Abstract
Background
In preterm infants, InSurE (Intubation–Surfactant–Extubation) and LISA (less invasive surfactant administration) techniques allow for exogenous surfactant administration while reducing lung injury associated with mechanical ventilation. We compared the acute pulmonary response and lung deposition of surfactant by LISA and InSurE in surfactant-depleted adult rabbits.
Methods
Twenty-six spontaneously breathing surfactant-depleted adult rabbits (6–7 weeks old) with moderate RDS and managed with nasal continuous positive airway pressure were randomized to 3 groups: (1) 200 mg/kg of surfactant by InSurE; (2) 200 mg/kg of surfactant by LISA; (3) no surfactant treatment (Control). Gas exchange and lung mechanics were monitored for 180 min. After that, surfactant lung deposition and distribution were evaluated monitoring disaturated-phosphatidylcholine (DSPC) and surfactant protein C (SP-C), respectively.
Results
No signs of recovery were found in the untreated animals. After InSurE, oxygenation improved more rapidly compared to LISA. However, at 180’ LISA and InSurE showed comparable outcomes in terms of gas exchange, ventilation parameters, and lung mechanics. Neither DSPC in the alveolar pool nor SP-C signal distributions in a frontal lung section were significantly different between InSurE and LISA groups.
Conclusions
In an acute setting, LISA demonstrated efficacy and surfactant lung delivery similar to that of InSurE in surfactant-depleted adult rabbits.
Impact
-
Although LISA technique is gaining popularity, there are still several questions to address. This is the first study comparing LISA and InSurE in terms of gas exchange, ventilation parameters, and lung mechanics as well as surfactant deposition and distribution.
-
In our animal study, three hours post-treatment, LISA method seems to be as effective as InSurE and showed similar surfactant lung delivery.
-
Our findings provide some clarifications on a fair comparison between LISA and InSurE techniques, particularly in terms of surfactant delivery. They should reassure some of the concerns raised by the clinical community on LISA adoption in neonatal units.
Similar content being viewed by others
Introduction
Preterm infants are at high risk for respiratory distress syndrome (RDS), mainly due to the immaturity of the lungs, including ineffective surfactant production and metabolism.1,2 RDS has been traditionally treated with a combination of early endotracheal intubation, followed by the administration of exogenous surfactant and mechanical ventilation (MV).3 Although this approach has been demonstrated to be lifesaving, tracheal intubation is an invasive procedure with attendant risks. Of note, prolonged MV increases the risk of ventilator-associated lung injury, pneumonia, and bronchopulmonary dysplasia (BPD).4 To minimize the risk of these complications, the use of non-invasive respiratory support (NRS), particularly nasal continuous positive airway pressure (nCPAP), has become a widespread strategy for early respiratory management of preterm infants. However, a high proportion of very low-gestational-age infants supported from birth with nCPAP still require MV within the first 72 h of life.5 Therefore, the primary use of NRS and the administration of exogenous surfactant without MV are major goals for clinicians that remain challenging. The Intubation–Surfactant administration–Extubation technique, known as InSurE, is traditionally widespread in neonatal units, combining the advantages of short intubation and MV with those of nCPAP and surfactant. More recently, new approaches for bolus surfactant instillation without concomitant MV have been described6,7,8,9 and have rapidly gained popularity. These strategies are known as minimally invasive surfactant therapy or less invasive surfactant administration (LISA). The LISA technique is performed using the laryngoscope with or without Magill forceps to introduce a thin catheter through the vocal cords to deliver a bolus of surfactant while the infant is managed with NRS, breathing spontaneously. Several clinical studies comparing the InSurE and LISA have found a decreased need of early MV need and a decrease in the composite outcome of death and BPD with LISA.10 Recently, the results of 2 years follow-up from the AMV (avoid mechanical ventilation) study showed no statistically significant differences in weight, length, or neurodevelopmental outcome between the LISA intervention group and the control group that received CPAP, rescue intubation, and surfactant treatment if needed.11 Also, remarkable results have been shown in the NINSAPP trial.12 Given these promising results, LISA is rapidly being adopted in neonatal intensive care units.13,14 However, the question still remains regarding several aspects of this technique, highlighting the need for further investigations of the LISA approach.15,16
The main potential delivery difference between InSurE and LISA is attributed to the effects of manual or MV and spontaneous breathing, respectively, on the surfactant bolus, which promotes its dispersion through the respiratory system. Nevertheless, clinical data comparing surfactant distribution in the lungs after InSurE or LISA administration are lacking. Niemarkt et al. compared surfactant lung deposition in preterm lambs after delivering a bolus of 200 mg/kg of surfactant (Poractant alfa) either using the LISA technique or the classic method with surfactant and MV.17 Unfortunately, no InSurE group was included in this study. Surprisingly, after 180 min of follow-up, the intrapulmonary exogenous surfactant amount was almost sixfold lower in the group of animals treated with LISA compared to the standard method.17 Also, Herting and colleagues showed that >30% of the preterm infants receiving LISA with a gestational age below 27 weeks required MV in the first 72 h following treatment.15 Even if there is still an ongoing debate on the reasons for this failure rate, this could be due to the reduced respiratory effort of this specific population of infants or to an insufficient lung deposition of the exogenous surfactant.
We have recently established a robust spontaneously breathing adult rabbit model of RDS to evaluate the acute effects of surfactant therapy in the context of NRS on pulmonary function through gas exchange and pulmonary mechanics. In this model, surfactant depletion by repeated broncho-alveolar lavages (BALs) reduces the recovered intrapulmonary desaturated-phosphatidylcholine (DSPC) pool from ~20 mg/kg to values <3 mg/kg,18 producing a significant reduction of lung compliance and a permanent gas exchange impairment if the animals are managed only with NRS.19 However, this induced respiratory failure can be reverted by the intratracheal instillation of 200 mg/kg of Poractant alfa using InSurE.19,20 After surfactant treatment by InSurE, the intrapulmonary DSPC pool was also restored to baseline.18
In the present study, we have used this established model to compare the efficacy of LISA and InSurE delivery in terms of acute pulmonary response to surfactant treatment as well as to compare the pulmonary delivery and distribution of surfactant by the two methods.
Materials and methods
Animal preparation
The experiments were carried out in 6–7-week-old male rabbits (Charles River Laboratories, Calco, Italy). All experimental procedures were approved by the intramural Animal Welfare Body, and the Italian Ministry of Health (Prot.no. 1300-2015-PR) and comply with the European regulations for animal care.
Rabbits (bodyweight of 1.5–2.5 kg) were initially sedated with intramuscular (i.m.) medetomidine (Domitor®, 2 mg/kg). The throat of the animals was first shaved, and local anesthesia was applied in the anterior neck with lidocaine gel (Luan® 2.5%). Thirty minutes later, the animals received 50 mg/kg of ketamine (Imalgene 1000®, Merial-Boehringer Ingelheim, France) and 5 mg/kg of xylazine (Rompun®, Bayer, Germany) i.m. Rabbits, in the supine position, were intubated and stabilized on positive pressure ventilation (Fabian HFO, Acutronic, Zug, Switzerland) as previously described.18,19,21,22 Fraction of inspired oxygen (FiO2) = 100%, flow = 10 L/min, respiratory rate (RR) = 40 breaths/min, positive end-expiratory pressure (PEEP) = 3 cmH2O, tidal volume (VT) targeted to 7 mL/kg (with the peak inspiratory pressure (PIP) not exceeding 15 cmH2O) and inspiratory time of 0.5 s. Airway flow, mean airway pressure, and VT were monitored as long as the animals were intubated. Body temperature was constantly measured with a rectal probe and maintained at 37 °C by placing a heating pad underneath the animal.
After endotracheal intubation, a catheter was inserted into the right jugular vein for continuous infusion of 1 mg/mL of ketamine and 0.1 mg/mL of xylazine in 0.9% saline solution (100 μL/min) to maintaining anesthesia at a steady level for the whole experimental period. Trometamol (tris-hydroxymethyl aminomethane, 1 M, Sigma-Aldrich) was also infused during the surfactant depletion procedure for maintaining CO2 level under control. A second catheter was inserted into the right carotid artery for blood sampling. After instrumentation, baseline blood gases were measured with a blood sample analyzer (Radiometer Medical, Denmark). Animals with an initial arterial oxygen partial pressure (PaO2) value >400 mmHg at PIP <15 cmH2O were included in the study. Spontaneous breathing was monitored using a multi-parameter monitor (IntelliVue, Philips, Amsterdam) and evaluating chest movements and continuous vital parameters (SpO2 and heart rate).
To induce respiratory distress, repeated BALs were performed by flushing the airways with 20 mL/kg of pre-warmed 0.9% NaCl solution, followed by a short recovery period in between, until a PaO2 value <150 mmHg was reached. Then, if after 15 min of stabilization on MV the respiratory failure was confirmed (PaO2 < 150 mmHg, with PIP not exceeding 23 cmH2O), the animal was included in the study.
Experimental groups
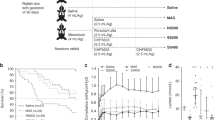
Twenty-six animals were allocated to one of the three experimental groups (figure of the experimental design available as supplemental material). Animals in the Control group (n = 6) were maintained on nCPAP (Fabian HFO, Acutronic; 10 cmH2O) for 180 min, using customized nasal prongs as an interface. We selected a CPAP level of 10 cmH2O because it generates a pharyngeal pressure of 3.9 ± 0.5 cmH2O in our rabbit model (data not shown), which is in line with the pressures used in clinical practice. Animals allocated to the InSurE group (n = 10) received an intratracheal bolus of Poractant alfa (200 mg/kg, Curosurf® Chiesi Farmaceutici) in the supine position followed by 10 min of MV (VT = 7 mL/Kg, RR = 40/min, and PEEP = 3 cmH2O).23,24 After that, animals in the InSurE group were managed with nCPAP (10 cmH2O) for 180 min. Animals in the LISA group (n = 10) received Poractant alfa (200 mg/kg) using the LISA technique. With the animal in the supine position and maintained on nCPAP (10 cmH2O), a nasogastric enteral feeding tube (Penta enteral SEGAP®, diameter = 2 mm and length = 50 cm) was introduced into the trachea with direct visualization of the vocal cords with the videoscope (Ambu® aView™). Briefly, the videoscope tip was introduced into the oral cavity until the oropharynx could be visualized in the monitor to obtain an unobstructed view of the epiglottis and vocal cords ventrally. Once the opening of the vocal cords was visualized, the catheter was advanced through the vocal cords and surfactant was instilled. After surfactant administration, the feeding tube was removed, and animals were maintained on nCPAP (10 cmH2O) for 180 min (Fig. 1). All animals were maintained on sedation (continuous infusion of 1 mg/mL of ketamine and 0.1 mg/mL of xylazine in 0.9% saline solution) during surfactant treatment and for the whole experimental time.
PaO2 (a) and PaCO2 (b) values in surfactant-depleted adult rabbits treated with nasal continuous positive pressure ventilation (nCPAP, white squares), Intubation–Surfactant–Extubation plus nCPAP (InSurE, black circles), or plus less invasive surfactant administration (LISA gray circles). Values are shown as mean ± SEM. †p InSurE and LISA vs. nCPAP <0.01; *p InSurE vs. LISA <0.05.
Gas exchange and respiratory indices
Arterial carbon dioxide and oxygen partial pressure (PaCO2, PaO2) and pH were measured right after the induction of anesthesia (baseline) and after the induction of respiratory distress (Radiometer Medical, Denmark). Arterial blood gases were also measured right after the animal was put on nCPAP, 15 and 30 min after the start of nCPAP, and then every 30 min until the end of the experiment.
PaO2 and PaCO2 were also used to compute the oxygenation index (OI) and the ventilation efficacy index (VEI). To perform a direct comparison of the ventilation indices at baseline with the post surfactant treatment values, animals were shifted from nCPAP to MV after completion of the 180 min observational period. The brief period of MV (5–10 min) was performed using the same ventilation settings as at baseline (FiO2 100%, flow = 10 L/min; RR = 40 bpm, PEEP = 3 cmH2O, VT set at 7 mL/kg, and inspiratory time of 0.5 s. OI and VEI were therefore determined at those time intervals in which the animals were ventilated with a tracheal tube: at baseline, after inducing the acute RDS, and at the end of the 180 min follow-up period. The OI was calculated as follows:
The VEI was calculated to evaluate the overall ventilation efficiency of mechanically ventilated animals independently from the ventilation settings:
Pulmonary mechanics
Dynamic compliance (Cdyn) was also determined at those time intervals in which animals were managed with MV: Cdyn was calculated dividing lung volume (∆V) by the changes in pressure (∆P) standardized by the animal’s weight.
After autoptic analysis performed to evaluate pneumothorax events, a post mortem pressure–volume (P/V) curve was also performed. The lungs were progressively inflated, introducing 5, 10, 15, 20, 25, and 30 mL of air volume through a syringe. The pressure recorded at each insufflation volume was annotated and the lungs were then deflated. The pressure recorded after the insufflation of 30 mL of air was used as an endpoint to compare the pulmonary mechanics between the groups.
Intrapulmonary DSPC quantification
As a proxy for lung deposition of exogenous surfactant, the intrapulmonary levels of DSPC after 180 min of nCPAP were determined, as previously described.25 BALs from three animals per control group and six animals per surfactant-treated groups were collected as described above, centrifuged, and stored at −80 °C until analysis. Lipids were extracted according to the method described by Bligh and Dyer.26 DSPC was separated by thin-layer chromatography after treatment with osmium tetraoxide.27 Fatty acids of DSPC were derivatized as methyl ester by adding 2 mL 3 M HCl methanol and extracted with hexane. Quantitative analysis of DSPC was performed by a FID gas chromatograph (Agilent 5890, Milan, Italy). Data are presented as mg/kg of DSPC.
Porcine SP-C lung distribution
Matrix-assisted laser desorption ionization (MALDI) mass spectrometry imaging (MSI) technique28,29,30 was used to investigate the lung distribution of porcine SP-C deriving from Poractant alfa administrations (InSurE group, n = 3; LISA group, n = 3). Fresh-frozen frontal sections of the animal’s left lung lobes were taken with a cryostat (CM1860 UV, Leica Microsystem, Wetzlar, Germany) at 20-µm thickness and posed on ITO conductive glasses (Bruker Daltonics, Bremen, Germany). A 50% ethanol solution with 0.2% of trifluoroacetic acid, of 2,5-dihydroxybenzoic acid in a concentration of 30 g/L, was sprayed all over the tissue surface with a pneumatic device (IMatrix Spray, Tardo Gmbh, Switzerland) with an in-house edited method. Instrumental analysis was carried out on an Ultraflex III MALDI-Tof/Tof mass spectrometer (Bruker Daltonics) in positive ion reflector mode, with full scan acquisition in the mass range 1500–5000 Da, rasterizing the tissue surface every 350 µm in both x and y directions. Two-dimensional (2D) ion intensity maps were created by taking into consideration the intensity of [porcine SP-C + H]+ ion at m/z 4188.5 extracted with a tolerance of 1 Da. Datafiles were exported in the open-source imzML format31 and individual ion images were extracted by using a custom Python library relying on the pyimzML parser. All data analyses were performed in R (https://www.R-project.org). Data normalization and preprocessing were the same as described by Zecchi et al.32 Briefly, all analyses were performed on the log-transformed MSI intensity to compensate for the expected heteroscedasticity of this type of data. An additional step of batch correction was implemented by setting to zero the average intensity measured on the background pixels. The two experimental groups were compared considering the interquartile range (IQR) of the imaging signal over the frontal sections as a quantitative proxy of the variability of the distribution of Poractant alfa within the lungs. Imaging data were also used to reconstruct drug distribution maps. In this case, the signal obtained for each animal was mean-centered and scaled in order to allow a direct comparison of the individual spatial distributions.
Statistical analysis
Group data are presented as mean ± standard error of the mean (SEM). Raw data were analyzed and compared by repeated-measures two-way analysis of variance as a function of group and time, followed by Dunnett’s and Tukey’s t post hoc tests (GraphPad software, version 6.0). p value <0.05 was considered statistically significant. In the MALDI-MSI experiments, the two experimental groups were compared considering the IQR of the imaging signal over the frontal sections as a quantitative proxy of the variability of the distribution of Poractant alfa within the lungs; the statistical comparison was performed by applying a standard Welch two-sample t test.
Results
No significant differences were found between groups in PaO2, PaCO2, pH, or Cdyn at baseline. The rabbits’ bodyweight and the number of BALs required to induce severe and sustained respiratory distress did not differ among the groups (Table 1).
Gas exchange and ventilator indices
The series of BALs produced a marked and significant impairment of gas exchange resulting in poor arterial oxygenation (mean PaO2 < 70 mmHg in all the groups) and a marked increase of arterial carbon dioxide (mean PaCO2 > 70 mmHg in all the groups). The successful endogenous surfactant depletion was persistent, as denoted by the sustained impairment of gas exchange in the untreated control group (Fig. 1).
Surfactant administration significantly improved PaO2. Regardless of the surfactant administration method, significant differences were registered at 15 min between the surfactant-treated groups and untreated control animals (p < 0.01), and these differences lasted for the whole experimental period (Fig. 1a). However, the increase in oxygenation was faster in the InSurE group compared to the LISA group, as highlighted by a significant difference between groups at 15–120 min (p < 0.05). PaCO2 remained high in all the groups. It remained fairly constant in the control and LISA groups but increased in the InSurE group following surfactant administration. The InSurE group registered the highest mean PaCO2 at all study points and was even significantly higher in comparison to the LISA group at 120, 150, and 180 min (p < 0.05, Fig. 1b).
The mean OI increased from 1.2 ± 0.2 to 15.7 ± 4.3 after depleting the endogenous surfactant (mean ± SD of all animals). OI values returned to baseline values after surfactant treatment, achieving mean OI values of 2.0 ± 0.8 and 2.3 ± 0.2 for the InSurE and LISA groups, respectively (Fig. 2a). OI values partially recovered after 180 min of nCPAP in the control group. Nevertheless, the mean OI value of the control group (8.0 ± 5.5) was significantly higher compared to the surfactant-treated groups (p < 0.01).
Box plots showing a the oxygenation index (OI) and b the ventilation efficacy index (VEI) at baseline (from all animals), after inducing respiratory distress (Post BALs, from all animals) and 180 min after treatment with nasal continuous positive pressure ventilation (nCPAP, Control), with Intubation–Surfactant–Extubation plus nCPAP (InSurE), or nCPAP plus with less invasive surfactant administration (LISA). The boxes encompass the 25–75 percentiles. The horizontal line within the boxes represents the median and the black dot the mean of each group. The whiskers indicate the maximum and minimum values observed for each group. †p vs. Control <0.01.
The increase of the OI was mirrored by a decrease of the VEI from 0.25 ± 0.05, at baseline, to 0.06 ± 0.01 in ±SD of all animals. Surfactant treatment, in particular, if delivered by the LISA method, slightly improved the mean VEI, although the mean values in all the groups were significantly lower compared to those recorded at baseline (Fig. 2b).
Lung mechanics
Surfactant depletion produced almost a 70% drop of Cdyn, from 0.97 ± 0.22 to 0.31 ± 0.10 mL/cmH2O/kg (mean ± SD of all animals). Cdyn improved in all the groups after the observational period. After 180 min, the highest mean Cdyn was registered in the LISA group (0.65 ± 0.29 mL/cmH2O/kg) followed by the InSurE and control groups (0.61 ± 0.17 and 0.48 ± 0.13 mL/cmH2O/kg, respectively, Fig. 3a). No significant differences were detected between the groups. Before P/V curve measurement, we performed an autoptic analysis and we did not report any pneumothorax events.
a Box plots showing the dynamic compliance (Cdyn) at baseline (from all animals), after inducing respiratory distress (Post BALs, from all animals) and 180 min after treatment with nasal continuous positive pressure ventilation (nCPAP, Control), with Intubation–Surfactant–Extubation (InSurE), or with less invasive surfactant administration (LISA). The boxes encompass the 25–75 percentiles. The horizontal line within the boxes represents the median and the black dot the mean of each group. The whiskers indicate the maximum and minimum values observed for each group. b Bar chart showing the mean pressure values recorded after insufflating 30 mL of air post mortem in the lungs of animals managed with nCPAP only, control, treated with Intubation–Surfactant–Extubation plus nCPAP (InSurE), or with nCPAP plus less invasive surfactant administration (LISA). Values are shown as mean ± SEM. †p vs. Control <0.05.
The P/V curve revealed stiffer lungs in the control group compared to surfactant-treated animals (Fig. 3b). The mean pressure registered after insufflating 30 mL of air into the lungs was significantly lower in the LISA and InSurE groups (20 ± 1.4 and 19.0 ± 2.1 cm H2O) compared to the control group (23.5 ± 2.5 cmH2O, p < 0.05).
Intrapulmonary DSPC quantification
A marginal DSPC signal was detected in untreated control animals (1.20 ± 0.51 mg/kg, Fig. 4). The mean intrapulmonary amount of DSPC detected in the surfactant-treated groups was significantly higher (p < 0.01) compared to untreated animals. No significant differences in terms of intrapulmonary DSPC amount were recorded between the InSurE (39.1 ± 3.5 mg/kg) and LISA groups (35.5 ± 6.3 mg/kg).
Bar chart showing the intrapulmonary amount of disaturated-phosphatidylcholine (DSPC) in the lungs of animals managed with nasal continuous positive pressure ventilation (nCPAP, Control), treated with Intubation–Surfactant–Extubation plus nCPAP (InSurE), or with nCPAP plus less invasive surfactant administration (LISA). Values are displayed as mean ± SEM. †p vs. Control <0.01.
SP-C lung distribution
The comparison of the IQR ranges between the study groups is shown in Fig. 5. The plot indicates that the two administration strategies give comparable results in terms of signal variability across the sections (p value = 0.30).
The absence of a strong difference between the two delivery methods is also supported by the visual inspection of the porcine SP-C distribution maps reconstructed from the MALSI-MSI imaging data. An example of the maps is shown in Fig. 6.
The image displays the normalized5 and scaled raw MALDI-MSI signal.
For both treatments, sparse high-intensity areas (yellow) are distributed over the lung parenchyma, which is characterized by large and similarly connected regions of intermediate intensity. As expected, low-intensity regions are visible in correspondence of the bronchial tubes.
Discussion
To our knowledge, this is the first study comparing LISA and InSurE concurrently in terms of oxygenation, lung mechanics, intrapulmonary surfactant availability, and distribution in a surfactant-depleted adult rabbit model.
Our study showed a similar 3-h recovery outcome in terms of oxygenation and lung mechanics in the LISA and InSurE groups. However, we found a relevant difference in the acute physiological response between these two techniques. InSurE was associated with an acute increase of arterial oxygenation, whereas PaO2 improved gradually after LISA. The different acute responses may be related to the use of MV after delivering the surfactant bolus. Animals allocated in the InSurE group received treatment followed by 10 min of MV, whereas the animals in the LISA group were managed with nCPAP during surfactant treatment. These data are in accordance with the results of Niemarkt et al. who reported a rapid increase of PaO2 values in intubated and mechanically ventilated lambs compared with the lambs that underwent LISA and were supported with nCPAP.17 Conversely, this difference in the acute response between LISA and InSurE was not observed in a similar recent study by Rey-Santano et al. conducted in surfactant-deficient newborn piglets, which can be partially explained by the fact that animals in the InSurE group were mechanically ventilated just for 1 min.33
Three hours after surfactant treatment, both surfactant delivery methods showed a significant increase of the intrapulmonary DSPC pool, highlighting the efficacy of the LISA procedure in surfactant deposition into the lungs. In this regard, our findings are not fully in line with Niemarkt’s study,17 who showed a significantly lower surfactant deposition in the lungs of lambs treated with LISA plus nCPAP compared with the animals treated with surfactant and MV. The authors speculated that the low intrapulmonary levels of surfactant might have been caused by surfactant reflux observed in some animals. This difference in lung surfactant deposition observed in the present work compared with the Niemarkt’s study may be explained by the duration of the LISA procedure in our protocol, which was extended to 2 min to reduce the surfactant reflux, compared to 1-min delivery in Niemarkt’s study.17 Moreover, we used a video laryngoscope to minimize the risk of incorrect surfactant delivery.
Notably, we found an amount of DSPC in the lungs of animals treated with LISA not significantly different from the amount detected in the InSurE group. Giambelluca et al. determined that the BAL-recovered mean DSPC pool in the lungs of healthy adult rabbits ranged between 15 and 25 mg/kg, whereas in rabbits with severe respiratory distress the mean DSPC was <2 mg/kg.18 According to these findings, the mean DSPC pool observed in the LISA group after 180 min of nCPAP (36.4 ± 6.39 mg/kg) resembles that of InSurE-treated and healthy rabbits and explains the significant improvement at 3 h in arterial oxygenation to the same level of the InSurE group. The preliminary MALDI-MSI findings at 3 h showing a quite extended distribution of exogenous SP-C in the “D” sections of the lung parenchyma, although obtained in small numbers of animals, provide a further qualitative explanation for the comparable arterial oxygenation recovery between the two groups.
We observed differences in the pharmacodynamics of surfactant therapy, in particular in terms of gas exchange, between LISA and InSurE. Due to the pushing effect of the ventilator, PaO2 rapidly increased in the InSurE group. We also registered a marked increase in the mean PaCO2 immediately after surfactant administration in this group. Similarly, Rey-Santano et al. also reported a hypercarbia phenomenon in preterm lambs immediately after receiving a surfactant bolus.34 The authors attributed the increase of PaCO2 to temporary airway obstruction due to the acute airway fluid load following surfactant instillation. In our study, the increase of PaCO2 was not limited to the immediate period following surfactant instillation, but it lasted until the end of the experiment in the InSurE group. We might hypothesize that the surfactant distribution process in the lungs could potentially be more physiological during the LISA procedure, since spontaneous breathing, supported by nCPAP, is the only driver for surfactant spreading. Moreover, a slower increase in oxygenation might reduce the risk of oxygen radical production, especially when FiO2 titration becomes challenging due to acute changes in arterial oxygenation. In this regard, the risk of multi-organ damages related to hyperoxia is well known in preterm infants.35,36
The present study has some limitations. First, we used surfactant-depleted adult rabbits instead of a preterm animal model with RDS. In this regard, the surfactant-depleted adult rabbit model does not present all the features of prematurity-related RDS. Second, we used customized nasal prongs, which have not been designed for the rabbit’s nostrils and may therefore enhance the risk for increased resistance and lead to gas exchange imbalances, in particular in terms of PaCO2, which remained high in all the groups despite surfactant treatment. Moreover, although we monitored that the spontaneous breathing remained effective during and after the whole procedure, the animals were under sedation. Finally, although we were able to study the overall intrapulmonary DSPC amount recovered from BALs and to have a preliminary evaluation of exogenous SP-C distribution in 2D longitudinal lung sections at the 3-h sacrifice point, we could not follow the regional distribution of exogenous surfactant within the lungs in real time, in the time frame encompassing treatment (t0) to sacrifice (t180min), which should be addressed in future studies.
Conclusions
Surfactant administration by the LISA procedure to spontaneously breathing, surfactant-depleted adult rabbits was effective as the InSurE technique in improving pulmonary function in a 3-h post-treatment time frame. Both methods allowed for a partially homogeneous intrapulmonary surfactant distribution and restored the intrapulmonary DSPC pools. Although oxygenation recovery was faster with InSurE, a comparable improvement in oxygenation was observed with LISA. These findings support the LISA procedure as a valid alternative to the InSurE technique for surfactant administration. Nevertheless, further studies are needed to confirm these results in preterm infants with RDS.
References
Cogo, P. E. et al. Pharmacokinetics and clinical predictors of surfactant redosing in respiratory distress syndrome. Intensive Care Med. 37, 510–517 (2011).
Speer, C. P. Neonatal respiratory distress syndrome: an inflammatory disease? Neonatology 99, 316–319 (2011).
Pfister, R. H. & Soll, R. F. Initial respiratory support of preterm infants: the role of CPAP, the INSURE method, and noninvasive ventilation. Clin. Perinatol. 39, 459–481 (2012).
Jensen, E. A. et al. Effects of multiple ventilation courses and duration of mechanical ventilation on respiratory outcomes in extremely low-birth-weight infants. JAMA Pediatr. 169, 1011–1017 (2015).
Dargaville, P. A. et al. Incidence and outcome of CPAP failure in preterm infants. Pediatrics 138, e20153985 (2016).
Gopel, W. et al. Avoidance of mechanical ventilation by surfactant treatment of spontaneously breathing preterm infants (AMV): an open-label, randomised, controlled trial. Lancet 378, 1627–1634 (2011).
Lista, G., Bresesti, I. & Fabbri, L. Is less invasive surfactant administration necessary or “only” helpful or just a fashion? Am. J. Perinatol. 35, 530–533 (2018).
Dargaville, P. A. et al. Minimally-invasive surfactant therapy in preterm infants on continuous positive airway pressure. Arch. Dis. Child. Fetal Neonatal Ed. 98, F122–F126 (2013).
Kribs, A. et al. Nonintubated surfactant application vs conventional therapy in extremely preterm infants: a randomized clinical trial. JAMA Pediatr. 169, 723–730 (2015).
Aldana-Aguirre, J. C., Pinto, M., Featherstone, R. M. & Kumar, M. Less invasive surfactant administration versus intubation for surfactant delivery in preterm infants with respiratory distress syndrome: a systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 102, F17–F23 (2017).
Herting, E. et al. Two-year outcome data suggest that less invasive surfactant administration (LISA) is safe. Results from the follow-up of the randomized controlled AMV (avoid mechanical ventilation) study. Eur. J. Pediatr. 179, 1309–1313 (2020).
Mehler, K. et al. Developmental outcome of extremely preterm infants is improved after less invasive surfactant application: developmental outcome after LISA. Acta Paediatr. https://doi.org/10.1111/apa.15565 (2020).
Klotz, D., Porcaro, U., Fleck, T. & Fuchs, H. European perspective on less invasive surfactant administration-a survey. Eur. J. Pediatr. 176, 147–154 (2017).
Bresesti, I., Zivanovic, S., Ives, K. N., Lista, G. & Roehr, C. C. National surveys of UK and Italian neonatal units highlighted significant differences in the use of non-invasive respiratory support. Acta Paediatr. 108, 865–869 (2019).
Herting, E., Härtel, C. & Göpel, W. Less invasive surfactant administration (LISA): chances and limitations. Arch. Dis. Child. Fetal Neonatal Ed. 104, F655–F659 (2019).
De Luca, D. et al. Less invasive surfactant administration: a word of caution. Lancet Child Adolesc. Health 4, 331–340 (2020).
Niemarkt, H. J. et al. Effects of less-invasive surfactant administration on oxygenation, pulmonary surfactant distribution, and lung compliance in spontaneously breathing preterm lambs. Pediatr. Res. 76, 166–170 (2014).
Giambelluca, S. et al. Tracing exogenous surfactant in vivo in rabbits by the natural variation of (13)C. Respir. Res. 20, 158 (2019).
Ricci, F. et al. Physiological, biochemical, and biophysical characterization of the lung-lavaged spontaneously-breathing rabbit as a model for respiratory distress syndrome. PLoS ONE 12, e0169190 (2017).
Ricci, F. et al. In vitro and in vivo characterization of poractant alfa supplemented with budesonide for safe and effective intratracheal administration. Pediatr. Res. 82, 1056–1063 (2017).
Agassandian, M. & Mallampalli, R. K. Surfactant phospholipid metabolism. Biochim. Biophys. Acta 1831, 612–625 (2013).
Ricci, F. et al. Surfactant replacement therapy in combination with different non-invasive ventilation techniques in spontaneously-breathing, surfactant-depleted adult rabbits. PLoS ONE 13, e0200542 (2018).
Verder, H. et al. Nasal continuous positive airway pressure and early surfactant therapy for respiratory distress syndrome in newborns of less than 30 weeks’ gestation. Pediatrics 103, E24 (1999).
Dani, C. et al. Early extubation and nasal continuous positive airway pressure after surfactant treatment for respiratory distress syndrome among preterm infants <30 weeks’ gestation. Pediatrics 113, e560–e563 (2004).
Cogo, P. E. et al. Dosing of porcine surfactant: effect on kinetics and gas exchange in respiratory distress syndrome. Pediatrics 124, e950–e957 (2009).
Bligh, E. G. & Dyer, W. J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917 (1959).
Facco, M. et al. In vivo effect of pneumonia on surfactant disaturated-phosphatidylcholine kinetics in newborn infants. PLoS ONE 9, e93612 (2014).
Caprioli, R. M., Farmer, T. B. & Gile, J. Molecular imaging of biological samples: localization of peptides and proteins using MALDI-TOF MS. Anal. Chem. 69, 4751–4760 (1997).
Chughtai, K. & Heeren, R. M. Mass spectrometric imaging for biomedical tissue analysis. Chem. Rev. 110, 3237–3277 (2010).
Balluff, B., Schöne, C., Höfler, H. & Walch, A. MALDI imaging mass spectrometry for direct tissue analysis: technological advancements and recent applications. Histochem. Cell Biol. 136, 227–244 (2011).
Schramm, T. et al. imzML–a common data format for the flexible exchange and processing of mass spectrometry imaging data. J. Proteomics 75, 5106–5110 (2012).
Zecchi, R. et al. Mass spectrometry imaging as a tool for evaluating the pulmonary distribution of exogenous surfactant in premature lambs. Respir. Res. 20, 175 (2019).
Rey-Santano, C. et al. Cerebral oxygenation associated with INSURE versus LISA procedures in surfactant-deficient newborn piglet RDS model. Pediatr. Pulmonol. 54, 644–654 (2019).
Rey-Santano, C. et al. Acute and sustained effects of aerosolized vs. bolus surfactant therapy in premature lambs with respiratory distress syndrome. Pediatr. Res. 73, 639–646(2013).
Vento, M. et al. Preterm resuscitation with low oxygen causes less oxidative stress, inflammation, and chronic lung disease. Pediatrics 124, e439–e449 (2009).
Bhandari, V. Hyperoxia-derived lung damage in preterm infants. Semin. Fetal Neonatal Med. 15, 223–229 (2010).
Acknowledgements
This study was supported by Chiesi Farmaceutici SpA. The company contributed to the study design but had no influence in the analysis and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
F.R.: conceptualization, data curation, formal analysis, investigation, methodology, project administration, supervision, validation, visualization, writing—original draft, writing—review and editing; I.B.: conceptualization, data curation, formal analysis, investigation, methodology, validation, writing—original draft, writing—review and editing; P.A.M.L.: conceptualization, data curation, formal analysis, investigation, methodology, validation; F.S.: conceptualization, data curation, formal analysis, investigation, methodology, project administration, supervision, validation, visualization, writing—original draft, writing—review and editing; C. Casiraghi: data curation, formal analysis, investigation, methodology, validation; A.M.: data curation, formal analysis, investigation, methodology, validation; M. Storti: data curation, formal analysis, investigation, methodology, validation; C. Catozzi: conceptualization, data curation, formal analysis, investigation, methodology, validation; L.T.: data curation, validation, writing—review and editing; R.Z.: data curation, formal analysis, investigation, methodology, validation, writing—review and editing; P.F.: data curation, formal analysis, investigation, methodology, validation, writing—review and editing; X.M.: data curation, formal analysis, writing—review and editing; M. Simonato: data curation, formal analysis, investigation, methodology, validation, writing—review and editing; P.C.: data curation, methodology, validation, writing—review and editing; V.C.: conceptualization, project administration, supervision, validation, writing—review and editing; G.L.: conceptualization, data curation, project administration, supervision, validation, visualization, writing—original draft, writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
F.R., F.S., C. Casiraghi, C. Catozzi, and L.T. are Chiesi Farmaceutici SpA employees. X.M. acts as Chiesi Farmaceutici SpA scientific consultant.
Patient consent
Patient consent was not required for this study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Ricci, F., Bresesti, I., LaVerde, P.A.M. et al. Surfactant lung delivery with LISA and InSurE in adult rabbits with respiratory distress. Pediatr Res 90, 576–583 (2021). https://doi.org/10.1038/s41390-020-01324-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-01324-2
This article is cited by
-
Ultrasound evaluation of diaphragm kinetics after minimally invasive surfactant administration
Journal of Ultrasound (2023)
-
Current Controversies and Advances in Non-invasive Respiratory Support for Preterm Infants
Current Treatment Options in Pediatrics (2022)