Abstract
Background
At the population level, there is a negative linear correlation between childhood body mass index (BMI) and pubertal height gain. However, in children with obesity, there are no studies showing whether the severity of obesity affects pubertal height gain. Moreover, how obesity in childhood affects pubertal timing is controversial, especially in boys. We aimed to investigate the impact of severe obesity in childhood on the pubertal growth spurt in both sexes.
Methods
The study group consisted of 68 patients (32 boys) with childhood onset obesity followed in a Spanish university hospital. The QEPS growth model was used to calculate pubertal growth function estimates for each individual. The highest individual prepubertal BMI SDS value was related to the age at onset of pubertal growth and pubertal height gain. Results were compared to analyses from individuals in a community-based setting (n = 1901) with different weight status.
Results
A higher peak BMI in childhood was associated with less specific pubertal height gain in children with moderate-to-extreme obesity. For boys, the higher the BMI, the earlier the onset of pubertal growth. For girls with obesity, this correlation was not linear.
Conclusions
Obesity in childhood impairs the pubertal growth spurt in a severity-related fashion.
Impact
-
The higher the BMI in childhood, the lower the pubertal height gain in children with moderate-to-extreme obesity.
-
For boys with obesity, the higher the BMI, the earlier the onset of pubertal growth.
-
The results contribute to the research field of how weight status in childhood is related to pubertal timing and pubertal growth.
-
The results have implications for understanding how childhood obesity is related to further growth.
Similar content being viewed by others
Introduction
Puberty is a biological process where an individual makes a transition from an immature child to a reproductively competent mature adult. Not going into puberty is dependent on active inhibition whereby the central nervous system suppresses the hypothalamic–pituitary axis, and it is still enigmatic how this suppression is released, and puberty begins. Puberty is dependent upon a rise in sex steroid secretion, which induces secondary sex characteristics and promotes growth, determining the pubertal growth spurt in a sex-specific fashion.1,2,3 Muscle and adipose tissue are also metabolic active, not only as target tissues but also as hormone-secreting organs. Growth hormone (GH), insulin, and insulin-like growth factor 1 (IGF-1) are important hormones for growth/height gain during childhood and puberty.1 During puberty, secondary sex characteristics develop and there are typical changes in body composition, with increased fat mass and altered fat distribution in females (hips and breasts) and increased muscle mass in males.1 During puberty, there is an increase in height gain velocity, known as the pubertal growth spurt; the slowly declining height velocity of childhood is changed to an acceleration of height velocity, followed by a decline in height velocity until adult height is reached. Puberty generally occurs earlier in girls, thus resulting in an earlier pubertal growth spurt, and the mean height gain is greater for boys during puberty.4,5
It has been a challenge to adequately describe and model pubertal growth due to variations in the timing and the S-shaped pattern of growth characterizing puberty.6 Onset of the pubertal growth spurt can be identified based on the lowest height velocity preceding the spurt, sometimes referred to as take-off.1,7 The QEPS-growth model is a tool for detailed investigation of growth patterns in general and pubertal growth in particular, thus making it possible to analyze timing and total height gain of the pubertal growth spurt in individuals.8,9
Body mass index (BMI), kg/m2, correlates with fat mass in children and is the most common proxy for defining whether a child or adolescent is thin, normal weight, overweight, or obese.10,11 BMI during childhood is generally much lower than in adulthood and has an age- and sex-specific pattern.12 Due to this fact, it is necessary to use age and sex standardized BMI (BMI SDS) instead of raw BMI values in pediatric clinics and research.11
Since the early–mid-twentieth century, it has been shown that prepubertal children with obesity in general are taller than normal-weight children and that being overweight or obese during childhood is associated with increased height velocity.13,14,15,16,17 This accelerated linear growth in children with obesity usually associates with advanced bone age (BA) maturation, assessed by X-ray of the non-dominant hand, routinely used in pediatric clinics.18,19,20 Nutritional factors affecting weight during childhood may also influence the timing of puberty.15,21 The trend for puberty to start earlier over time as seen during the past 100 years in westernized societies is postulated to be related to a parallel increase in weight/BMI during childhood.22,23 Indeed, previous studies have shown an association between obesity/high BMI during childhood and early puberty in girls; for boys, this correlation is more heterogeneous.14,15,24,25 Our study in a community-based longitudinal setting (including a limited number of children with obesity) showed an inverse linear correlation between the highest childhood BMI SDS and specific pubertal height gain (estimated by the QEPS model); the higher the BMI during childhood, the less the pubertal height gain.26 To our knowledge, how the pattern of pubertal growth is related to the severity of obesity in a cohort of children with moderate-to-extreme obesity has not been investigated. It is possible that a decrease in pubertal height gain in children with obesity is related to accelerated prepubertal growth, associated with an accelerated BA during late childhood. The increase in standardized height above that determined by target height on the basis of an advanced BA observed in most patients with childhood obesity may limit the potential growth remaining for puberty.
The aims of the present study were:
-
1.
To investigate whether higher standardized childhood BMI in children with moderate/extreme obesity is associated with a further reduction in specific pubertal height gain compared to the findings from individuals with a wide range of BMI levels,26
-
2.
To study whether this association is determined by the degree of obesity-induced BA acceleration, and
-
3.
To investigate whether the age at the onset of pubertal growth is related to the severity of obesity in children with moderate-to-extreme obesity.
Methods
Patients
Ninety-eight adolescents with childhood onset of obesity (BMI > +2 SDS according to the International Obesity Task Force references) from the obesity clinic at the Department of Endocrinology of the University Hospital Niño Jesús, Madrid, Spain were studied.11,20 All patients and their parents or guardians gave informed written consent as required by the local ethics committee, which had previously approved the study in accordance with the “Ethical Principles for Medical Research Involving Human Subjects” adopted in the Declaration of Helsinki by the World Medical Association (64th WMA General Assembly, Fortaleza, Brazil, October 2013). Patients were studied to rule out any underlying pathological condition or syndromic cause of obesity prior to their enrolment in this study. Longitudinal measures of height and weight together with information of pubertal development according to Tanner staging was obtained from clinical visits.4,5 The BMI values from each visit were transformed to BMI SDS to yield age- and sex-specific BMI scores.11,27 The maturation of the bones of the non-dominant hand and wrist was evaluated by X-ray for estimation of BA.19 Every BA was determined by the same pediatric endocrinologist and later blindly re-read by a second pediatric endocrinologist, in order to ensure quality control. In this study, the highest BMI SDS recorded between 3.5 years and the onset of puberty (i.e., Tanner II) was used for analysis. This was done to reflect the stable period of childhood growth in height. The methodology used has been previously published26; hence, comparisons are straight forward with the same methodology as in the previous paper. Longitudinal height data were analyzed by the QEPS growth model.8,9 This analysis was performed on 68 patients (36 girls and 32 boys) all of whom had reached adult height and had sufficient growth data in the prepubertal period recorded in their longitudinal follow-up to adequately fit the model. The analyses included visual curve analysis for each individual and re-evaluation of height/weight values to select individuals with growth data of high quality (30 patients did not adequately fit the model requirements due to insufficient longitudinal growth data availability and a few registration or measurement errors were corrected/withdrawn). The children had taller heights at onset of puberty (+0.7 in females to +0.8 SDS in males), compared to the national Spanish reference27 (Table 1). As seen in Table 1, there were no statistically significant differences between the predicted adult heights from mid-parental heights and the achieved adult heights.
Community-based study group for comparison
A subgroup from the GrowUp 1990 Gothenburg cohort consisting of healthy individuals, as well as children/adolescents with obesity, born at full term (gestational age 37–42 week) in Sweden with longitudinal growth data was analyzed.26 Weight and height had been measured by experienced nurses at well-baby clinics and at schools in Gothenburg, Sweden and surrounding municipalities.28 All individuals, as well as their parents/guardians for individuals aged <18 years, gave informed consent as required by the local ethics committee, which had previously approved the study. In total, the study group included 1901 individuals (929 females, 972 males). The BMI values from each visit were transformed to BMI SDS to yield age- and sex-specific BMI scores.11 For the analyses in the present study, the highest BMI SDS obtained between 3.5 and 7 years of age for girls and 8 years of age for boys was used and for categorizing the subjects into underweight, normal weight, overweight, and obese subgroups.11 The use of a different age range for boys and girls was due to the difference between the sexes in the timing of puberty, to be sure that no individual had entered puberty at the time when the highest prepubertal BMI SDS was recorded. This was done to reflect the stable period of childhood growth in height. The methodology used has been previously published26; hence, comparisons are straight forward with the same methodology as in the previous paper. Longitudinal height data were analyzed by the QEPS growth model.8,9 This study group has been described in detail in a previous publication.26 In the current study, the data from children with overweight and obesity (187 girls and 190 boys) were used for comparisons with the clinical study group of children with moderate-to-extreme obesity. The children had taller heights at onset of puberty (+0.5 in females to +0.6 SDS in males), compared to the national Swedish reference29 (Table 1).
The QEPS growth model
QEPS describes individual growth using a combination of four basic growth functions modeling total height (T) in cm as a function of age; T(age) = Q(age) + E(age) + P(age) − S(age). The Q-function and the negative exponential E-function both start during fetal life, 8 months before birth; the E-function levels off after birth, whereas the Q-function continues until the end of growth.8,9 A specific pubertal P-function starts at the onset of puberty, and growth during puberty is determined by the continuing Q-function and the specific P-function (Fig. 1). A stop S-function ends growth. For the E-, Q-, and P-functions, an individual height-scale parameter is defined, and for the E- and P-functions, a time-scale parameter, together with individual timing of puberty (AgeP50) giving six modifying parameters, which makes it possible to model individual growth curves from birth to adult height. The QEPS growth model was used in the present study to analyze individual growth patterns. The Mathlab® software program (version 7.13.0 R2012b, The Mathworks) was applied with the QEPS model formulae to construct individual growth curves and calculate QEPS variables for each studied individual. The model has been described in detail in other publications.8,9 In the present study, the specific pubertal gain in height (Pmax, height gain due to the P-function in cm) and onset of the pubertal growth spurt (AgeP5, the time in years where 5% of the specific pubertal height gain is reached) related to the highest prepubertal BMI SDS for each individual was analyzed.
Left: The four mathematical functions of the QEPS model that are combined to describe total gain in height (T) from fetal life to adulthood: Quadratic (Q), Exponential (E), Pubertal (P), and Stop (S). B = birth, marked with a vertical line. t0 = about 6 weeks after conception. The three vertical arrows indicate the fitted individual height-scale parameters, from left to right Eheightscale, Qheightscale, and Pheightscale. The two horizontal arrows indicate the time-scale parameters, from left to right Etimescale and Ptimescale, and the sixth parameter is the location of mid-puberty, AgeP50, indicated with a dot. Right: height and height velocity graphs where onset of pubertal growth—ageP5, representing the time where 5% of the specific pubertal height gain is reached, is indicated. The specific pubertal height gain, Pmax, what the P-function adds to the ongoing Q-function growth is noted in the upper right of the figure.
Statistical analyses
Mann–Whitney U test was used for to compare growth estimates from the QEPS model and the highest prepubertal BMI SDS (non‐normally distributed values in this study population). A p value <0.05 was considered statistically significant. The relationships between quantitative normal variables were studied by linear correlation analysis (ANOVA/Pearson’s r), whereas Spearman’s rho was used for non‐normally distributed variables. The analyses were performed both in relation to the highest individual prepubertal BMI SDS and related to subgroups where the clinical study groups where divided into moderate obesity (BMI SDS <+4.0) and extreme obesity (BMI SDS >+4.0). The individuals in the community-based study group with overweight (BMI SDS +1.2) and obesity (BMI SDS +2) was one subgroup. Statistical analyses were performed using the software Statistical Package for Social Sciences (SPSS v. 15.0. MapInfo Corporation, Troy, NY, USA). SAS software was used for the analyses/graph building in Figs. 2–4 (SAS Institute Inc., Cary, NC, USA, version 9.3).
The specific pubertal gain in adult height, Pmax, is related to the highest prepubertal BMI SDS for each girl (red) and boy (blue). Pmax is the growth in cm during the pubertal years due to the specific P-function from QEPS model. Girls in the clinical obesity cohort are shown as red triangles, girls in the community-based cohort as red cross. Boys in the clinical obesity cohort are shown as blue cross, boys in the community-based cohort as blue circles. Mean values for girls are shown with a red regression line, for boys with a blue regression line, for each cohort. The shaded red/blue areas indicate 95% CI of the regression lines. For boys, regression lines in the community-based cohort: Pmax = 17.83 + BMI SDS × −1.44 (p < 0.0001); in the clinical obesity cohort: Pmax = 17.99 + BMI-SDS × −1.44 (p = 0.0047). For girls, the regression lines in the community-based cohort: Pmax = 13.43 + BMI SDS × −1.22 (p < 0.0001), in the clinical obesity cohort: Pmax = 12.08 + BMI SDS × −0.57 (p = 0.39).
The specific pubertal gain in adult height, Pmax (cm), is related to the difference between bone age (BA) and chronological age (CA) in years for girls (red crosses) and boys (blue circles) in the clinical obesity cohort. Children with the most accelerated BA had the lowest height gain (r2 linear = 0.287, p = 0.006 in boys, r2 linear = 0.154, p = 0.02 in girls). The shaded red (girls) and blue (boys) areas indicate 95% CI of the regression lines.
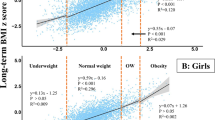
The onset of pubertal growth in years AgeP05, is related to the highest prepubertal BMI SDS for each girl (red) and boy (blue). Girls in the clinical obesity cohort are shown as red triangles, girls in the community-based cohort as red crosses. Boys in the clinical obesity cohort are shown as blue crosses, boys in the community-based cohort as blue circles. Mean values for girls are shown with a red regression line, for boys with a blue regression line, for each cohort. The shaded red/blue areas indicate the 95% CI of the regression lines. For boys, the regression lines in the community-based cohort AgeP05 = 11.8 + BMI SDS × −0.12 (p < 0.0001); in the clinical obesity cohort: AgeP05 = 12.05 + BMI SDS × −0.17 (p = 0.086). For girls, the regression lines in the community-based cohort: AgeP05 = 9.83 + BMI SDS × −0.14 (p < 0.0001); in the clinical obesity cohort: AgeP05 = 9.14 + BMI SDS × 0.025 (p = 0.82).
Results
Specific pubertal height gain versus highest childhood BMI SDS
In both sexes, children with extreme obesity had the lowest specific pubertal height gain (Pmax) compared to individuals with overweight–moderate obesity (p < 0.01; Table 2 and Fig. 2). For boys, there was an inverse linear correlation across the entire BMI spectrum between the highest childhood BMI SDS and pubertal height gain, with almost identical regression lines in the two different study groups (Pmax = 17.83 + BMI SDS × −1.44 versus Pmax = 17.99 + BMI SDS × −1.44) as seen in Fig. 2. In the subgroup of boys with extreme obesity, the specific pubertal height gain was 5.74 cm less compared to boys with overweight and obesity in the community-based cohort. For girls, the regression lines were not identical, with smaller differences in the group of girls with extreme obesity (Fig. 2). The difference of Pmax (1.43 cm) between girls with obesity in the clinical setting and girls with overweight and obesity in the community-based study group was just below statistical significance (p = 0.056), whereas the difference between girls with extreme obesity and the group of girls with overweight and obesity of 2.3 cm was significant (p = 0.024) as seen in Table 2.
Specific pubertal height gain versus BA advancement
In the whole cohort from the clinical setting, there was a significant advance in BA versus chronological age (Table 1, p < 0.001). For both boys and girls, there were statistically significant differences in the specific pubertal height gain (Pmax) related to BA. The difference between chronological age and BA, with most individuals having some degree of advanced BA, were related to the specific pubertal height gain. Children with the most accelerated BA had the lowest height gain (p = 0.006 in boys, p = 0.02 in girls; Fig. 3). Due to lack of BA data in the community-based study group, this analysis was performed only in the clinical study group.
Onset of pubertal growth versus highest childhood BMI SDS
For both sexes, children with obesity had the earliest onset of pubertal growth (Table 2 and Fig. 4). For boys, there were statistically significant differences regarding the onset of pubertal growth (AgeP5) related to the highest childhood BMI SDS. An inverse linear correlation across the entire BMI spectrum was found between the highest childhood BMI SDS and onset of pubertal growth, with similar regression lines in the two different study groups (AgeP5 = 11.80 + BMI SDS × −0.12 versus AgeP5 = 12.05 + BMI SDS × −0.17) as seen in Fig. 4. Boys with the highest degree of obesity (the subgroup of boys with extreme obesity with BMI >4 SDS) demonstrated an onset of pubertal growth 7.2–8.4 months earlier compared to the boys with moderate obesity in the clinical subgroup and boys with overweight and obesity in the community-based subgroup. For girls, the regression line was flattened in the group of girls with moderate-to-extreme obesity (the clinical study group), meaning no further statistically significant advance in the onset of pubertal growth with higher degrees of obesity (Fig. 4).
Discussion
The main finding of this study was that children of both sexes with severe obesity (>+4 BMI SDS) had the lowest pubertal height gain and that boys with severe obesity had a significantly earlier onset of pubertal growth. To our knowledge, this is the first study to investigate how different degrees of overweight and obesity during childhood are related to the pubertal height gain. This study confirms the relationship of high BMI in childhood and a diminished pubertal growth spurt for both sexes suggested in some previous studies.16,17,26,30,31 The children in the present study also had pronounced growth during childhood, with taller heights at onset of puberty (compared to national references27,29), confirming the results from other studies.14,15,16,17,26,31
The general sex differences in pubertal growth, with a more pronounced growth spurt in boys, was less apparent in the subgroup of children with extreme obesity where boys with extreme obesity (>+4 BMI SDS) had a smaller pubertal growth spurt similar to girls with extreme obesity (8.93 versus 8.86 cm). An explanation for the reduction in the specific pubertal height gain associated with a higher childhood BMI could be that obesity during childhood accelerates bone maturation. With an older (more advanced) BA before puberty, the remaining growth potential before reaching adult height is reduced, with less height gain left for the pubertal growth spurt. The present study confirms this theory since there was a strong linear correlation between accelerated BA and reduced specific pubertal height gain. There was a lack of difference between the adult height for both girls and boys and their mid-parental height, reinforcing the concept that the patients finally reach their target height, however, growing more in the prepubertal period and less during the pubertal years. Children with obesity have low levels of GH despite growing at a normal or high growth velocity.32 IGF-I levels of the patients with obesity were in the normal range.32 During puberty, there is a marked increase in GH secretion rate,33 which coincides with and probably explains part of the pubertal growth spurt. Our results, showing a low pubertal height gain in boys with extreme obesity, may be a result of a diminished increase in GH secretion compared to boys with normal weight/more moderate obesity.
Earlier pubertal onset associated with childhood obesity has been reported in numerous other studies, particularly for girls.14,15,23,24,25,31,34,35 For boys, the results have been incongruous, where some studies, mainly from the US, have reported late puberty in boys with obesity,24,36 whereas most studies from other parts of the world have reported earlier puberty associated with childhood obesity.15,16,23,25,30,31 A recent study, also based on two different populations (from the Belarus and the US), supports the role of higher BMI in accelerating linear growth in early life with earlier pubertal development and a reduced pubertal growth spurt.37 A somewhat surprising result in our investigation is that boys, but not girls, in this study setting appear to have a linear correlation between both diminished pubertal height gain and earlier puberty with a higher degree of obesity (higher BMI SDS).
The somewhat contradictory findings for girls in this study regarding pubertal timing may be due to a nonlinear correlation between childhood BMI and pubertal timing at the extreme end of the spectrum, when obesity is very pronounced. Excess calorie intake and being affected with obesity could both stimulate linear growth and lead to diminished inhibition from the hypothalamus to the pituitary that initiates puberty.38 In girls with extreme obesity, the higher secretion of estradiol from adipose tissue could have an inhibitory effect on gonadotropin secretion modulating the onset of puberty. This mechanism has been postulated as a reason for late puberty in boys with obesity, seen in some studies.36 Another possible explanation could be that the secretion of estrogens from adipose tissue in contrast to estradiol from the ovaries lack the circadian rhythm, thus interfering with gonadotropin secretion from the pituitary. High estradiol levels or a disturbed circadian rhythm of estradiol secretion could then counteract a reduction in hypothalamic inhibition, explaining why the correlation of obesity and early onset of pubertal growth ceases to be linear in girls with extreme obesity. It should also be kept in mind that there is a sex-specific feedback mechanisms of estrogens at the hypothalamic level involving both positive and negative feedback in males; a possible explanation of the sex differences.39 The majority of studies on the relationship between the timing of puberty and weight status during childhood have been based on secondary sex characteristics, including age of menarche in girls. The age of menarche, which is employed in many studies of pubertal timing in girls as the main outcome, may also have a different correlation to pubertal growth patterns in girls with mild-to-moderate obesity compared to girls with extreme obesity. Here a possible explanation for the inconsistent findings regarding BMI and puberty for girls compared to other studies could be that the correlation between secondary sex characteristics and pubertal height gain differ in girls with extreme obesity compared with girls of normal weight and girls with mild-to-moderate obesity.
These possibilities and uncertainties call for further studies to attain more insights into the mechanisms involved relating childhood obesity and the timing of puberty and the pubertal pattern of growth. At the individual level, BMI is sometimes not an optimal measure of obesity as it is not always well correlated with fat mass and may also be influenced by bone and muscle mass. Future research related to the results of the present study may be to study growth patterns, as described by the QEPS model, and other measures of weight status/adiposity, such as waist circumference, skin fold measurements, dual-energy X-ray absorptiometric measures such as fat tissue and fat free mass, or magnetic resonance imaging evaluations of fat mass.
The QEPS model enables detailed investigation of pubertal growth. In previous studies, the model has been used to characterize growth patterns in longitudinal community-based cohorts. For the first time, the QEPS model now has been applied in a clinical context, demonstrating the usefulness of the model in this new setting. In contrast to Preece–Baines and other methods describing pubertal growth, the QEPS model can both delineate the onset of pubertal growth (AgeP5) and calculate the amount of specific pubertal height gain (Pmax), separated from the ongoing basic growth.1,9,40 The results of the study, showing a diminished pubertal growth spurt related to high childhood BMI, indicate a tendency to overestimate future height gain in children with obesity. Hence, these results are not only of scientific value but may also be useful in the clinical assessments of growth in individual children.
The current study is not without limitations. The two different study groups forming the basis of our investigation are very different from each other. The mean adult heights of the clinical study group were shorter than the Spanish population references,27 however, similar or slightly taller than target heights (non-significant difference), whereas the individuals with childhood obesity and overweight in the community-based group were of similar adult heights as the Swedish references.29 The difference between the two study groups may be regarded as a limitation; on the other hand, the almost identical regression lines for boys from the two study cohorts may be seen as an indication of a universal connection between BMI status during childhood and further height gain during puberty in boys. The results of our study should also be interpreted with some caution since the number of patients with extreme obesity included is still rather limited. This calls for further investigation in different cohorts and groups of patients to confirm or modify the insights gained by the current study.
In conclusion, the results of the present study show that children of both sexes with extreme obesity had the lowest specific pubertal height gain compared to individuals with less severe obesity; childhood obesity impairs the pubertal growth spurt in a severity-related fashion. For boys, our results regarding both pubertal height gain and the onset of pubertal growth indicate a linear correlation from normal weight, through overweight and mild-to-extreme obesity. The findings of the present study validate childhood BMI as an important modifier of pubertal growth in both children with normal weight and children with obesity.
References
Holmgren, A. Human Growth Patterns -With Focus on Pubertal Growth and Secular Changes (Department of Pediatrics, Institute of Clinical Sciences, University of Gothenburg, Gothenburg, 2018).
Albertsson-Wikland, K. et al. Twenty-four-hour profiles of luteinizing hormone, follicle-stimulating hormone, testosterone, and estradiol levels: a semilongitudinal study throughout puberty in healthy boys. J. Clin. Endocrinol. Metab. 82, 541–549 (1997).
Ankarberg, C. & Norjavaara, E. Diurnal rhythm of testosterone secretion before and throughout puberty in healthy girls: correlation with 17beta-estradiol and dehydroepiandrosterone sulfate. J. Clin. Endocrinol. Metab. 84, 975–984 (1999).
Marshall, W. A. & Tanner, J. M. Variations in pattern of pubertal changes in girls. Arch. Dis. Child. 44, 291–303 (1969).
Marshall, W. A. & Tanner, J. M. Variations in the pattern of pubertal changes in boys. Arch. Dis. Child. 45, 13–23 (1970).
Merrell, M. The relationship of individual growth to average growth. Hum. Biol. 3, 37–70 (1931).
Taranger, J. & Hagg, U. The timing and duration of adolescent growth. Acta Odontol. Scand. 38, 57–67 (1980).
Nierop, A. F. et al. Modelling individual longitudinal human growth from fetal to adult life - QEPS I. J. Theor. Biol. 406, 143–165 (2016).
Holmgren, A. et al. Insight into human pubertal growth by applying the QEPS growth model. BMC Pediatr. 17, 107 (2017).
Cole, T. J., Bellizzi, M. C., Flegal, K. M. & Dietz, W. H. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 320, 1240–1243 (2000).
Cole, T. J. & Lobstein, T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr. Obes. 7, 284–294 (2012).
Karlberg, J. & Albertsson-Wikland, K. in Recent Developments in Infant Nutrition 112–127 (Springer, 1996).
Bauer, J. Einige bemerkungen uber “fettkinder”. Med. Welt 3, 1467 (1929).
Mossberg, H. Obesity in children. Acta Paediatr. XXXV (1948).
Wolff, O. H. Obesity in childhood; a study of the birth weight, the height, and the onset of puberty. Q. J. Med. 24, 109–123 (1955).
He, Q. & Karlberg, J. Bmi in childhood and its association with height gain, timing of puberty, and final height. Pediatr. Res. 49, 244–251 (2001).
Johnson, W. et al. Patterns of linear growth and skeletal maturation from birth to 18 years of age in overweight young adults. Int. J. Obes. 36, 535–541 (2012).
Bayley, N. & Pinneau, S. R. Tables for predicting adult height from skeletal age: revised for use with the Greulich-Pyle hand standards. J. Pediatr. 40, 423–441 (1952).
Greulic, W. W. & Pyle, S. I. Radiographic Atlas of Skeletal Development of the Hand and Wrist (Stanford University Press, 1959).
Martos-Moreno, G. A., Martinez-Villanueva, J., Gonzalez-Leal, R., Chowen, J. A. & Argente, J. Sex, puberty, and ethnicity have a strong influence on growth and metabolic comorbidities in children and adolescents with obesity: report on 1300 patients (the Madrid Cohort). Pediatr. Obes. 14, e12565 (2019).
Frisch, R. E. & Revelle, R. Height and weight at menarche and a hypothesis of critical body weights and adolescent events. Science 169, 397–399 (1970).
Parent, A. S. et al. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr. Rev. 24, 668–693 (2003).
Aksglaede, L., Olsen, L. W., Sorensen, T. I. & Juul, A. Forty years trends in timing of pubertal growth spurt in 157,000 Danish school children. PLoS ONE 3, e2728 (2008).
Wang, Y. Is obesity associated with early sexual maturation? A comparison of the association in American boys versus girls. Pediatrics 110, 903–910 (2002).
Dai, Y. L. et al. Association between obesity and sexual maturation in Chinese children: a muticenter study. Int. J. Obes. 38, 1312–1316 (2014).
Holmgren, A. et al. Pubertal height gain is inversely related to peak BMI in childhood. Pediatr. Res. 81, 448–454 (2017).
Hernández, M., Castellet, J. & Narvaiza, J. in Curvas y Tablas de Crecimiento (ed. Garsi, S. A.) 5–31 (Fundación Faustino Orbegozo, Madrid, 1988).
Sjöberg, A. et al. Body size and lifestyle in an urban population entering adulthood: the ‘Grow up Gothenburg’ Study. Acta Paediatr. 101, 964–972 (2012).
Albertsson-Wikland, K., Luo, Z. C., Niklasson, A. & Karlberg, J. Swedish population-based longitudinal reference values from birth to 18 years of age for height, weight and head circumference. Acta Paediatr. 91, 739–754 (2002).
Denzer, C. et al. Pubertal development in obese children and adolescents. Int. J. Obes. 31, 1509–1519 (2007).
Sandhu, J. et al. The impact of childhood body mass index on timing of puberty, adult stature and obesity: a follow-up study based on adolescent anthropometry recorded at Christ’s Hospital (1936-1964). Int. J. Obes. 30, 14–22 (2006).
Argente, J. et al. Multiple endocrine abnormalities of the growth hormone and insulin-like growth factor axis in prepubertal children with exogenous obesity: effect of short- and long-term weight reduction. J. Clin. Endocrinol. Metab. 82, 2076–2083 (1997).
Albertsson-Wikland, K., Rosberg, S., Libre, E., Lundberg, L. O. & Groth, T. Growth hormone secretory rates in children as estimated by deconvolution analysis of 24-h plasma concentration profiles. Am. J. Physiol. 257, E809–E814 (1989).
Biro, F. M. et al. Onset of breast development in a longitudinal cohort. Pediatrics 132, 1019–1027 (2013).
Yokoya, M. & Higuchi, Y. Geographical differences in the population-based cross-sectional growth curve and age at peak height velocity with respect to the prevalence rate of overweight in Japanese children. Int. J. Pediatr. 2014, 867890 (2014).
Lee, J. M. et al. Timing of puberty in overweight versus obese boys. Pediatrics 137, 1–10 (2016).
Aris, I. M. et al. Association of BMI with linear growth and pubertal development. Obesity 27, 1661–1670 (2019).
Sanchez-Garrido, M. A. & Tena-Sempere, M. Metabolic control of puberty: roles of leptin and kisspeptins. Horm. Behav. 64, 187–194 (2013).
Rosenfield, R. L. & Bordini, B. Evidence that obesity and androgens have independent and opposing effects on gonadotropin production from puberty to maturity. Brain Res. 1364, 186–197 (2010).
Preece, M. A. & Baines, M. J. A new family of mathematical models describing the human growth curve. Ann. Hum. Biol. 5, 1–24 (1978).
Acknowledgements
The authors are grateful for the contribution by Andreas F.M. Nierop in performing the estimations of the QEPS growth functions of the study groups and for careful editing and language revision by Dr. Julie A. Chowen. The authors acknowledge financial support from the governmental grants under the ALF agreement, ALFGBG-719041 and ALFGBG-812951 (to K.A.-W.), the Region Halland Grants (to A.H.), the Foundation Växthuset for children (to K.A.W.), grants from Fondo de Investigación Sanitaria (PI-16/00485 and PI-19/00166 to J.A.), Fondos FEDER, and Centro de Investigación Biomédica en Red Fisiopatología de Obesidad y Nutrición (CIBEROBN), Instituto de Salud Carlos III (to J.A.).
Author information
Authors and Affiliations
Contributions
All six authors developed the study concept and design. G.Á.M.-M. and J.M.-V. were responsible for data collection from the clinical obese study group. K.A.-W. and J.A. are principal investigators of the study populations used. A.N. made Figs. 2–4. A.H. performed the statistical analyses and made the tables and wrote the first draft. All six authors critically revised the paper for important intellectual content and approved the final manuscript to be submitted for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Statement regarding the patient consent
All patients/study subjects and their parents or guardians gave informed written consent to participate in research studies as required by the local ethics committees.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Holmgren, A., Martos-Moreno, G.Á., Niklasson, A. et al. The pubertal growth spurt is diminished in children with severe obesity. Pediatr Res 90, 184–190 (2021). https://doi.org/10.1038/s41390-020-01234-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-01234-3
This article is cited by
-
Growth pattern evaluation of the Edinburgh and Gothenburg cohorts by QEPS height model
Pediatric Research (2022)