Abstract
Background
To determine normative data by forced oscillation technique (FOT) in non-sedated normal term neonates and test the hypothesis that infants with transient tachypnea of the newborn (TTN) have higher resistance (R) and lower reactance (X) on day 1.
Methods
Healthy term infants (n = 138) and infants with TTN (n = 17) were evaluated on postnatal days 1 through 3 (NCT03346343). FOT was measured with a mask using a TremoFlo C-100 Airwave System™. R, X, and area under the reactance curve (AX) were measured at prime frequencies 7–41 Hz for 8 s.
Results
In all, 86% of control infants had adequate measurements (coherence >0.8, CV < 0.25) on day 1. Infants with TTN had higher resistance at 13 Hz (TTN 32.5 cm H2O·s/L [95% CI 25.5–39.4]; controls 23.8 cm H2O·s/L [95% CI 22.2 to 25.3], P = 0.007) and lower reactance from 17 to 37 Hz (TTN −35.1 to −10.5; controls −26.3 to −6.1, P < 0.05). In healthy controls, lung mechanics were unchanged from days 1 to 3. In TTN, lung mechanics normalized on days 2 and 3.
Conclusions
FOT is feasible in neonates and distinguishes normal control infants from those with TTN on postnatal day 1. Oscillometry offers a non-invasive, longitudinal technique to assess lung mechanics in newborns.
Similar content being viewed by others
Introduction
Pulmonary function testing (PFT) detects respiratory diseases and evaluates response to therapeutic intervention. Unfortunately, these measures are typically only available in older children and adults who are able to perform spirometry.1 Currently available PFT for neonates requires sedation with methods such as forced inhalation and exhalation or requires sophisticated and frequently cumbersome equipment such as the single occlusion and nitrogen washout techniques, limiting their widespread use outside of research centers.2,3 Therefore, PFT techniques that can be performed non-invasively, reproducibly, and longitudinally at the bedside in neonates without these difficulties are desirable.
The forced oscillation technique (FOT) is a method in which a multi-frequency oscillatory signal is super-imposed on spontaneous tidal breathing to provide measurements of respiratory mechanics.4 FOT can be performed in less than half a minute, non-invasively, without sedation, and longitudinally,5 and usually takes only a few minutes for set-up time. FOT can measure total resistance (R), which relates to the impedance of signal through the respiratory system (e.g., the airways and tissues), and reactance (X), which corresponds to elasticity and inertia in the airway tree and tissues.5,6,7 The measures of R and X depend on the frequency of the transmitted oscillatory signal, with the medium range frequency from 5 to 40 Hz providing the greatest information about airway R and X; lower or higher frequencies measure tissue properties and acoustic wave propagation.6 The area under the reactance curve (AX) is a calculated value that reflects the frequency dependence of these changes.8 Hence, FOT is an attractive tool to quantify pulmonary function and track changes in disease trajectory. However, uptake of FOT in routine neonatal practice has been slow due to lack of reproducible normative neonatal values that distinguish health from disease as well as limited commercially available FOT equipment suitable for neonates.
Transient tachypnea of the newborn (TTN) is a frequently encountered neonatal respiratory disorder that is characterized by pulmonary edema resulting from delayed resorption and clearance of fetal alveolar fluid.9 Using nitrogen washout testing in combination with volume displacement plethysmography and an airway interrupter technique, it has been previously found that infants with TTN often have decreased compliance, in addition to other abnormalities of lung mechanics and function.10 As TTN is a common respiratory illness that usually resolves without therapeutic escalation, this disease was selected as the initial condition to evaluate the utility of FOT to detect disease and monitor improvement. Our objective was to determine feasibility and obtain normative data for FOT in non-sedated, term neonates without respiratory disease and to test the hypothesis that neonates with TTN have decreased X (which shares properties with compliance) and increased R compared to controls soon after birth.
Methods
Study design and participants
This was a single-center prospective observational cohort study evaluating the FOT in healthy term infants and infants with TTN. Data were reported according to the Strengthening the Reporting of Observational Studies in Epidemiology reporting guidelines for cohort studies (www.strobe-statement.org). This study was approved by the Institutional Review Board at the University of Alabama at Birmingham (UAB), Birmingham, AL. The study is part of a project registered with Clinicaltrials.gov (NCT03346343) evaluating the FOT method in preterm and term infants. From January 1 through November 16, 2018, 175 infants born at UAB hospital were eligible and enrolled following informed consent from an available parent. Inclusion criteria for the control cohort included a gestational age >37 weeks and enrollment within 24 h from birth; exclusion criteria included one or more of the following: receiving supplementary oxygen, signs of respiratory distress (defined as respiratory rate (RR) >60 breaths/min (bpm), subcostal retractions, or nasal flaring), and congenital malformations. Inclusion criteria for the TTN cohort included a diagnosis of TTN (defined as tachypnea with maintained RR >60 bpm at 2 h of age) and enrollment within 24 h from birth; exclusion criteria included congenital malformations and a diagnosis of other causes of respiratory distress (e.g., respiratory distress syndrome (RDS) or meconium aspiration syndrome).11,12 Tachypnea was defined as RR > 60 bpm at the time of exam. Our previous evaluation of 488 term infants13 indicated that, although 50% of infants had RR > 60 bpm in the first hour after birth, subsequent measurements were 50 bpm (SD 7). Hence, we anticipated that only about 5% of normal infants would have a RR > 60 bpm at the time point of evaluation. We used the most recent charting for initial screening of infants, followed by study team evaluation for confirmation.
While most cases of TTN do not require escalation of support, infants in the TTN group were not tested if receiving endotracheal intubation or continuous positive airway pressure (CPAP), but testing was permissible once weaned from such respiratory support. Chest X-ray findings were used to substantiate the diagnosis of TTN but were not a prerequisite as not all infants required an X-ray per the clinical team. Gestational age was confirmed by earliest ultrasonographic measurements available through prenatal records. Patient characteristics were collected through chart review.
Materials and procedures
All enrolled infants were tested after enrollment by one of the authors on postnatal days 1–3 (as available). Measurements were obtained using a TremoFlo C-100 Airwave System™ (THORASYS Thoracic Medical Systems Inc., Montreal, QC, Canada), custom fitted, and calibrated (by the manufacturer) with a filter (Humid-Vent Filter Small A, Teleflex Medical, Ireland) and infant face mask (Portex infant, large). The device was calibrated daily. Calibration was performed by using a dynamic method that takes into account the shunt impedance through the filter. The mask is regarded as a static part of the respiratory system in this set-up. The calibrated range of the device used in this study has been modified; however, the hardware is identical to the commercially available TremoFlo C-100 device. The dynamic calibration impedance range of the device used in this study is from 0 to 90 cm H2O/L/s. The factory calibration was checked daily by measuring a test load with a known impedance of 2 cm H2O/L/s, which is the standard reference for the TremoFlo SystemTM. The instrument has been found to be linear in calibration.14 A fault in the system, including the dynamic calibration, would be detected by the daily check. The software used was the standard TremoFlo supplied by the manufacturer without any modifications. The mask is air cushioned and is compliant to fit snugly over the nose and mouth of the infant, hence minimizing the risk for leaks. If there was a leak at the relatively high impedances seen in infants, this would be quickly detected as the impedance would drop precipitously. The software also allows for post hoc inspection of the measurement time course and any measurement with a leak was discarded.
To perform a test, the device and mask were placed on the infant’s face covering the nose and slightly open mouth, while the infant was in the supine, “sniffing” position. Control infants were tested at the bedside in the mother’s room, in the well infant nursery, or in the neonatal intensive care unit. Infants in the TTN cohort were tested in the intensive care unit unless moved to the nursery. Initial testing occurred immediately after enrollment, and ensuing daily tests were then scheduled in accordance with parental preferences. Timing of testing was not standardized to occur only while asleep or after a feed but instead reflected availability for evaluation, as would occur in common clinical scenarios. Infants undergoing testing in the intensive care unit had their vital signs continuously monitored for any adverse events, such as apnea, desaturations, or bradycardia. Infants tested in the nursery were monitored before and after testing.
Each FOT measurement was performed for 8 s using prime frequencies from 7 to 41 Hz for each test. Prime frequencies were used to prevent interference in measurements caused by harmonic amplifications of non-prime oscillatory signals. The frequency range from 7 to 41 Hz was based on data from previous studies, which demonstrated the greatest utility in measuring airway R and X with decreased amount of noise from respiratory testing.6,8 On each testing day, up to five measurements were made per infant, with the mean results of at least two adequate measurements being used for analysis. Total time for set-up of equipment, preparing the patient, and measurement of the 5 tests was <5 min for each testing event. A measurement was considered adequate if there was an appropriate seal around the infant’s nose and mouth throughout the entire test, no vocalizations made during testing, no swallowing or obvious glottis closure during testing, and the coherence function value at each tested frequency was at least 0.80. The coherence function, which evaluates the amount of noise and abnormalities present in the measured signal, was calculated for all tested frequencies.15 The tested Rs must also have a coefficient of variation (CV) of <25% among them at each frequency. These coherence and CV thresholds for validity were based on our preliminary results and success rates in testing. Other studies have used coherence of at least 0.90 and CV of at least 15%.16 Data collected at time of testing included respiratory R and X at each prime frequency 7–41 Hz for each measurement. AX across all frequencies was also obtained with testing. If patients had been discharged early, were unavailable for testing, or uncooperative with testing, that day’s tests were not included for analysis.
Statistical analysis
We analyzed the changes in R, X, and AX for the 3 days’ data for control infants. Each day’s data were compared between the control and TTN groups (e.g., day 1 TTN compared to day 1 control). Averages and 95% confidence intervals (CIs) were calculated for each day based on the individual patients’ mean data. Background and demographic data were also compared between the two groups. Categorical variables were compared by Fisher’s exact test or χ2 test. Continuous variables were compared between TTN and control groups by two-sample t tests with two tails or Wilcoxon rank-sum test if applicable. A generalized mixed model was used to compare the R and X at each frequency among the controls and between the two groups from days 1 through 3. P values of ≤0.05 were considered to indicate statistical significance. Statistical analysis was performed using SAS, version 9.4 (SAS Institute). Where data were missing or not obtained, the appropriate denominator was indicated in the tables.
To determine the required sample size for evaluating differences in infants with TTN vs. controls, assuming initial measurements of lung X at 7 Hz would have an absolute difference of 50% (more negative) in infants with TTN compared to term controls, the minimum sample size required was 34 infants (17 per arm) assuming power of 80%, type I error rate of 5%, and standard deviation of 0.5 of the mean (based on initial preliminary data in 6 infants during initial testing of the device). To improve the power of the study, the entire sample of control infants was compared to the TTN group. However, for development of normative data in normal control infants, a larger sample size was required to reduce the width of CIs. With low sample size, CIs are wide; with increasing sample size, the width of the confidence intervals get narrower; and after 50–75 subjects, additional reductions are minimal even with doubling or tripling the sample size.17 As normative data and variability in measurements by FOT in normal term infants have not been determined in prior literature, and the feasibility and rate of drop-outs could not be predicted in advance, a sample size of approximately 125 control infants (in excess of the 50–75 needed, assuming a 50% failure rate) was empirically chosen and considered sufficient to generate reference data in this study.
Results
During the study period from January to November 2018, we enrolled 175 infants, with 158 in the control cohort and 17 in the TTN cohort. Of the 158 infants enrolled in the control group, 138 (87%) successfully completed at least one set of adequate tests; all 17 (100%) in the TTN cohort had at least 1 day of adequate tests. In all, 96% of infants in the control cohort and 29% of infants in the TTN cohort were enrolled from the well infant nursery. Enrollment and attainment of FOT results over the 3 days for control and TTN infants are shown in Fig. 1. Among the 138 healthy control infants who had FOT testing performed, 86% (118/137) had adequate tests on day 1, 75% (90/120) had adequate tests on day 2, and 61% (33/54) had adequate tests on day 3. Most of these unavailable infants on days 2 and 3 were discharged home as is routine for normal infants, prior to completion of testing. Infants who remained for all 3 days were more likely to be delivered via Cesarean section, born to previously nulliparous mothers, or had an underlying reason to be kept (e.g., maternal illness). The increase in poor-quality tests among controls coincided with an increase in infant agitation with testing—infants on days 2 and 3 were more likely to be awake during testing or vocalized during the tests. We attempted to only obtain measurements when most convenient for the parents and families, but this was not always optimal as some infants were tested awake or before feeds when they were more likely to develop agitation, rather than when they were sleeping or resting after feeds. As mentioned previously, we used a coherence of 0.8 and CV of 25%. In all, 28% of our data points were above the previously used CV of 15%, and 35% of our data were above the previously used coherence of 0.9. We did not see any sudden changes in breathing pattern over the 8 s of each measurement, and the mask was removed immediately after the measurement was complete and reapplied at least 30 s later for the next measurement. No adverse events (e.g., bradycardia, apnea, or desaturations) associated with testing were noted with any of the studied infants.
Reasons for missing tests are infant agitation during testing, infant unavailability (due to discharge, unavailability during testing times, or need for nasal CPAP for TTN infants), or parental refusal. Participants who were unavailable for testing were excluded from the final adequate test result numbers.
Demographic and clinical variables are described in Table 1. As anticipated, infants with TTN were more often delivered by Cesarean section, born at a lower gestational age, received lower 1- and 5-min Apgar scores, had higher RRs at the time of enrollment, and required additional respiratory support. Five out of the 17 infants in the TTN cohort received supplementary respiratory support beyond oxygen administration (i.e., nasal cannula or CPAP) at some point during their hospitalization. Other demographic and background variables did not differ between the two groups.
Mean R and X measurements for the control infants over the first 3 days are shown in Fig. 2. R on day 1 ranged from 10.5 to 31.2 cm H2O·s/L, while X ranged from −46.6 to −6.1 cm H2O·s/L. On each day, there was an overall trend toward decreasing R with increasing frequency. X also steadily increased (became less negative) with increasing frequency. The largest differential from R to X was at the low frequencies, specifically 7 Hz. Analysis of data of control infants who were enrolled in all 3 days indicated that infants available on day 3 differed in characteristics from the initial study population. These infants were more likely to have been born via Cesarean section (48% vs. 22%, P < 0.001) or had other clinical reasons to remain hospitalized (e.g., neonatal abstinence syndrome or hyperbilirubinemia) (12% vs. 6%, P < 0.001).
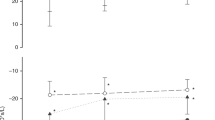
R on day 1 in TTN infants ranged from 12.9 to 35.6 cm H2O·s/L, and X ranged from −49.4 to −10.2 cm H2O·s/L. Like the controls, the TTN infants had higher R at lower frequencies that decreased with higher frequency measurements, and X was more negative at low frequencies and approached 0 at the higher tested frequencies. Comparison of R and X between control and TTN infants for each day is shown in Fig. 3. R was higher in TTN patients on day 1 with a statistically significant difference at 13 Hz with TTN at 32.5 cm H2O·s/L (95% CI 25.5–39.4) vs. controls at 23.8 cm H2O·s/L (95% CI 22.2–25.3), P = 0.007. X was also lower (more negative) in infants with TTN on day 1 compared to controls with significant differences from 17 through 37 Hz, (all P < 0.02). Receiver-operating characteristic (ROC) curves were generated at each reportedly significant frequency for both R and X on day 1, and the area under the curve (AUC) of the ROC curves were estimated (Table 2). The measured differences in R and X between TTN and controls were no longer present on days 2 and 3 with no statistically significant differences in R and X at any frequency. This coincided with clinical improvement also seen in the TTN infants on those days. The median RRs for the TTN cohort decreased from 70 bpm (interquartile range (IQR) 66–76) on day 1 to 53 (IQR 48–60) on day 2 and 52 (IQR 43–67) on day 3. Median RRs for controls did not significantly differ among the 3 days and were 44 (IQR 40–49) on day 1, 44 (IQR 40–50) on day 2, and 42 (IQR 40–46) on day 3. RRs significantly differed between the TTN and control cohorts on day 1 (P < 0.001) and on day 2 (P < 0.001). RRs on day 3 were not significantly different between the two groups.
a day 1, b day 2, and c day 3 for control (day 1 n = 118; day 2 n = 75, day 3 n = 33) and TTN (day 1 n = 13; day 2 n = 13, day 3 n = 6) infants. Error bars represent 95% CI. Asterisk (*) represents measurements in which the difference between TTN and controls are P < 0.05 as determined by the mixed model.
The AX was calculated across all tested frequencies for each group on each day. AX ranged from 624 to 765 cm H2O/L for term infants from day 1 to day 3. There was no statistically significant change in AX among control infants over days 1–3. Mean AX was not significantly higher on day 1 in the TTN group, 834 cm H2O/L, compared to controls, 624 cm H2O/L, P = 0.08. There were once again no statistically significant differences in AX between TTN and control infants on days 2 and 3. Figure 4 shows comparisons of AX between the two groups.
R and X were modeled by generalized linear mixed models for each frequency (7, 13, 17, 19, 23, 29, 31, 37, 41) taking birth weight, length, and sex of term infants into account. As birth weight and length were highly correlated (collinear), we generated separate models using birth weight and length (Supplementary Tables). However, birth weight and length were not strongly correlated with lung mechanics within the relatively narrow range of birth weight and length evaluated in this study. We also modeled AX by birth length and sex (Supplementary Tables).
Discussion
We performed FOT using a modified commercially available device in a cohort of spontaneously breathing, non-sedated, healthy term neonates on days 1, 2, and 3 and determined normative data for healthy neonates on the first postnatal day. We were able to follow some of these infants over time and evaluate respiratory mechanics longitudinally. This FOT data was also compared to infants with TTN and demonstrated feasibility in differentiating health from disease. As disease improved, the changes in respiratory mechanics found with oscillometry also improved and matched the normative data. In addition, although RRs still differed between the two groups on day 2, R and X no longer significantly differed, suggesting that RRs alone cannot explain the differences detected in R and X on day 1. Previous FOT studies have used a very limited range of non-prime frequencies, which may not offer the same information on underlying respiratory mechanics in the neonatal population, or used custom-built FOT devices.15,18 In addition, other neonatal FOT studies have used respiratory pauses by evoking the Hering–Breuer inflation reflex during oscillometry19,20 rather than evaluate FOT during quiet breathing, have evaluated only sedated infants,21 or evaluated premature neonates.22
FOT in healthy term infants demonstrated small changes in R and X from day 1 through 3 at some frequencies. These results may indicate that, even in healthy neonates without clinical respiratory disease, there may be small changes in respiratory mechanics as they continue to transition to extra-uterine life or these differences may be due to flow-dependent changes in breathing patterns among infants from days 1 to 3. R was higher across all 3 days in normal infants at lower frequencies. X was also lower at the lower frequencies. This frequency dependence is commonly observed in forced oscillometric measurements and may be amplified by airway obstruction.23 The R and X curves generated are similar to that found in the study by Nowowiejska et al. in children aged 3–18 years.24 However, the absolute R in our control infants is about four times higher than that of the older children; similarly, the X is lower (more negative) by about four times that of the older children. This difference in R is similar to the findings by Gochicoa et al.25 who measured airway resistance by the interrupter technique and compared results in neonates and children. Interestingly, mean R was lower than reported by Gray et al.26 who tested unsedated infants at 6–10 weeks of age. Mean R values were similar in magnitude to those reported by Hantos et al.18 who had previously tested a small subset of neonates in the first 3 days of life.
As anticipated, infants with TTN were more likely to have been born via Cesarean section, be of a younger gestational age, require more respiratory support, have lower Apgar scores, and have higher RRs at enrollment, compared to the control group. These are all known risk factors for infants who develop TTN.9 Otherwise, demographics between the groups did not differ. When compared to healthy term infants on day 1, TTN infants had decreased X with statistically significant differences from 11 to 37 Hz suggesting reduced compliance. The study by Sandberg et al. showed similar changes of reduced compliance in infants with TTN.10 Lung edema is preferentially around airways and blood vessels in the lung interstitium,27 and such accumulation of interstitial fluid may not only reduce compliance but also cause airway compression, increasing airway resistance. Previously, it had been shown that X may be more specific than R in assessing bronchial obstruction in children,24 so airway edema may also possibly contribute to the finding of decreased X in TTN infants. Infants with TTN also had increased R, specifically at 13 Hz, suggesting increased airway and viscous resistance, which may be due to airway compression. The changes in R and X found on day 1 in TTN infants as compared to controls were not seen on days 2 and 3, indicating that, as the clinical disease of TTN improves, FOT quantification of respiratory mechanics also normalizes. Hence, FOT as performed in this study offers a non-invasive method to obtain information about respiratory mechanics, which can be performed longitudinally, and differentiate disease from healthy states.
There were a few limitations to our study. One of the main limitations was the reduction in number of measurements from day 1 to day 3. Early discharge from the hospital limited the number of patients who could be tested sequentially. This potentially provides a source of bias in infants who remained in the hospital on day 3 for testing. In addition, infants who remained hospitalized by day 3 differed in method of delivery and clinical reasons for prolonged admission from the total population initially enrolled. However, despite the change in the number of tests obtained, the results from day 1 to day 3 in controls differed only minimally. Furthermore, even though the test is not invasive and only requires an 8 s interval to perform, the test requires a baseline level of cooperation from the infant. One cannot test the infant if they are crying or vocalizing. Our study had a number of infants who could not be tested while sleeping or in the quiet fed state but were crying or otherwise agitated during testing on days 2 and 3. Testing should be performed by someone who is experienced in testing with neonatal patients to overcome these limitations. It must be noted that these figures are comparable or better than that of spirometry in preschool children of whom only 80% were able to produce at least one acceptable spirometry effort, with 54% able to repeat similar efforts,28 and acceptable and repeatable spirometry could be obtained in only 42% of children aged 4–5 years.29 As mentioned previously, a coherence of at least 0.90 and CV of at least 15% in three replicate measures provided the most accurate estimates in prior studies.16 However, others have shown that a coherence of at least 0.6 only at 5 Hz would provide adequate measurements.30 Our study elected to use a threshold for coherence of 0.8 and CV of 25% for validity based on our preliminary measurements and success rates, and even with these increased measures of validity, only about a third of the total measurements were outside of the threshold. Based on the results obtained from this study, and practical issues in testing, we provided feedback to THORASYS Thoracic Medical Systems Inc., who have since developed a new model, the TremoFlo N-100 “Neo” Airwave Oscillometry device, which has a flexible connecting element and a button to initiate measurement close to the mask, which should provide similar data but be easier to use in newborn infants.
In adults, oscillometry testing in supine vs. sitting has been shown to provide different resistance-flow gradients based on body position.31 All infants tested were in the supine position and did not have a respiratory support device. It is possible that some infants had upper airway narrowing during supine testing, but this is difficult to determine or quantify, and it is possible that pulmonary mechanics measurements would be different if infants were positioned prone or on their side. Infants with TTN who qualified for testing did not require respiratory support above an oxygen hood or an incubator environment servo-controlled with oxygen. Testing of infants who were intubated, on CPAP, or nasal cannula would have required a different interface and technique, which was outside the scope of our study. Previous studies have suggested using a “head generator technique” to reduce upper airway resistance and artifact.32 However, nasal passages and upper airways are dominant areas of resistance in infants, such that it may be important to include the upper airways during respiratory testing in neonates.33 It is also possible that changes in upper airway resistances may have occurred or that total measurement of the infant’s tidal volume may have been lost due to the use of the infant face mask and filter applied to the device. This could potentially provide different R and X measurements when compared to alternative methods of FOT testing, such as performed by Hantos et al.18 However, because all infants in our study were tested in the same manner (i.e., using the TremoFloTM device with mask and filter), results within our study and those replicated using the same technique should provide consistent results and similar measurements of R and X. It is certain that the use of the mask plus filter and the FOT device adds to the dead space, which may be a considerable percentage of the infant’s tidal volume. We did not see any sudden changes in breathing pattern over the 8 s of each measurement. We did not separately account for dead space as a percentage of the infants’ tidal volume, which would vary from infant to infant. Although higher RR in neonates may reduce the signal-to-noise ratio, this will be applicable to future studies using FOT in a comparable age group. Finally, we did not specifically evaluate lung mechanics during inspiration vs. expiration, although some differences are likely. We noted that birth weight and length were not strongly correlated with lung mechanics within the relatively narrow range of birth weight and length noted in this study. It is likely that evaluation of older and larger infants or smaller preterm infants will require considerations of anthropometry.
In conclusion, this study provides normative non-invasive FOT data in non-sedated healthy term neonates on the first postnatal day. FOT can be performed longitudinally and reproducibly in this patient population. Our study demonstrates that testing is not only feasible in infants using non-invasive methods using a slightly modified commercially available device but also that differences in respiratory mechanics can be detected when investigating clinical disease. In addition, improvements in respiratory mechanics coincide with resolution of the disease. The data support our hypothesis that TTN infants have lower X and higher R compared to healthy term infants on day 1. FOT presents a promising opportunity to longitudinally assess lung disease in neonates. Further studies in additional neonatal lung diseases such as RDS, bronchopulmonary dysplasia, and meconium aspiration syndrome may prove valuable to monitor disease trajectory and response to therapy.
References
Beydon, N. et al. An official American Thoracic Society/European Respiratory Society statement: pulmonary function testing in preschool children. Am. J. Respir. Crit. Care Med. 175, 1304–1345 (2007).
Hjalmarson, O. & Sandberg, K. Abnormal lung function in healthy preterm infants. Am. J. Respir. Crit. Care Med. 165, 83–87 (2002).
Vogt, B., Falkenberg, C., Weiler, N. & Frerichs, I. Pulmonary function testing in children and infants. Physiol. Meas. 35, R59–R90 (2014).
Dubois, A. B., Brody, A. W., Lewis, D. H. & Burgess, B. F. Jr Oscillation mechanics of lungs and chest in man. J. Appl. Physiol. 8, 587–594 (1956).
Skylogianni, E., Douros, K., Anthracopoulos, M. B. & Fouzas, S. The forced oscillation technique in paediatric respiratory practice. Paediatr. Respir. Rev. 18, 46–51 (2016).
Frey, U. Forced oscillation technique in infants and young children. Paediatr. Respir. Rev. 6, 246–254 (2005).
Oostveen, E. et al. The forced oscillation technique in clinical practice: methodology, recommendations and future developments. Eur. Respir. J. 22, 1026–1041 (2003).
Goldman, M. D. Clinical application of forced oscillation. Pulm. Pharm. Ther. 14, 341–350 (2001).
Guglani, L., Lakshminrusimha, S. & Ryan, R. M. Transient tachypnea of the newborn. Pediatr. Rev. 29, e59–e65 (2008).
Sandberg, K., Sjoqvist, B. A., Hjalmarson, O. & Olsson, T. Lung function in newborn infants with tachypnea of unknown cause. Pediatr. Res. 22, 581–586 (1987).
Fleming, S. et al. Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: a systematic review of observational studies. Lancet 377, 1011–1018 (2011).
Heimann, K. et al. Basic values for heart and respiratory rates during different sleep stages in healthy infants. Biomed. Tech. (Berl.) 58, 27–34 (2013).
Kelleher, J. et al. Oronasopharyngeal suction versus wiping of the mouth and nose at birth: a randomised equivalency trial. Lancet 382, 326–330 (2013).
Lundblad, L. K. A., Miletic, R., Piitulainen, E. & Wollmer, P. Oscillometry in chronic obstructive lung disease: in vitro and in vivo evaluation of the impulse oscillometry and TremoFlo devices. Sci. Rep. 9, 11618 (2019).
Desager, K. N. et al. Measurement of total respiratory impedance in infants by the forced oscillation technique. J. Appl. Physiol. (1985) 71, 770–776 (1991).
Landser, F. J., Nagles, J., Demedts, M., Billiet, L. & van de Woestijne, K. P. A new method to determine frequency characteristics of the respiratory system. J. Appl. Physiol. 41, 101–106 (1976).
Bridges, A. J. & Holler, K. A. How many is enough? Determining optimal sample sizes for normative studies in pediatric neuropsychology. Child Neuropsychol. 13, 528–538 (2007).
Hantos, Z. et al. Assessment of respiratory mechanics with forced oscillations in healthy newborns. Pediatr. Pulmonol. 50, 344–352 (2015).
Pillow, J. J., Stocks, J., Sly, P. D. & Hantos, Z. Partitioning of airway and parenchymal mechanics in unsedated newborn infants. Pediatr. Res. 58, 1210–1215 (2005).
Sly, P. D., Hayden, M. J., Petak, F. & Hantos, Z. Measurement of low-frequency respiratory impedance in infants. Am. J. Respir. Crit. Care Med. 154, 161–166 (1996).
Marchal, F., Haouzi, P., Gallina, C. & Crance, J. P. Measurement of ventilatory system resistance in infants and young children. Respir. Physiol. 73, 201–209 (1988).
Zannin, E., Neumann, R. P., Dellaca, R. & Schulzke, S. M. Forced oscillation measurements in the first week of life and pulmonary outcome in very preterm infants on noninvasive respiratory support. Pediatr. Res. 86, 382–388 (2019).
Tomalak, W., Radlinski, J., Pawlik, J., Latawier, W. & Pogorzelski, A. Impulse oscillometry vs. body plethysmography in assessing respiratory resistance in children. Pediatr. Pulmonol. 41, 50–54 (2006).
Nowowiejska, B. et al. Transient reference values for impulse oscillometry for children aged 3-18 years. Pediatr. Pulmonol. 43, 1193–1197 (2008).
Gochicoa, L. G. et al. Reference values for airway resistance in newborns, infants and preschoolers from a Latin American population. Respirology 17, 667–673 (2012).
Gray, D. et al. Respiratory impedance in healthy unsedated South African infants: effects of maternal smoking. Respirology 20, 467–473 (2015).
Staub, N. C., Nagano, H. & Pearce, M. L. Pulmonary edema in dogs, especially the sequence of fluid accumulation in lungs. J. Appl. Physiol. 22, 227–240 (1967).
Gaffin, J. M., Shotola, N. L., Martin, T. R. & Phipatanakul, W. Clinically useful spirometry in preschool-aged children: evaluation of the 2007 American Thoracic Society Guidelines. J. Asthma 47, 762–767 (2010).
Loeb, J. S. et al. Acceptability and repeatability of spirometry in children using updated ATS/ERS criteria. Pediatr. Pulmonol. 43, 1020–1024 (2008).
Soares, M., Richardson, M., Thorpe, J., Owers-Bradley, J. & Siddiqui, S. Comparison of forced and impulse oscillometry measurements: a clinical population and printed airway model study. Sci. Rep. 9, 2130 (2019).
Drews, D., Vogel, J., Wilke, A. & Smith, H. J. [Impulse oscillometry and body position]. Pneumologie 51(Suppl 2), 478–482 (1997).
Peslin, R., Duvivier, C., Didelon, J. & Gallina, C. Respiratory impedance measured with head generator to minimize upper airway shunt. J. Appl. Physiol. (1985) 59, 1790–1795 (1985).
Solow, B. & Peitersen, B. Nasal airway resistance in the newborn. Rhinology 29, 27–33 (1991).
Acknowledgements
We would like to acknowledge the assistance of THORASYS Thoracic Medical Systems Inc., Montreal, QC, Canada for assistance with customization of the TremoFlo C-100 Airwave System™ using specific adaptors and prime frequencies for use in newborn infants. The TremoFlo C-100 system was purchased using a one-time non-transferrable investigational discount of 20%. No additional discount/free use was provided, and THORASYS did not have any role in the drafting of this manuscript. The authors did not receive any payment or financial compensation from THORASYS. This study was supported in part by grants from the National Institutes of Health (NIH), Bethesda, MD (U01 HL133536).
Author information
Authors and Affiliations
Contributions
Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data: A.P.K., C.P.T., H.-C.K., N.A. Drafting the article or revising it critically for important intellectual content and final approval of the version to be published: all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Prior presentation: Southern Society of Pediatric Research 2019 and Pediatric Academic Society 2019
Supplementary information
Rights and permissions
About this article
Cite this article
Klinger, A.P., Travers, C.P., Martin, A. et al. Non-invasive forced oscillometry to quantify respiratory mechanics in term neonates. Pediatr Res 88, 293–299 (2020). https://doi.org/10.1038/s41390-020-0751-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-0751-7
This article is cited by
-
Prematurity-associated wheeze: current knowledge and opportunities for further investigation
Pediatric Research (2023)
-
Oscillatory mechanics trajectory in very preterm infants: a cohort study
Pediatric Research (2023)