD (44 vs. 49%, RR (95% CI) 0.90 (0.78–1.02), p = 0.09), EOS (4 vs. 4%, RR (95% CI) 1.24 (0.71–2.15), p = 0.55), LOS (13 vs. 12%, RR (95% CI) 1.06 (0.78–1.33), p = 0.93), and PDA (57 vs. 62%, RR (95% CI) 0.93 (0.84–1.02), p = 0.15).
Subjects were divided into two GA groups: 22–23 weeks and 24–25 weeks. After stratification by GA, there were no statistical differences in mortality and morbidities between LGA and AGA extremely premature infants (Table 2).
Mortality and morbidities in LGA infants
Subgroup analyses of mortality and morbidities were also conducted among LGA extremely premature infants stratified by GDM and PI (Table 3). Between LGA infants born from GDM and non-GDM mothers, there were no statistical differences in mortality (27 vs. 18%, RR (95% CI) 1.55 (0.58–4.18), p = 0.67) and morbidities, including IVH (38 vs. 36%, RR (95%) 1.04 (0.42–2.59), p = 1.00), cystic PVL (0 vs. 5%), treated ROP (63 vs. 50%, RR (95% CI) 1.25 (0.72–2.17), p = 0.73), NEC (0 vs. 4%), BPD (14 vs. 45%, RR (95% CI) 0.28 (0.04–1.74, p = 0.14), EOS (0 vs. 4%), LOS (0 vs. 12%), and PDA (63 vs. 57%, RR (9% CI) 1.10 (0.63–1.90), p = 1.00).
In LGA extremely premature infants, PI did not affect mortality (15 vs. 17%, RR (95% CI 0.89 (0.53–1.50), p = 0.77) and morbidities: IVH (40 vs. 33%, RR (95% CI) 1.24 (0.90–1.71), p = 0.24), cystic PVL (6 vs. 5%, RR (95% CI) 1.16 (0.43–3.16), p = 0.99), treated ROP (48 vs. 48%, RR (95% CI) 0.93 (0.72–1.19), p = 0.10), NEC (4 vs. 3%, RR (95% CI) 1.16 (0.34–4.01), p = 1.00), and BPD (47 vs. 45%, RR (95% CI) 1.05 (0.80–1.36), p = 0.83), EOS (5 vs. 5%, RR (9% CI) 1.09 (0.37–3.23), p = 1.00), LOS (11 vs. 12%, RR (95% CI) 0.96 (0.48–1.91), p = 1.00), and PDA (61 vs. 55%, RR (95% CI) 1.10 (0.90–1.35), p = 0.46).
Discussion
In this comparative study of outcomes in LGA and AGA extremely premature infants in Japan, differences in mortality and morbidities were investigated between the two groups. The study highlighted that there were no statistical differences in mortality and morbidities between LGA and AGA extremely premature infants. Furthermore, subgroup analyses between LGA extremely premature infants showed that existence of maternal GDM and the neonatal PI did not affect mortality and morbidities in LGA extremely premature infants.
Previously, Baer and colleagues also examined the effect of SGA or LGA status on mortality and morbidity by GA among infants born between 25 and 44 weeks. They reported that there was a decreased mortality risk for LGA infants born between 25 and 27 weeks and the decreased risk of preterm morbidity (any of IVH, NEC, BPD, ROP, or PVL) for LGA infants born before 37 weeks.20 Recently, Boghossian and colleagues have also reported in-hospital outcomes in LGA infants born at 22–29 weeks of gestation. They concluded that infants born with LGA were associated with lower risks for all examined outcomes, including mortality, respiratory distress syndrome, PDA, NEC, LOS, severe ROP, and BPD, except for EOS and severe IVH.21 They explained these benefits of LGA compared with AGA by a 100-g increment in BW across the entire GA range, which could affect perceptions about impairment and consequent life support provisions, such as surfactant therapy, ventilator support, epinephrine, and cardiac compressions. Furthermore, they found higher rates of maternal hypertension among AGA infants compared to LGA infants both during and after 24 weeks. On the other hand, the increased risks of EOS and severe IVH among LGA infants were explained by higher rates of chorioamnionitis.
In Japan, the limit of viability moved from 24 to 22 weeks of gestation in 1991. A national survey conducted in Japan in 2012 reported that active resuscitation of extremely preterm infants born at 22 and 23 weeks of gestation was performed in 81 and 85% of NICUs.22 In contrast, active treatment was provided by only 22% of infants born at 22 weeks and by 71% born at 23 weeks in 11 participating sites in the National Institute of Child Health and Human Development Neonatal Research Network in the US.23 Therefore, one of the reasons for the distinction between results from the current study and previous studies might be explained by the difference in decision-making pertaining to the active treatment of periviable infants, suggesting that an increment of 100–150 g in BW in LGA extremely premature infants does not reduce the risk of neonatal morbidities in this Japanese cohort.
In Boghossian’s study, the differences in neonatal morbidities between LGA and AGA infants were also explained by the rates of maternal hypertension and chorioamnionitis. In the current study, there was no significant difference observed in the rate of maternal hypertension was between LGA (2.25%) and AGA (2.75%) (RR (95% CI) 1.22 (0.61–2.47), p = 0.70), as well as in the rate of chorioamnionitis (35.7 vs. 34.1%, RR (95% CI) 0.96 (0.83–1.11), p = 0.62), which could also explain the reason for the different results from the Boghossian’s study.
Infants who are born with LGA are seemingly more common among diabetic pregnancies. Infants of diabetic mothers are at an increased risk of mortality and various neonatal adverse outcomes, including macrosomia, preterm birth, hypoglycemia, hypocalcemia, hyperbilirubinemia, polycythemia, respiratory distress syndrome, hypertrophic cardiomyopathy, cardiac malformations, and neurologic impairment due to perinatal asphyxia and birth traumas.20, 24, 25 To examine the effects of GDM on neonatal outcomes in LGA extremely premature infants, a subgroup analysis was performed according to the presence/absence of GDM. In the current study, LGA preterm infants with GDM did not increase the risks of mortality and any prematurity-related morbidities compared to those without GDM. The adverse neonatal outcomes are not constant in all GDM cases, but their frequency and severity are significantly influenced by maternal care quality and maternal health condition.24 Similar to our results, in the international cohort study of singleton infants who were born very preterm, Persson and colleagues have reported that very preterm infants born to mothers with diabetes are not at a higher risk of in-hospital mortality or morbidity compared to the population without diabetes.26 This is mostly consistent with data from previous studies,27,28,29,30 even though some studies report an increased risk of NEC in infants of mothers with GDM.28, 29 Possible explanations for the lack of increase in mortality and morbidities in LGA extremely premature infants by the presence of GDM are (1) short-term exposure to hyperglycemia in utero due to extremely preterm birth, (2) recent improvements in management of maternal GDM, or (3) a more intense monitoring of high-risk pregnancies, such as pregnancies with maternal GDM.
PI has generally been the traditional measure used to assess proportionality at birth and to discriminate between asymmetrical and symmetrical intrauterine growth restriction types. Recently, it was also calculated as a marker of adiposity.31 Armangil and colleagues calculated PI of LGA infants and found that the median PI of infants with diabetic mothers was significantly higher than that of infants with non-diabetic mothers. However, mean BW, height, and head circumference were similar in both groups, suggesting that PI can provide useful information on the proportionality of fetal growth in LGA infants.32 Therefore, we divided LGA infants into two groups according to the proportionality of fetal growth using PI values. There were no differences in mortality and morbidities between LGA infants with higher PI (PI > 2.5) and those with lower PI (PI ≤ 2.5). Persson and colleagues also examined whether disproportionate body composition was a risk factor for perinatal complications in preterm and term LGA infants born to mothers with type 1 diabetes using PI and concluded that disproportionality was not a risk factor for neonatal complications in LGA infants, as was also observed in our current study.33
We acknowledge that there are several limitations to this study. First, because it was a retrospective study, maternal and neonatal complications were defined before the study; thus the evaluation of these complications was performed at each participating center. Therefore, the precision of these diagnoses was difficult to assess. Second, an essential aspect of extremely premature infants’ management is the long-term outcome, but this could not be evaluated as part of this study. Third, because the number of LGA infants born to mothers with GDM was small and information on the impact of maternal GDM on AGA infants was lacking, we could not draw definitive conclusions from mortality and morbidities.
We conclude that (i) compared with AGA extremely premature infants, LGA infants were not likely to die or have prematurity-related morbidities in a Japanese nationwide cohort; (ii) maternal GDM and PI did not seem to affect mortality and morbidities in LGA infants.
References
Katz, J. et al. Mortality risk in preterm and small-for-gestational-age infants in low-income and middle-income countries: a pooled country analysis. Lancet 382, 417–425 (2013).
Mendez-Figueroa, H., Truong, V. T., Pedroza, C. & Chauhan, S. P. Morbidity and mortality in small-for-gestational-age infants: a secondary analysis of nine MFMU Network studies. Am. J. Perinatol. 34, 323–332 (2017).
Longo, S. et al. Short-term and long-term sequelae in intrauterine growth retardation (IUGR). J. Matern. Fetal Neonatal Med. 26, 222–225 (2013).
Wasiluk, A. et al. Thrombopoiesis in small for gestational age newborns. Platelets 20, 520–524 (2009).
Fustolo-Gunnink, S. F. et al. Early-onset thrombocytopenia in small-for-gestational-age neonates: a retrospective cohort study. PLoS ONE 11, e0154853 (2016).
Boghossian, N. S., Geraci, M., Edwards, E. M. & Horbar, J. D. Morbidity and mortality in small for gestational age infants at 22 to 29 weeks’ gestation. Pediatrics 141, e20172533 (2018).
Berard, J. et al. Fetal macrosomia: risk factors and outcome. A study of the outcome concerning 100 cases >4500 g. Eur. Obstet. Gynecol. Reprod. Biol. 77, 51–59 (1998).
Seely, E. W. Current opinion in endocrinology, diabetes & obesity. Editorial overview. Curr. Opin. Endocrinol. Diabetes Obes. 17, 187 (2010).
Weissmann-Brenner, A. et al. Maternal and neonatal outcomes of large for gestational age pregnancies. Acta Obstet. Gynecol. Scand. 91, 844–849 (2012).
Schellong, K., Schulz, S., Harder, T. & Plagemann, A. Birth weight and long-term overweight risk: systematic review and a meta-analysis including 643,902 persons from 66 studies and 26 countries globally. PLoS ONE 7, e47776 (2012).
Johnsson, I. W., Haglund, B., Ahlsson, F. & Gustafsson, J. A high birth weight is associated with increased risk of type 2 diabetes and obesity. Pediatr. Obes. 10, 77–83 (2015).
Harder, T., Rodekamp, E., Schellong, K., Dudenhausen, J. W. & Plagemann, A. Birth weight and subsequent risk of type 2 diabetes: a meta-analysis. Am. J. Epidemiol. 165, 849–857 (2007).
Yu, Z. B. et al. Birth weight and subsequent risk of obesity: a systematic review and meta-analysis. Obes. Rev. 12, 525–542 (2011).
Nakanishi, H. et al. Trends in the neurodevelopmental outcomes among preterm infants from 2003-2012: a retrospective cohort study in Japan. J. Perinatol. 38, 917–928 (2018).
Itabashi, K., Miura, F., Uehara, R. & Nakamura, Y. New Japanese neonatal anthropometric charts for gestational age at birth. Pediatr. Int. 56, 702–708 (2014).
Bell, M. J. et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann. Surg. 187, 1–7 (1978).
Papile, L. A., Munsick-Bruno, G. & Schaefer, A. Relationship of cerebral intraventricular hemorrhage and early childhood neurologic handicaps. J. Pediatr. 103, 273–277 (1983).
Lubchenco, L. O., Hansman, C. & Boyd, E. Intrauterine growth in length and head circumference as estimated from live births at gestational ages from 26 to 42 weeks. Pediatrics 37, 403–408 (1966).
Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 48, 452–458 (2013).
Baer, R. J. et al. Population-based risks of mortality and preterm morbidity by gestational age and birth weight. J. Perinatol. 36, 1008–1013 (2016).
Boghossian, N. S., Geraci, M., Edwards, E. M. & Horbar, J. D. In-hospital outcomes in large for gestational age infants at 22-29 weeks of gestation. J. Pediatr. 198, 174.e13–180.e13 (2018).
Isayama, T. The clinical management and outcomes of extremely preterm infants in Japan: past, present, and future. Transl. Pediatr. 8, 199–211 (2019).
Younge, N. et al. Survival and neurodevelopmental outcomes among periviable infants. N. Engl. J. Med. 376, 617–628 (2017).
Mitanchez, D., Yzydorczyk, C. & Simeoni, U. What neonatal complications should the pediatrician be aware of in case of maternal gestational diabetes? World J. Diabetes 6, 734–743 (2015).
Lai, F. Y., Johnson, J. A., Dover, D. & Kaul, P. Outcomes of singleton and twin pregnancies complicated by pre-existing diabetes and gestational diabetes: a population-based study in Alberta, Canada, 2005-11. J. Diabetes 8, 45–55 (2016).
Persson, M. et al. Association of maternal diabetes with neonatal outcomes of very preterm and very low-birth-weight infants: an international cohort study. JAMA Pediatr. 172, 867–875 (2018).
Rehan, V. K., Moddemann, D. & Casiro, O. G. Outcome of very-low-birth-weight (<1,500 grams) infants born to mothers with diabetes. Clin. Pediatr. 41, 481–491 (2002).
Grandi, C., Tapia, J. L. & Cardoso, V. C. Impact of maternal diabetes mellitus on mortality and morbidity of very low birth weight infants: a multicenter Latin America study. J. Pediatr. 91, 234–241 (2015).
Boghossian, N. S. et al. Outcomes of extremely preterm infants born to insulin-dependent diabetic mothers. Pediatrics 137, e20153424 (2016).
Bental, Y. et al. Impact of maternal diabetes mellitus on mortality and morbidity of preterm infants (24-33 weeks’ gestation). Pediatrics 128, e848–e855 (2011).
Olsen, I. E. et al. Use of a body proportionality index for growth assessment of preterm infants. J. Pediatr. 154, 486–491 (2009).
Armangil, D., Yurdakök, M., Korkmaz, A., Yiğit, S. & Tekinalp, G. Ponderal index of large-for-gestational age infants: comparison between infants of diabetic and non-diabetic mothers. Turk. J. Pediatr. 53, 169–172 (2011).
Persson, M., Pasupathy, D., Hanson, U. & Norman, M. Disproportionate body composition and perinatal outcome in large-for-gestational-age infants to mothers with type 1 diabetes. BJOG 119, 565–572 (2012).
Acknowledgements
Institutions enrolled in the study of the Neonatal Research Network, Japan were as follows: Sapporo City General Hospital, Asahikawa Kosei General Hospital, Engaru-Kosei General Hospital, Kushiro Red Cross Hospital, Obihiro-Kosei General Hospital, Tenshi Hospital, NTT Higashinihon Sapporo Hospital, Nikko Memorial Hospital, Nayoro City General Hospital, Sapporo Medical University, Asahikawa Medical University, Aomori Prefectural Central Hospital, Iwate Medical University, Iwate Prefectural Ofunato Hospital, Iwate Prefectural Kuji Hospital, Iwate Prefectural Ninohe Hospital, Sendai Red Cross Hospital, Akita Red Cross Hospital, Tsuruoka Municipal Shonai Hospital, Yamagata University, Yamagata Prefectural Central Hospital, Fukushima Medical University, Takeda General Hospital, Fukushima National Hospital, Tsukuba University, Tsuchiura Kyodo Hospital, Ibaraki Children’s Hospital, Dokkyo Medical University, Jichi Medical University, Ashikaga Red Cross Hospital, Gunma Children’s Medical Center, Kiryu Kosei General Hospital, Fuji Heavy Industries Health Insurance Society Ota Memorial Hospital, Gunma University, Saitama Children’s Medical Center, Nishisaitama-chuo National Hospital, Saitama Medical University Saitama Medical Center, Kawaguchi Municipal Medical Center, Jichi Medical University Saitama Medical Center, Asahi General Hospital, Chiba Kaihin Municipal Hospital, Kameda Medical Center, Tokyo Women’s Medical University Yachiyo Medical Center, Juntendo University Urayasu Hospital, Tokyo Metropolitan Children’s Medical Center, Tokyo Women’s Medical University, Aiiku Hospital, Nihon University Itabashi Hospital, National Center for Global Health and Medicine, Tokyo Medical University, Teikyo University, Showa University, Japan Red Cross Medical Center, National Center for Child Health and Development, Tokyo Metropolitan Otsuka Hospital, Toho University, Tokyo Metropolitan Bokuto Hospital, Tokyo Jikei Medical University, Tokyo Medical and Dental University, Saint Luku’s International Hospital, Juntendo University, Sanikukai Hospital, Katsushika Red Cross Hospital, Yokohama Rosai Hospital, Yokohama City University Medical Center, St. Marianna University School of Medicine Hospital, Kanagawa Children’s Medical Center, Tokai University, Kitazato University, Odawara Municipal Hospital, Nippon Medical School Musashi Kosugi Hospital, Saiseikai Yokohamashi Tobu Hospital, National Hospital Organization Yokohama Medical Center, Yamanashi Prefectural Central Hospital, Nagano Children’s Hospital, Shinshu University, Iida Municipal Hospital, National Hospital Organization Shinshu Ueda Medical Center, Saku General Hospital, Niigata University, Niigata Prefectural Central Hospital, Niigata Municipal Hospital, Nagaoka Red Cross Hospital, Koseiren Takaoka Hospital, Toyama Prefectural Central Hospital, Toyama University, Ishikawa Medical Center for Maternal and Child Health, Kanazawa Medical University, Kanazawa Medical Center, Fukui Prefectural Hospital, Fukui University, Gifu Prefectural General Medical Center, National Hospital Organization Nagara Medical Center, Takayama Red Cross Hospital, Seirei Hamamatsu Hospital, Shizuoka Saiseikai Hospital, Shizuoka Children’s Hospital, Hamamatsu Medical University, Numazu Municipal Hospital, Yaizu City Hospital, Fujieda Municipal General Hospital, Nagoya Red Cross Daini Hospital, Nagoya University, Nagoya Red Cross Daiichi Hospital, Toyohashi Municipal Hospital, Nagoya City West Medical Center, Anjo kosei Hospital, Tosei General Hospital, Komaki Municipal Hospital, TOYOTA Memorial Hospital, Okazaki Municipal Hospital, Konan Kosei Hospital, National Mie Central Medical Center, Ise Red Cross Hospital, Yokkaichi Municipal Hospital, Otsu Red Cross Hospital, Shiga University of Medical Science Hospital, Nagahama Red Cross Hospital, Uji Tokushukai Hospital, The Japan Baptist Hospital, Kyoto University, Kyoto Red Cross Daiichi Hospital, National Maizuru Medical Center, Fukuchiyama City Hospital, Kyoto Prefectural University of Medicine Hospital, Kyoto City Hospital, Mitsubishi Kyoto Hospital, Yodogawa Christian Hospital, Osaka Medical Center and Research Institute for Maternal and Child Health, Osaka University, Takatsuki General Hospital, Kansai Medical University, Osaka City General Hospital, Osaka City Sumiyoshi Hospital, Aizenbashi Hospital, Toyonaka Municipal Hospital, National Cerebral and Cardiovascular Center, Kitano Hospital, Saiseikai Suita Hospital, Chifune Hospital, Bellland General Hospital, Rinku General Medical Center, Osaka Red Cross Hospital, Yao Municipal Hospital, Osaka General Medical Center, Osaka City University, Hyogo Prefectural Kobe Children’s Hospital, Kobe University, Kakogawa West City Hospital, Saiseikai Hyogoken Hospital, Kobe City Medical Center General Hospital, Hyogo College of Medicine Hospital, Himeji Red Cross Hospital, Toyooka Public Hospital, Hyogo Prefectural Awaji Medical Center, Nara Medical University, Wakayama Medical University, Tottori Prefectural Central Hospital, Tottori University, Shimane Prefectural Central Hospital, Matsue Red Cross Hospital, Kurashiki Central Hospital, Tsuyama Central Hospital, Kawasaki Medical School Hospital, National Hospital Organization Okayama Medical Center, Okayama Red Cross Hospital, Hiroshima City Hiroshima Citizens Hospital, Hiroshima Prefectural Hospital, Hiroshima University, Tsuchiya General Hospital, National Hospital Organization Kure Medical Center, Yamaguchi University, Yamaguchi Grand Medical Center, Tokushima University, Tokushima Municipal Hospital, Kagawa University, National Hospital Organization Kagawa Children’s Hospital, Matsuyama Red Cross Hospital, Ehime Prefectural Central Hospital, Kochi Health Science Center, St. Mary’s Hospital, National Kyushu Medical Center, Kurume University, Kitakyushu Municipal Medical Center, University of Occupational and Environmental Health, Fukuoka University, Kyushu University, Iizuka Hospital, National Hospital Organization Kokura Medical Center, National Hospital Organization Saga Hospital, National Hospital Organization Nagasaki Medical Center, Kumamoto City Hospital, Kumamoto University, Oita Prefectural Hospital, Almeida Memorial Hospital, Nakatsu Municipal Hospital, Miyazaki University, National Hospital Organization Miyakonojo Medical Center, Kagoshima City Hospital, Imakiire General Hospital, Okinawa Prefectural Nanbu Medical Center & Children’s Medical Center, Okinawa Prefectural Chubu Hospital, Naha City Hospital, Okinawa Red Cross Hospital
Author information
Authors and Affiliations
Consortia
Contributions
K.K., F.N., and Neonatal Research Network, Japan made substantial contributions to conception, design, and acquisition of data. J.O. and K.T. analyzed the data. J.O. and F.N. contributed to drafting the article. J.O., K.T., K.K., and F.N. contributed to interpretation of data, revising the article critically for important intellectual content, and approved the final version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent statement
Patient consent was not required to this study, because it met the criteria that “the research involves no more than minimal risk to the subjects.”
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Ozawa, J., Tanaka, K., Kabe, K. et al. Impact of being large-for-gestational-age on neonatal mortality and morbidities in extremely premature infants. Pediatr Res 90, 910–916 (2021). https://doi.org/10.1038/s41390-021-01375-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01375-z