Abstract
Background
To define the role of topical timolol maleate (TTM) in the treatment of infantile haemangiomata (IH).
Methods
In this single-centre randomised controlled trial, we included all <1-year-old infants within a 13-month period presenting with small (<2 cm) superficial IH located at high-risk areas (i.e. tip of ears, tip of nose, eyelids, acral areas, facial areas, scalp, neck, buttocks, perineum and axilla). Patients either received 12 months of 0.5% TTM solution (study group) or watchful waiting (control group). The primary outcome was IH with development of complications that required additional interventions. The secondary outcomes included side effects of TTM and change in IH size.
Results
Forty-two children were eligible to the study. Patients who received TTM were noted to have significantly fewer complications than the control group (4.2% versus 29%, odds ratio 9.58 [95% confidence interval: 1.01–91.62], p = 0.04). Mean IH volume percentage reduction was significantly more for the TTM group and no-TTM group at 3, 6 and 12 months.
Conclusions
TTM is an effective and safe treatment option to reduce complications, IH volume and the need for further intervention for infants with small superficial IH located at high-risk areas.
Impact
-
There is a lack of reliable signs to predict ulceration, disfigurement and other complications for high-risk IH.
-
Treatment options range from watchful waiting to early systemic treatment, with TTM a novel and promising treatment.
-
The exact role of TTM remains unanswered due to a lack of evidence-based research.
-
TTM is effective and safe for infants with superficial IH of <2 cm in high-risk areas.
-
Early TTM use on IH can reduce complications, IH volume and the need for further treatment.
Similar content being viewed by others
Introduction
As the most common vascular tumour of infancy, infantile haemangiomata (IH) are a major source of concern and stress for parents around the world, affecting ~1–12.7% of all infants worldwide.1,2,3 They often proliferate rapidly in the first year of life and then involute in young children over years.4 Some patients may develop ulceration and haemorrhage that can result in disfigurement, functional impairment, tissue necrosis and life-threatening complications, with 10–38% warranting specialist assessment and further intervention.3,4,5 Ulcerations risks are reported to be ~16%, with those located at areas of frequent mechanical trauma (i.e. tip of ears, tip of nose, ends of phalanges, facial areas, buttocks, perineum and axilla) considered as higher risk.1,6 Ulcerations further can cause pain, haemorrhage, irritability, secondary infection, scarring and disfigurement and can lead to decreased appetite and sleep, as well as parental stress. Corticosteroids, propranolol and laser therapy are currently the main therapeutic modalities for IH that require intervention,3,7 while watchful waiting with caregiver education and guidance is the mainstay of treatment for non-complicated IH.3,4
An increasing number of treatment options have been introduced for IH,8 and topical timolol maleate (TTM) eye drops—a non-selective beta blocker used for glaucoma patients—has been increasingly used in view of its good safety profile and potential benefits.1,9,10,11,12
Although the action of beta blockers on IH is not completely understood,9 proposed mechanisms include vasoconstriction, downregulation of vascular endothelial growth factor and basic fibroblast growth factor, and/or triggering of apoptosis.2,3,9 One drop of 0.5% TTM is estimated to contain 0.25 mg of the drug.2,9 It is accepted that TTM does not penetrate deeply and with no systemic effects unlike oral propranolol (with 13.7% of patients reporting systemic effects).13 Studies on systemic absorption have shown clinically insignificant timolol levels in the bloodstream of patients who were given the medication topically.2,13,14
Due to a lack of predictive indicators for degree of growth and involution, studies have used a wide range of measurements to assess efficacy of interventions, including size reduction, growth cessation, completeness of involution, visual analogue scales for colour and haemangioma activity scores, among others. Moreover, the large variation in IH size, thickness, location, superficial versus deep components and stage of growth create further confusion for clinicians. There is currently no consensus on the optimal preparation, dose and duration for TTM use,2,11,14,15 with the definite indications and benefits for TTM usage for IH still unknown and clinical significance still unclear.2,11,12,15,16 There is also a paucity of high-quality evidence on TTM, with only one single randomised controlled trial identified by guidelines, meta-analyses and systematic reviews.11,12,13
Response rates of IH to TTM are quoted to range from 47% to 100%.13 While published studies have focused on the safety and optimal dosing regimen of TTM for IH, the vital question of the exact role of TTM in preventing complications and need for further intervention remains unanswered.
In this study, we aimed to evaluate the effectiveness of TTM in the prevention of complications and need for more aggressive interventions for IH located at high-risk areas. We expected TTM to reduce complications and the subsequent need for further interventions for IH. TTM solution (0.5%) was chosen for our study based on its superior safety profile and availability at our unit,1,2,17 as well as its perceived superiority in efficacy as compared to more dilute formulations.9,18 A treatment duration of at least 6 months was shown to be effective in prior studies.17,18 We focused on small superficial IH of <20 mm based on current evidence favouring a better outcome for these lesions with higher surface area-to-volume ratio, while study subjects of <1 year old were chosen due to data demonstrating more effective response in early proliferation stage IH.9,15
Methods
In 2016, a single-centre, prospective randomised study was initiated at United Christian Hospital in Hong Kong. Patients were enrolled over a 14-month period. Informed consents were obtained from all parents of the patients.
All patients referred to the hospital’s paediatric outpatient clinic for the management of IH in the study time period were recruited into the study. The inclusion criteria included Chinese patients, patients <1 year old at first consultation within the study period, superficial IH, IH <2 cm in its longest diameter and IH located in high-risk areas (specifically, tip of ears, tip of nose, eyelids, acral areas, facial areas, scalp, neck, buttocks, perineum and axilla).
The exclusion criteria included patients with pre-treated IH, IH with mixed or deep components, non-IH such as non-involuting congenital haemangiomata, syndromal haemangiomata (e.g. PHACES (posterior fossa anomalies, hemangioma, arterial anomalies, cardiac anomalies and eye anomalies) syndrome) and IH that were already complicated or ulcerated at the first consultation.
Accepted patients were randomised by simple randomisation by coin toss to either the TTM group or the no-TTM group. TTM (0.5%) ophthalmological solution was prescribed at 1 drop per 10 mm in length/width of lesion twice daily for 12 months. Patients were followed up at 1, 3, 6 and 12 months after study uptake, with documentation of baseline patient demographics, details on TTM dosing, IH characteristics, IH complications and drug side effects. Blood pressure, respiratory rate and heart rate were performed for new cases, but not routinely arranged afterwards. The lesion was then photographed by the trained investigators to document its colour, superficial and deep components and margin regularity. The width, length and depth of the IH were measured to the nearest millimetre and documented. Haemangioma size was estimated by assuming the IH is a hemisphere.
Intervention group (TTM group)
TTM was initiated on parental consent to study recruitment. Local application of 0.5% TTM solution at 1 drop per 10 mm in length or width of lesion for 6 months was prescribed for all patients in this group. Caregivers were taught how to apply TTM and gently rub it in a circular motion for 1 min. The lesion was not occluded. Follow-up was arranged at 1, 3, 6 and 12 months after initiation of the drug.
Non-intervention group (no-TTM group)
All treatment modalities and follow-up intervals were identical to the intervention group albeit without the use of TTM.
Outcome measures
Primary outcome was the number of IH with rapid increase in size, development of ulceration or impairment of vital functions (e.g. breathing, vision). Additional interventions including oral propranolol, laser therapy, corticosteroid injection or surgical excision, were arranged should the aforementioned features occur.
Secondary outcomes included incidence of side effects of TTM, and the change in IH size. These were objectively measured and recorded by designated investigators in follow-up clinics.
Study participants who failed to return for follow-up sessions were contacted by phone for any development of IH complications.
Statistical analysis
We hypothesised that TTM is superior to watchful waiting for treatment of IH. Assuming that the percentage of uncomplicated IH without need for further intervention would be 62%,5 while that after the use of TTM would be 99%,13 and the superiority margin 0.05, the required sample size with equal (1:1) allocation to achieve an 80% power (=0.2) and α = 0.05 is 60 subjects (30 subjects in each group). Assuming a 5% dropout rate, we planned to recruit a total of 64 subjects into this study, with 32 subjects in the TTM group and 32 subjects in the no-TTM group. The primary and secondary analyses of all outcomes followed the intention-to-treat principle.
Patient characteristics were presented as frequencies and percentages for categorical data and means (SD) or medians (interquartile range) for continuous data.
Statistical analysis was performed using the Statistical Package SPSS 23 software. Categorical data was compared using the χ2 test or the Fisher’s exact test (for cells <5), and odds ratio (OR) with 95% confidence interval (CI) was calculated. Continuous variables were compared using the independent t test or Mann–Whitney U test. For the secondary outcome, percentage changes over time at 3, 6 and 12 months after study uptake were compared. Flat IH and subjects who defaulted the 12-month follow-up session were excluded from the secondary outcome analysis, while missing data were kept the same as the last measured size. IH were excluded from the secondary outcome analysis upon receiving additional treatment. A two-sided p value of ≤0.05 was considered significant.
Results
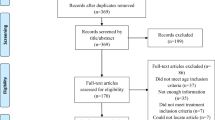
Of the 43 children who were included in the study, one patient was ineligible due to beta-blocker usage prior to study uptake. Of those eligible, parents of one patient declined consent to enter the study. Twenty-four patients were randomised into the TTM group and 17 patients in the no-TTM group (Fig. 1). The intragroup analysis for gender and age was similar among the two groups, but there were more acral lesions in the TTM group (Table 1). All patients were <7 months of age at study enrolment.
The primary outcome comparing IH complications (Table 2a, b) showed significant results for the TTM group (1/24 [4.2%]) as opposed to the no-TTM group (5/17 [29%]; OR 9.58 [95% CI: 1.01–91.62]; p = 0.04*], with complications including ulceration (5/41 [12%]) and one showing rapid progression of >150% per month increase in volume (1/41 [2.4%]). No patients suffered from impairment of vital functions due to IH complications. Propranolol and/or laser treatment were indicated and offered to the parents of all six patients. No patients required corticosteroid injection or surgical excision of IH. Analysis of mean IH volume percentage change for TTM group and no-TTM group from 0 to 3, 6 and 12 months all showed significant results (Table 2c, Fig. 2). No side effects were reported in the TTM group. The most significant effect was noted in the first 3 months of TTM usage.
Discussion
“To treat or not to treat?”—this has always been a question in the management of IH located at high-risk areas. While experts have agreed on the IH locations that pose higher risks of complications, the lack of reliable signs to predict ulceration, disfigurement and other complications creates a treatment dilemma between watchful waiting (with anxiety and complication risks) versus early treatment with systemic agents (with risks of discomfort and side effects). The emergence of TTM offers hope to resolve this dilemma.
To the best of our knowledge, this is the first randomised controlled trial that shows reduction in complications in small superficial high-risk IH, as well as the first Chinese randomised controlled trial on timolol for the treatment of IH. The first reported use of TTM for IH was in 2010 by Guo and Ni,10 although it had been used for adult and paediatric ophthalmology patients for over 30 years.1 Since then, TTM has become increasingly popular as a safe alternative to conservative treatment with mixed results for deep, large or even ulcerated haemangiomata in certain studies.2
In particular, a randomised placebo controlled trial of 41 patients by Chan et al.9 has shown a significant reduction in size, colour and proportional growth in the TTM group compared with the placebo group for lesions of <100 mm3 volume, albeit slower than oral propranolol. It concluded that two drops per day application of 0.5% TTM gel is a safe and effective treatment for IH that do not require systemic treatment, and that further studies may be needed to investigate “factors such as site-dependent efficacy […, duration of treatment and age-group-specific data”.
A review by Khan et al.11—the first meta-analysis and systematic review focusing on TTM for IH treatment—analysed 31 studies of 691 patients in total who used TTM for IH, concluding that the various response rates using clinical scores and photograph comparisons showed significant improvement with a 91% resolution rate for a mean treatment duration of 4.11 months in pooled meta-analysis, with the quality of evidence being low to moderate. The study suggested adequately powered randomised controlled trials and less biased studies using clearer diagnostic criteria and validated outcome measures.
Although earlier studies have suggested that TTM improves IH progression, the results of our single-centre randomised controlled trial help to shed light on the vital question of which IH should be treated with TTM. We have demonstrated that for patients <12 months old with superficial small IH of <20 mm in longest diameter located over high-risk areas, the use of TTM successfully reduced complications and the need for further interventions.
The early regression of IH demonstrated by our treatment group illustrates the effectiveness of TTM. Regression at this early age is not typical of that of the natural course of IH.15 The early regression and the reduction in the need for further systemic interventions demonstrated by our study highly supports the use of TTM for patients <1 year old with superficial IH located at high-risk areas.
Our study also supports the safety of TTM, with no reported side effects in a period of 12 months from all subjects. Most other studies have also demonstrated minimal adverse effects.1,9,10,11,13,14,15,17 Larger studies have pointed out that potential side effects include local irritation, rebound growth and sleep disturbance, while evidence for the safety of TTM for ulcerated, mucosal and large IH are lacking.11,15,16,18 It must however be stressed that TTM usage must be supported with diligent follow-up and caregiver education.
While a recent retrospective multi-centred study by Puttgen et al.15 suggested TTM could prevent potential permanent disfigurement and is indicated for superficial IH without aggressive growth or threat of functional impairment, our study suggests that early TTM treatment has the additional benefit of reducing the need for further intervention on these IH, thereby sparing young patients from the risk of systemic beta blockers and more invasive procedures. However, as the above study focused on low-risk patients only, the clinical significance of the study in treatment for IH that have minimal risks of complications from watchful waiting is debatable. At the time of publication of this article, several other clinical trials from around the world have been registered on ClinicalTrials.gov and will hopefully provide more high-quality evidence that support the use and the role of TTM.
The strengths of the study include the prospective design, randomisation, photo documentation of IH, objective outcome measurements, presence of an observation group and significant power from the results. However, despite the sample size being sufficient for a statistically significant primary outcome, it may not be adequate for detection of rare but significant adverse effects. Although the outcomes are significant, objectively defined and professionally assessed whenever possible, blinding would further reduce selection bias. A longer follow-up period beyond 1 year old may also be of benefit to monitor the IH until regression sets in, as well as to assess for any rebound IH growth upon cessation of TTM.17 Preliminary phone contact 2 years after our study has shown no rebound growth of IH.
Conclusion
This study demonstrates that 0.5% TTM solution is an effective and safe treatment option for infants presenting with small superficial IH of <2 cm in largest diameter located in high-risk areas to reduce complications, IH volume and the need for further intervention. These findings should help paediatricians, dermatologists and family doctors by shedding light on the treatment dilemma for the management of IH, specifically on the use of TTM for this group of patients.
Data availability
Data are available on reasonable request. Deidentified participant data are available on reasonable request, contact Dr. James Wesley Cheng (cch278@ha.org.hk).
References
Yu, L. et al. Treatment of superficial infantile hemangiomas with timolol: evaluation of short-term efficacy and safety in infants. Exp. Ther. Med. 6, 388–390 (2013).
Painter, S. L. & Hildebrand, G. D. Review of topical beta blockers as treatment for infantile hemangiomas. Surv. Ophthalmol. 61, 51–58 (2016).
Sethuraman, G., Yenamandra, V. K. & Gupta, V. Management of infantile hemangiomas: current trends. J. Cutan. Aesthet. Surg. 7, 75–85 (2014).
Drolet, B. A. et al. Initiation and use of propranolol for infantile hemangioma: report of a consensus conference. Pediatrics 131, 128–140 (2013).
Cheng, C. E. & Friedlander, S. F. Infantile hemangiomas, complications and treatments. Semin. Cutan. Med. Surg. 35, 108–116 (2016).
Chamlin, S. et al. Multicenter prospective study of ulcerated hemangiomas. J. Pediatr. 151, 684–689 (2007).
Chik, K. K., Luk, C. K., Chan, H. B. & Tan, H. Y. Use of propranolol in infantile haemangioma among Chinese children. Hong Kong Med. J. 16, 341–346 (2010).
Chambers, C. B., Katowitz, W. R., Katowitz, J. A. & Binenbaum, G. A controlled study of topical 0.25% timolol maleate gel for the treatment of cutaneous infantile capillary hemangiomas. Ophthal. Plast. Reconstr. Surg. 28, 103–106 (2012).
Chan, H., McKay, C., Adams, S. & Wargon, O. RCT of timolol maleate gel for superficial infantile hemangiomas in 5- to 24-week-olds. Pediatrics 131, e1739–e1747 (2013).
Guo, S. & Ni, N. Topical treatment for capillary hemangioma of the eyelid using β-blocker solution. Arch. Ophthalmol. 128, 255–256 (2010).
Khan, M. et al. The role of topical timolol in the treatment of infantile hemangiomas: a systematic review and meta-analysis. Acta Derm. Venereol. 97, 1167–1171 (2017).
National Institute for Health and Care Excellence. Infantile Haemangioma: Topical Timolol https://www.nice.org.uk/advice/esuom47/ (2015).
Ovadia, S. A., Landy, D. C., Cohen, E. R., Yang, E. Y. & Thaller, S. R. Local administration of β-blockers for infantile hemangiomas: a systematic review and meta-analysis. Ann. Plast. Surg. 74, 256–262 (2015).
Semkova, K. & Kazandjieva, J. Topical timolol maleate for treatment of infantile haemangiomas: preliminary results of a prospective study. Clin. Exp. Dermatol. 38, 143–146 (2013).
Puttgen, K. et al. Topical timolol maleate treatment of infantile hemangiomas. Pediatrics 138, e20160355 (2016).
Wu, H. W. et al. Topical application of 0.5% timolol maleate hydrogel for the treatment of superficial infantile hemangioma. Front. Oncol. 7, 137 (2017).
Ge, J., Zheng, J., Zhang, L., Yuan, W. & Zhao, H. Oral propranolol combined with topical timolol for compound infantile hemangiomas: a retrospective study. Sci. Rep. 6, 19765 (2016).
Chakkittakandiyil, A. et al. Timolol maleate 0.5% or 0.1% gel-forming solution for infantile hemangiomas: a retrospective, multicenter, cohort study. Pediatr. Dermatol. 29, 28–31 (2012).
Author information
Authors and Affiliations
Contributions
J.W.C.H.C. was the lead investigator. J.W.C.H.C. conceptualised and designed the study, carried out data collection, data curation, data analysis and drafted the initial manuscript, as well as the subsequent revisions. Y.-Y.L. and D.C.K.L. contributed to the design of the study, carried out the data collection and contributed to the revised manuscripts. D.C.K.L. also oversaw the study. C.S. contributed to the design of the study. G.P.G.F. contributed to the data curation and analysis. B.H.B.C. and W.-K.C. contributed to the formal analysis of the final manuscript. All authors approved the final manuscript as submitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and clinical trial registration
Hospital Authority Kowloon East Cluster Clinical Research Ethics Committee (Reference number: KC/KE-16-0187/FR-2; Prospective approval in 2016). Australian New Zealand Clinical Trials Registry (Registration number: ACTRN12619001685101; Retrospective approval in 2019).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cheng, J.W.C.H., Lam, YY., Fung, G.P.G. et al. Randomised controlled trial: Can topical timolol maleate prevent complications for small superficial infantile haemangiomata in high-risk areas?. Pediatr Res 88, 756–760 (2020). https://doi.org/10.1038/s41390-020-0917-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-0917-3