Abstract
Background
Previous studies describe a short-term decrease in cerebral oxygen saturation (StO2) after intraventricular hemorrhage (IVH) in premature infants; little is known about long-term implications.
Methods
Infants born <30 weeks gestational age (GA) were included. Clinical characteristics, hemoglobin measurements, the highest grade of IVH, and white matter injury (WMI) were noted. NIRS monitoring occurred daily or every other day for 4 weeks; weekly through 36 weeks GA. Recordings were error-corrected before calculation of mean StO2 and fractional tissue oxygen extraction (FTOE). Mean StO2 and FTOE were plotted by postnatal age and injury group (IVH/no IVH; WMI/no WMI). Non-linear regression by locally estimated scatterplot smoothing was used to generate the best-fit line and CI.
Results
A total of 1237 recordings from 185 infants were included; mean length = 6.5 h; mean GA = 26.3 w; mean BW = 951 g; overall/severe IVH incidence was 29/8%, WMI incidence was 16%. IVH was independently associated with an acute drop in StO2, which remained lower for 68 d. Severe IVH was associated with lower StO2 values than mild IVH. WMI was associated with early and persistent elevation of FTOE.
Conclusion
IVH of any grade is associated with a prolonged cerebral desaturation and WMI is associated with prolonged elevation of FTOE. This finding is exacerbated for infants with severe IVH.
Impact
-
The longitudinal impact of IVH on cerebral oxygenation has not been previously studied.
-
IVH is associated with persistent cerebral desaturation, months in length, and is independent of anemia.
-
More severe IVH is associated with worsened cerebral hypoxia.
-
Infants later diagnosed with white matter injury have an early and persistent elevation of cerebral oxygen extraction (cFTOE).
-
This cerebral desaturation, below previously identified normative ranges, may provide insight into the mechanistic link between IVH and white matter injury.
Similar content being viewed by others
Introduction
Brain injury is common and substantial morbidity of prematurity. The most frequently identified form of brain injury, intraventricular hemorrhage (IVH), occurs in an acute or subacute fashion over the course of the first few days following birth.1 Although IVH is readily apparent on cranial ultrasound, the exact timing is often difficult to identify due to the episodic nature of sonographic evaluation. IVH, even of a low grade,2 is associated with long-term neurodevelopmental consequences including intellectual disability and cerebral palsy.3,4,5 Mechanisms of long-term impairment include direct neuronal injury,1,6 post-hemorrhagic ventricular dilation,7 and associated white matter injury.8
Studies examining cerebral blood flow and oxygenation over short time courses have demonstrated that IVH alters cerebral autoregulation and contributes to a metabolic mismatch of oxygen demand and delivery.9,10 It is well known that systemic hypoxia is strongly linked to the development of IVH,11,12 but the impact of IVH on regional oxygenation in the brain is understudied. This is particularly problematic as IVH quickly evolves into a chronic condition, requiring months for intraventricular blood to be fully resorbed;1 the impact of this chronic insult is not known. In this study, we hypothesized that IVH induces persistent, long-term alterations in cerebral oxygenation, inducing chronic cerebral hypoxia independent of other causes of hypoxia, namely anemia of prematurity.
Methods
Cohort selection
Preterm infants admitted to St. Louis Children’s Hospital, a level IV NICU serving urban, suburban, and rural populations, were prospectively recruited for a longitudinal NIRS monitoring study between 2012 and 2019. Inclusion criteria were preterm birth before 30 weeks gestation and enrollment within 48 h of birth. The parents of eligible infants were approached when members of the study team and equipment were available. Infants were excluded if there were known congenital or genetic anomalies or if the infant was not expected to survive. The study was reviewed and approved by the Washington University Human Protection Office and written informed consent was obtained from the parents prior to the start of recording.
Maternal and infant clinical characteristics
Comprehensive demographic and outcome data were collected for all included infants, including gestational age (GA) at birth, birth weight, sex, race, mode of delivery, and need for ventriculo-peritoneal shunt. Exposure to inotropic medications (i.e., dopamine, epinephrine), opioid sedation (fentanyl or morphine), and antenatal steroids were recorded. As anemia is known to be an important modifier of cerebral saturation,13,14,15 all hemoglobin values measured over the study period were recorded. The presence of a persistent patent ductus arteriosus (PDA) requiring treatment (inclusive of medical [indomethacin, ibuprofen], surgical, and catheter-based approaches) was recorded.
Approach to neuroimaging
Institutional guidelines indicate that routine screening head ultrasounds should be performed for all infants born before 30 completed weeks of gestation;16 those born after 30 weeks only have ultrasound screening performed in the case of traumatic birth and/or extensive resuscitation. As an imaging outcome was a primary endpoint of this study, we limited participation to those infants who were routinely screened clinically. Screening head ultrasounds were performed at least once in the first 3 days following birth, once between 7 and 10 days of life, and again at 30 days of life. Additional ultrasound imaging may have been performed based on the infant’s clinical course. Our institutional protocol for neonatal ultrasound includes imaging through the anterior fontanel and the mastoid window for evaluation of the posterior fossa.
Term-equivalent brain MRI was not a part of the study design, although it was performed for many of the infants. Research and clinical MR imaging practices at our institution share a common approach, being performed at term-equivalent age (36–42 weeks PMA), without contrast or sedation, and including a minimum of T1, T2, diffusion, and susceptibility-weighted sequences. MR imaging was included where available.
IVH was classified based on the highest grade of hemorrhage in the first 10 days on either side using the Papile scale17 coded as values between 0 and 4. Cerebellar hemorrhage was classified as present or absent using ultrasound or MR imaging. Post-hemorrhagic ventricular dilation (PHVD) was diagnosed on monitoring ultrasounds, typically performed twice weekly for at-risk infants. White matter injury was classified as none, punctate, or cystic using the 30-day cranial ultrasound or term-equivalent MRI. All imaging findings were extracted from the clinical radiology report.
Clinical practices
Although not compulsory, the typical institutional practice is to obtain a hemoglobin measurement on admission and generally once weekly until 34 weeks post-menstrual age (PMA). Individual infants may have hemoglobin monitoring more frequently, depending on the clinical context. Enteral iron supplementation, at 2 mg/kg, is started between 14 and 30 days of life, once the infant is tolerating full volume, fortified feedings. Infants are generally transfused with packed red blood cells (pRBC) in 15 mL/kg aliquots when the hemoglobin is less than 10 mg/dL (for mechanically ventilated or sick infants) or less than 8 mg/dL for (extubated infants with a more stable clinical status). As with the measurement of hemoglobin, transfusions may be given outside of these thresholds based on the individual infant’s clinical course and context.
During the study, a delayed cord clamping protocol was initiated (ca. 2014) for infants born at less than 32 weeks estimated gestational age. The goal delay was 30–60 s and cord milking was not used.
NIRS recording
As continuous NIRS monitoring for weeks on end is not logistically feasible, the approach to monitoring was structured to obtain shorter recordings with brief inter-recording intervals under the premise that a sufficiently large sample is representative of the overall pattern of cerebral oxygenation. All infants underwent regular NIRS monitoring with the goal of monitoring infants daily or every other day in the first 4 weeks following birth, then weekly until 36 weeks PMA or hospital discharge (whichever was earliest). Each monitoring session was a minimum of 6 h in length and up to a maximum of 24 h, depending on the stability of the infant and availability of equipment and research staff.
NIRS recordings were made using three different devices, the ForeSight (Edwards Lifesciences, Irvine, CA), the ForeSight Elite (Edwards Lifesciences, Irvine, CA), and the INVOS 5100c (Medtronic, Minneapolis, MN). The appropriate neonatal sensors were used in each device instance. ForeSight data were captured using a laptop computer connected to the NIRS monitor and the FS-DAQ software package (CAS Medical, Branford, CT). INVOS monitors were interfaced with the bedside patient monitor (IntelliVue MP70 or MX800; Philips Medical, Andover, MA) and the integrated data sample was recorded using IxTrend version 2.1 (ixellence, Wildau, Germany). Default manufacturer sampling rates were used (0.5 Hz for ForeSight, 0.2 Hz for INVOS).
Inter-device data transformation
Although the ForeSight and ForeSight Elite monitors are calibrated to provide equivalent measurements in the same infant, the INVOS 5100c has a different measurement profile and, if measurements are not adjusted, will provide lower oximetry values compared to the ForeSight monitors, given the same level of tissue oxygenation. Through the work of Hyttel-Sorensen and Kleiser, the sensitivity to changes in oxygen as well as the linear correlation with a gold standard device have been developed for numerous device-probe combinations18,19,20 using a blood-lipid model system. Using the coefficients for linear transformation from Kleiser 2018,18 all INVOS measurements were converted to ForeSight-equivalent measurements.
Data cleaning and processing
Noise and artifact are incredibly common in NIRS recordings made in the NICU. A two-step cleaning algorithm, similar to those in prior publications by the authors,10 was applied to each recording. In the first step, missing or unmeasurable values were removed from the recordings. For recordings made using the INVOS device, this was defined as StO2 < 15%, as this is the lower limit of device detection. For the ForeSight devices, measurements of 0 or missing measurements were removed. In all cases, missing or non-physiologic measurements were replaced with NaN (not-a-number) so as not to influence statistical calculations. In the second step, sudden non-physiologic shifts in the recording as occur with motion artifact were removed. Motion artifact induces a sudden increase in variance in a localized region of the recording. Using the sliding-motion artifact correction algorithm described by Ayaz et al.,21 the coefficient of variation (CV) of the entire recording is calculated after division into small, overlapping windows. Windows with motion artifact have elevated CV, and those windows where CV exceeds the threshold are removed from the analysis. As recommended by Ayaz, we have empirically derived this threshold \(\left( {\tau _d^{{\mathrm{upper}}}} \right)\) using 17,000 10-min windows of representative NIRS data finding that \(\tau _d^{{\mathrm{upper}}} = 0.068\). While this value is somewhat higher than the 0.015 found by Ayaz et al., this is not surprising given our use of commercial NIRS systems in a real clinical environment, with a lower signal to noise ratio when compared to a research fNIRS system in an experimental setting.
Data averaging and analysis
As the impact of IVH, WMI, anemia of prematurity, and development of cerebrovascular autoregulation develop over long time scales, individual recordings were summarized by a single mean value. The relatively long recording length and comprehensive data cleaning allow for calculation of a value that represents the average degree of oxygen saturation in the brain at that moment in time. These values can be plotted over time to better understand the overall trajectory of cerebral oxygenation. As anemia is known to strongly influence cerebral saturation, the relationship between the measured StO2, and measured hemoglobin concentration in peripheral blood were compared using simple linear correlation.
Cerebral fractional tissue oxygen extraction (cFTOE) is the ratio of cerebral oxygen consumption to oxygen delivery. In a large study by Alderliesten et al.,22 the mean cFTOE in the days following birth was noted to be 0.32. As cFTOE increases, it reflects the increased metabolic demand of the cerebral tissue and may eventually exceed the available oxygen supply, increasing the risk of hypoxic injury. Chronic elevation of cFTOE in the setting of cerebral desaturation may represent chronic cerebral hypoxia, a proposed mechanism of white matter injury in preterm infants,23 however, longitudinal measurements of cFTOE have not been previously reported. For those infants with simultaneously captured pulse oximetry (SpO2) data, calculation of cFTOE was performed using the standard equation: FTOE = (SpO2 − StO2)/SpO2.22 As with StO2, each recording was summarized with a single mean FTOE value.
Although the relationship between hemoglobin concentration and cerebral saturation is linear, anemia of prematurity does not progress in a linear fashion.24 Thus, while the trajectories of StO2 and cFTOE are a function of post-menstrual age, this relationship is likely complex and poorly captured by traditional linear and non-linear regression techniques. Given a sufficiently dense dataset, such complex signals can be modeled using a conditional mean function, where the outcome variable (e.g., StO2) is modeled as a function of the independent variable (postnatal age in days). In this approach, a larger dataset is convoluted or broken into smaller subsets, to which low-order polynomials are fit using a least-squares approach, weighting points closer to the subset more than those distant.
Smoothed conditional means were calculated using the ggplot2 package25 for the R statistical package (R Foundation for Statistical Computing, Vienna, Austria). When datasets were larger than 1000 elements, modeling was done using generalized additive models with integrated smoothness estimation (GAM); those with fewer than 1000 elements were modeling using locally estimated scatterplot smoothing (LOESS). For this analysis, infants were first divided into those who had IVH of any grade and those who did not. To understand the impact of worsening grades of IVH, infants were divided into three groups: without IVH, grade I/II, and grade III/IV. The line of best fit and the 95% confidence interval were estimated. A similar approach was used to model the relationship between longitudinal cFTOE and white matter injury status where infants were cluster by the presence or absence of white matter injury on neuroimaging.
Univariate analysis of relevant demographic and clinical variables was performed using Fisher’s exact test for categorical variables and Mann–Whitney U-test for continuous variables. Statistical tests were considered significant where p < 0.05. All statistical testing was performed using R version 3.6.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Demographics and clinical characteristics
A total of 185 infants were included in the study with a mean gestational age of 26.3 weeks, mean birthweight of 951 g, and 51% were male (Table 1). Most infants received antenatal steroids (74%), 24% received inotropic medications, 48% received transfusions, and 11% of infants died. During the 7-year study period, 215 infants were screened for study eligibility and 30 infants were excluded from enrollment (7 due to known or suspected congenital anomalies and the remainder due to moribund state).
Neurologic injury outcomes
The overall IVH incidence of any grade was 54/185 (29%), whereas high-grade IVH incidence was 8%. Most cases were diagnosed within 3 days of birth (58%) and the bulk of the remainder (28%) diagnosed between 4 and 10 days. Post-hemorrhagic ventricular dilation occurred in 16/54 (29%) of IVH cases and required placement of a ventricular shunt in 3/54 (6%) of cases. Cerebellar hemorrhage was noted in 16/185 infants (9%) and white matter injury was noted in 30/185 (16%) infants (Table 2).
As expected, there were notable differences between infants with and without IVH, with hemorrhage more common in sicker, less mature infants. Those infants with IVH were born at an earlier gestational age (25.5 vs. 26.7 weeks), had worse lung disease (median ventilator days 23.5 vs. 2), received antenatal steroids less often, received more inotropes, sedation, and transfusions (Table 1). There was no difference in the use of delayed cord clamping or the need for PDA treatment between infants with and without IVH.
Recording quality and characteristics
A total of 1237 recordings were made from the 185 infants included in the study. The mean ± SD recording length was 6.5 ± 5.3 h. The device used for recording was distributed roughly in thirds with 370/1237 (30%) of recordings made using the INVOS device, 295/1237 (23%) made with the ForeSight, and 572/1237 (46%) made using the ForeSight Elite monitor. Error correction removed a mean ± SD of 10.6 ± 0.9% of each recording. Simultaneous SpO2 data were available for (1140/1237, 92%) of recordings and were used for cFTOE calculations.
Hemoglobin and cerebral saturations
Hemoglobin concentration was measured 1927 times in this cohort of infants. Overall, the mean hemoglobin at birth was 14.5 g/dL and was slightly higher in the IVH group than the non-IVH group (15.2 vs 14.2 g/dL, p = 0.03). The hemoglobin was measured a median of 8 times (IQR 4-17) per infant. Those infants in the IVH group were transfused more often (59% vs. 44% of instances) and received a greater median number of transfusions (1.5 vs 0). Using all recorded hemoglobin values, the non-linear decay of hemoglobin in anemia of prematurity was visualized. Hemoglobin decreased in a biphasic pattern, with a rapid early drop followed by a slower decline before eventual recovery (Supplemental Fig. 1). A rise in hemoglobin was noted at ~2 weeks of age, right as the hemoglobin approached the transfusion threshold of 10 g/dL. A similar rise at this threshold is not noted later in postnatal age, likely a reflection that the older infants are less critically ill and have been transitioned to the lower threshold of 8 g/dL. Importantly, the pattern and trajectory of anemia were unaffected by IVH, even severe grade IVH (Fig. 1). Plotting mean values of hemoglobin against the mean cerebral saturation revealed the expected linear decrease in StO2 as the hemoglobin drops (r = 0.38, p < 0.01; Fig. 2).
Cerebral saturation and IVH status
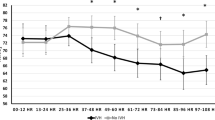
As previously noted, all error-corrected StO2 measurements were plotted by postnatal age in days, and then infants were divided into two groups by IVH status. Smoothed means via GAM were used to identify the line of best fit and 95% confidence interval for the two groups. Overall, both groups showed a similar trajectory in StO2 over time, with a biphasic drop in StO2, a plateau, and the beginning of recovery. Although this pattern closely resembles the observed decrease in total hemoglobin concentration, a gap greater than the 95% confidence interval was noted between the two groups, with the IVH infants at lower cerebral saturation until postnatal day 68 where the curves re-converged (Fig. 3). Of note, postnatal day 68 corresponds to a median corrected PMA of 36 weeks, a rough approximation of term-equivalent age.
StO2 by postnatal age, IVH vs. no IVH. Each point represents a single recording, plotted by PNA in days on the x axis and StO2 on the y axis. Infants without IVH are blue, infants with IVH are black. Best-fit lines by LOESS non-linear regression are solid lines, bounded by the 95% CI shown in gray shading. For infants with IVH, cerebral desaturation occurs soon after birth and is distinct from infants without IVH until postnatal day 70.
Infants were then further subdivided into those without IVH, those with mild IVH (grade 1 or 2), and those with severe IVH (grade 3 or 4). Smoothed conditional means were again used to identify the trajectory of StO2 over time. All three groups exhibited a similar overall pattern, but infants with severe IVH demonstrated the lowest saturations, infants with mild IVH had intermediate saturations, and those without IVH had the highest saturation. Confidence intervals did not fully re-converge until day 62 (Fig. 4).
StO2 by postnatal age, severe vs. mild vs. no IVH. Each point represents a single recording, plotted by PNA in days on the x axis and StO2 on the y axis. Best-fit lines by LOESS non-linear regression are solid lines, bounded by the 95% CI shown in gray shading. Infants with no IVH are shown in green, mild IVH in yellow, and severe IVH in red.
cFTOE and white matter injury status
All error-corrected cFTOE measurements were plotted by postnatal age in days and then infants were divided into two groups by WMI status. The small number of infants with WMI in this study precluded subgroup analysis by the severity of the injury. Smoothed means via GAM were again used to identify the line of best fit and 95% confidence interval for the two groups. A consistent pattern was demonstrated between the two groups, with relatively stable values of cFTOE in the first 20 days followed by a rapid increase until a second plateau around day 50 (Fig. 5). These patterns correspond to those found in the average hemoglobin concentration during those same periods, with the significant rise in cFTOE matching the worsening anemia during this period. Although all infants followed this pattern, those with a later diagnosis of WMI injury had evidence of increased oxygen extraction with an earlier start in the cFTOE increase phase and higher peak cFTOE values.
cFTOE by postnatal age, WMI vs. no WMI. Each point represents a single recording, plotted by PNA in days on the x axis and cFTOE on the y axis. Infants without WMI are blue, infants with WMI are black. Best-fit lines by LOESS non-linear regression are solid lines, bounded by the 95% CI shown in gray shading. For infants with WMI, elevated extraction occurs soon after birth and is largely distinct from infants without WMI until postnatal day 45.
Discussion
There are numerous studies that have identified a link between hypoxia and the development of IVH over an acute period. In this study, for the first time, we have described prolonged cerebral hypoxia following intraventricular hemorrhage. The degree of hypoxia is graded on the degree of hemorrhage, apparently independent of the degree of anemia, and the effect is months in length. Infants with IVH spend extended periods with cerebral saturation below what is currently considered to be the normal range,22 potentially exposing them to the risk of secondary injury.
There is a paucity of longitudinal cerebral oxygenation studies in the literature. There are numerous reports of the trajectory of StO2 in the first 3 days following birth, most notably the cohort of nearly 1000 infants reported by Alderliesten et al.22 Roche-Larabe et al.26 measured cerebral oxygenation over 6 weeks following birth, but their cohort was small (11 infants) and much more mature (28–34 weeks). In both cases, the authors reported a slow decrease in cerebral saturations, although there was no differentiation between injured and uninjured infants, and the degree of anemia was not considered.
Studies of short-term trends of cerebral oxygenation in the setting of brain injury have generated mixed results. Several authors have found increased cerebral saturation and decreased fractional extraction of oxygenation around the time of IVH, suggesting under-utilization in the acute setting of injury.27,28 In contrast, other studies have demonstrated decreased cerebral oxygenation in the setting of IVH, which worsened with increasing severity.29,30 Of note, the monitoring times in all these studies were brief, ranging between 3 h (Ying Zhang) and 76 h (Noori).
Infants with and without delayed cord clamping experienced IVH in similar proportions (29% vs 26%). The exact mechanism by which DCC confers a reduction in IVH is not well understood but is thought to be related to improvement in cerebral autoregulation,31 which was not measured in this study. When comparing cerebral saturations between those with and without DCC, there is a slight difference between the groups, with higher StO2 in the DCC group and only for the first week of life (Supplementary Fig. 2), confirming previous reports.32 One of the purported benefits of DCC is a greater starting hemoglobin concentration, leading to fewer transfusions.33 Our data suggest that any differences in cerebral saturation conferred by the higher hemoglobin do not persist beyond the first week of life.
There are two potential mechanisms through which IVH may influence cerebral oxygenation. First, by definition, infants with IVH have extravascular blood. Although this blood is capable of absorbing infrared light (and could contribute to the measurement of “cerebral” saturation by the NIRS monitor) it is permanently in a deoxygenated state. A single-sensor NIRS device is not capable of differentiating intravascular from extravascular blood, thus this pool of desaturated blood has the theoretical potential to cause a downward shift in measured cerebral saturation as it gets averaged with blood still in circulation. The degree to which intraventricular blood contributes to NIRS measurement is not known and cannot easily be measured. However, given the known optical properties of the human cranium and the performance characteristics of the NIRS monitors, it is not expected that there would be substantial penetration of near-infrared light into the intraventricular space. The median combined skin, skull, and CSF thickness in preterm infants is 5.7 mm,34 and the cortico-ventricular distance is 15–17 mm, the majority of which is sub-cortical white matter.35,36 Photon path length, and thus the depth of NIR light penetration, can be calculated using known physical properties and is a function of the separation distance between the NIR light emitter and the receiver.37 For commercial NIRS devices used in this study, estimated near-infrared light penetration depths range between 10 and 18 mm19,38,39 well short of the intraventricular space. Furthermore, even in rare instances where the ventricle is in the field of view of the NIRS probe, intraventricular blood tends to pool in the posterior aspect of the ventricles and would likely not be captured in the measurement (assuming the standard supine positioning of neonates).
Post-hemorrhagic ventricular dilation (PHVD), a form of hydrocephalus which is an important complication of intraventricular hemorrhage, distorts the architecture of the brain and may disrupt these anatomical assumptions. Although the number of infants with PHVD in this cohort is too small for adequate statistical power, an exploratory secondary analysis demonstrates significant differences between infants who had IVH without PHVD to those who had IVH with PHVD (Supplemental Fig. 3). However, unlike the steady, progressive changes in ventricular size associated with PHVD, the StO2 undergoes a steep decline before plateauing. This suggests that PHVD is more likely a marker of the most severe IVH and that it is the severity of injury driving cerebral desaturation rather than the detection of blood in the ventricles.
A second possible mechanism by which IVH might alter cerebral oxygenation is the disruption of cerebral autoregulation. Cerebral autoregulation is not monolithic but is instead composed of multiple different elements—intrinsic vascular control, circulating vasoactive compounds, and tissue-level metabolism40—each of which might be injured or impaired after IVH. There is strong evidence to suggest that this system is less robust and developmentally immature in preterm neonates and is significantly impacted by IVH.9,10,41 Data from adults with subarachnoid hemorrhage suggest that, while all patients experience an acute impairment of autoregulation after the initial insult, only a subset have a persistent impairment and go on to develop secondary ischemic injury as a result.42,43 It is less likely that infants with IVH have prolonged subacute loss of vascular control and more likely that they have chronic changes in metabolism in the deep cerebral white matter. The cFTOE data support this theory, demonstrating prolonged cerebral desaturation with simultaneously elevated oxygen extraction, a scenario that places enormous metabolic strain on the brain, particularly regions with already tenuous vascular supplies (e.g., periventricular white matter). The early and persistent elevation of cFTOE in infants later found to have WMI suggests that this measure may be a useful marker of future injury risk.
Although not directly measured in this study, the findings suggest that autoregulatory function may be impaired for an extended period, reflecting diminished blood flow with decreased metabolic activity or increased consumption by repair or reorganization. It is of note that the injured infants spent a period of many weeks with cerebral saturations more than two standard deviations below published normative values (defined as StO2 < 63% using the Alderliesten normative data adjusted for ForeSight values). The combination of cerebral desaturation and increased oxygen extraction place these infants in significant danger of hypoxic injury.
Direct measurement of autoregulation was not a part of this study design, as the commonly used approaches to quantifying autoregulation utilize simultaneously captured NIRS and invasive arterial blood pressure data.9,10,44,45 Similar longitudinal measures of autoregulation by IVH status may illuminate potential strategies for intervention and limiting the injurious impact of the hemorrhage. Given the current push to minimize prolonged high-risk central arterial access, there is an urgent need to develop and validate alternative, fully non-invasive approaches that are feasible in preterm infants.
These data provide some illumination into the long-term sequelae of IVH, namely white matter injury. Deep cerebral white matter, which has a limited vascular supply in preterm infants46 is also the most vulnerable to injury.47 This injury may progress in two stages; first from ischemia during the hemodynamically turbulent period following birth and second from prolonged cerebral hypoxia. Previous studies have linked the burden of systemic and cerebral hypoxia to the development of IVH11,48 and white matter injury;49,50,51 persistently altered autoregulation may be the common link between the two.
Like all studies, there are limitations to this present one. Ideally, the record of cerebral oximetry would be continuous from birth through discharge. However, the fragile clinical condition and thin, easily damaged skin of VLBW infants present significant safety challenges that preclude continuous monitoring. This study strikes a compromise between this patient safety consideration and the bulk collection of data. Given the large sample size of the recording library, it is anticipated that these data are representative of the true trajectory of cerebral saturation. This assumption is supported by the previous empiric work of the authors which showed that an accurate estimate of the daily mean cerebral saturation can be obtained with as little as a single hour of high-quality NIRS data.13 Second, while the overall sample of infants is large, the number with severe IVH (n = 15) and white matter injury (n = 16) was still relatively small. While this is a desirable outcome from the perspective of the infants, it contributed to greater than expected uncertainty about the true trajectory of StO2 and cFTOE, as reflected in the wider confidence intervals than for the much larger group of infants without injury. Third, the fraction of inspired oxygen provided to the infants was not captured. Evaluating delivered oxygen is an important component of developing future interventions—infants with severe IVH may require different oxygen saturation targets to overcome cerebral desaturation and elevated cFTOE. Future studies should also consider the use of simultaneous EEG, permitting interrogation of the link between electrical brain activity and oxygen consumption after IVH injury. Investigation of neurovascular coupling and oxygen extraction will shed additional light on potential mechanisms behind this chronic cerebral hypoxia. Fourth, term-equivalent MRI imaging was not a part of the study design, thus an outcome that could be evaluated using imaging obtained consistently across the entire cohort (cranial ultrasound) was used. Future lines of investigation will include the relationship between cerebral hypoxia and brain injury on term-equivalent MRI.
An additional limitation worthy of separate mention is the uncertainty of tissue interrogated by the NIRS device. Although estimates of skin, bone, gray, and white matter would suggest that the expected path of the photons would primarily reflect oxygenation in gray and white matter, this cannot be directly confirmed. The NIRS devices used in this study utilize multiple receivers at different distances from the emitter. The sensor closer to the emitter detects changes in scalp blood flow, while the receiver further away detects changes in deeper cerebral structures. The first value is subtracted from the second to filter out superficial blood flow changes.52 NIRS recordings were conducted by research staff with years of experience and who ensured consistent placement of the probe to repeatedly interrogate the same tissue. Nevertheless, free intraparenchymal hemoglobin or static and dynamic changes in ventricular size and volume have the theoretical capacity to independently influence measured cerebral saturation. A detailed study of photon path length and the expected proportion of interrogated tissue should be investigated in future studies.
Conclusion
IVH of any grade is associated with a prolonged cerebral desaturation of nearly 70 d in length and is independent of hemoglobin concentration. WMI is associated with prolonged elevation of cerebral oxygen extraction over the same time period. This combination of desaturation and over-extraction exposes the brain to prolonged metabolic stain. Interventions to temporize or reverse this process (e.g., pRBC transfusion) need further investigation.
References
Volpe J. J. Neurology of the Newborn 5th edn (Saunders/Elsevier, Philadelphia, 2008).
Klebermass-Schrehof, K. et al. Impact of low-grade intraventricular hemorrhage on long-term neurodevelopmental outcome in preterm infants.Childs Nerv. Syst. ChNS Off. J. Int. Soc. Pediatr. Neurosurg. 28, 2085–2092 (2012).
Bolisetty, S. et al. Intraventricular hemorrhage and neurodevelopmental outcomes in extreme preterm infants. Pediatrics 133, 55–62 (2014).
Papile, L. A., Munsick-Bruno, G. & Schaefer, A. Relationship of cerebral intraventricular hemorrhage and early childhood neurologic handicaps. J. Pediatr. 103, 273–277 (1983).
Stoll, B. J. et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 126, 443–456 (2010).
Douglas-Escobar, M. & Weiss, M. D. Biomarkers of hypoxic-ischemic encephalopathy in newborns. Front. Neurol. 3, 144 (2012).
de Vries, L. S. et al. Treatment thresholds for intervention in posthaemorrhagic ventricular dilation: a randomised controlled trial. Arch. Dis. Child Fetal Neonatal Ed. 104, F70–F75 (2019).
Adler, I. et al. Mechanisms of injury to white matter adjacent to a large intraventricular hemorrhage in the preterm brain. J. Clin. Ultrasound 38, 254–258 (2010).
Soul, J. S. et al. Fluctuating pressure-passivity is common in the cerebral circulation of sick premature infants. Pediatr. Res. 61, 467–473 (2007).
Vesoulis, Z. A. et al. A novel method for assessing cerebral autoregulation in preterm infants using transfer function analysis. Pediatr. Res. 79, 453–459 (2016).
Vesoulis, Z. A. et al. Early hypoxemia burden is strongly associated with severe intracranial hemorrhage in preterm infants. J. Perinatol. J. Calif. Perinat. Assoc. 39, 48–53 (2019).
Dani, C., Cecchi, A. & Bertini, G. Role of oxidative stress as physiopathologic factor in the preterm infant. Minerva Pediatr. 56, 381–394 (2004).
Whitehead, H. V. et al. Anemia of prematurity and cerebral near-infrared spectroscopy: should transfusion thresholds in preterm infants be revised? J. Perinatol. J. Calif. Perinat. Assoc. 38, 1022–1029 (2018).
Whitehead, H. V. et al. Progressive anemia of prematurity is associated with a critical increase in cerebral oxygen extraction. Early Hum. Dev. 140, 104891 (2019).
Mintzer, J. P., Parvez, B., La & Gamma, E. F. Regional tissue oxygen extraction and severity of anemia in very low birth weight neonates: a pilot NIRS analysis. Am. J. Perinatol. 35, 1411–1418 (2018).
Ment, L. R. et al. Practice parameter: neuroimaging of the neonate: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology 58, 1726–1738 (2002).
Papile, L. A., Burstein, J., Burstein, R. & Koffler, H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J. Pediatr. 92, 529–534 (1978).
Kleiser, S. et al. Comparison of tissue oximeters on a liquid phantom with adjustable optical properties: an extension. Biomed. Opt. Express 9, 86–101 (2018).
Kleiser, S. et al. Comparison of tissue oximeters on a liquid phantom with adjustable optical properties. Biomed. Opt. Express 7, 2973–2992 (2016).
Hyttel-Sorensen, S., Kleiser, S., Wolf, M. & Greisen, G. Calibration of a prototype NIRS oximeter against two commercial devices on a blood-lipid phantom. Biomed. Opt. Express 4, 1662–1672 (2013).
Ayaz, H., Izzetoglu, M., Shewokis, P. A. & Onaral, B. Sliding-window motion artifact rejection for functional near-infrared spectroscopy. Annu Int Conf. IEEE Eng. Med. Biol. Soc. 2010, 6567–6570 (2010).
Alderliesten, T. et al. Reference values of regional cerebral oxygen saturation during the first 3 days of life in preterm neonates. Pediatr. Res. 79, 55–64 (2015).
Back, S. A. White matter injury in the preterm infant: pathology and mechanisms. Acta Neuropathol. 134, 331–349 (2017).
Martin, R. J., Fanaroff, A. A. & Walsh, M. C. Fanaroff and Martin’s Neonatal-perinatal Medicine: Diseases of the Fetus and Infant (Saunders/Elsevier, Philadelphia, 2011).
Wickham, H. Ggplot2: Elegant Graphics for Data Analysis (Springer, New York, 2009).
Roche-Labarbe, N. et al. Noninvasive optical measures of CBV, StO2, CBF index, and rCMRO2 in human premature neonates’ brains in the first six weeks of life. Hum. Brain Mapp. 31, 341–352 (2010).
Alderliesten, T. et al. Cerebral oxygenation, extraction, and autoregulation in very preterm infants who develop peri-intraventricular hemorrhage. J. Pediatr. 162, 698–704.e2 (2013).
Zhang, Y. et al. in 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society 1937–1940 (IEEE, Boston, MA, 2011).
Sorensen, L. C. et al. Neonatal cerebral oxygenation is not linked to foetal vasculitis and predicts intraventricular haemorrhage in preterm infants. Acta Paediatr. 97, 1529–1534 (2008).
Noori, S. et al. Changes in cardiac function and cerebral blood flow in relation to peri/intraventricular hemorrhage in extremely preterm infants. J. Pediatr. 164, 264–270.e3 (2014).
Hofmeyr, G. J., Bolton, K. D., Bowen, D. C. & Govan, J. J. Periventricular/intraventricular haemorrhage and umbilical cord clamping. Findings and hypothesis. South Afr. Med J. Suid-Afr. Tydskr. Vir. Geneeskd. 73, 104–106 (1988).
Baenziger, O. et al. The influence of the timing of cord clamping on postnatal cerebral oxygenation in preterm neonates: a randomized, controlled trial. Pediatrics 119, 455–459 (2007).
Oh, W. et al. Effects of delayed cord clamping in very-low-birth-weight infants. J. Perinatol. J. Calif. Perinat. Assoc. 31(Suppl 1), S68–71 (2011).
Demel, A. et al. Correlation between skin, bone, and cerebrospinal fluid layer thickness and optical coefficients measured by multidistance frequency-domain near-infrared spectroscopy in term and preterm infants. J. Biomed. Opt. 19, 017004 (2014).
Zubiaurre-Elorza, L. et al. Cortical thickness and behavior abnormalities in children born preterm. PLoS ONE 7, e42148 (2012).
Borenstein-Levin, L. et al. Neonatal frontal lobe: sonographic reference values and suggested clinical use. Pediatr. Res. 87, 536–540 (2020).
Cui, W., Kumar, C. & Chance, B. Experimental study of migration depth for the photons measured at sample surface. Proc. SPIE 1431, 180–191 (1991).
Gunadi, S., Leung, T. S., Elwell, C. E. & Tachtsidis, I. Spatial sensitivity and penetration depth of three cerebral oxygenation monitors. Biomed. Opt. Express 5, 2896 (2014).
Pichler, G., Schmölzer, G. M. & Urlesberger, B. Cerebral tissue oxygenation during immediate neonatal transition and resuscitation. Front. Pediatr. 5, 29 (2017).
Paulson, O. B., Strandgaard, S. & Edvinsson, L. Cerebral autoregulation. Cerebrovasc. Brain Metab. Rev. 2, 161–192 (1990).
Wong, F. Y. et al. Impaired autoregulation in preterm infants identified by using spatially resolved spectroscopy. Pediatrics 121, e604–e611 (2008).
Hijdra, A. et al. Delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage: clinicoanatomic correlations. Neurology 36, 329–333 (1986).
Jaeger, M. et al. Continuous monitoring of cerebrovascular autoregulation after subarachnoid hemorrhage by brain tissue oxygen pressure reactivity and its relation to delayed cerebral infarction. Stroke 38, 981–986 (2007).
Lee, J. K. et al. A pilot study of cerebrovascular reactivity autoregulation after pediatric cardiac arrest. Resuscitation 85, 1387–1393 (2014).
Chock, V. Y., Ramamoorthy, C., Van & Meurs, K. P. Cerebral autoregulation in neonates with a hemodynamically significant patent ductus arteriosus. J. Pediatr. 160, 936–942 (2012).
Inage, Y. W., Itoh, M. & Takashima, S. Correlation between cerebrovascular maturity and periventricular leukomalacia. Pediatr. Neurol. 22, 204–208 (2000).
Greisen, G. & Børch, K. White matter injury in the preterm neonate: the role of perfusion. Dev. Neurosci. 23, 209–212 (2001).
Ng I. H. X. et al. Burden of hypoxia and intraventricular haemorrhage in extremely preterm infants. Arch. Dis. Child Fetal Neonatal Ed. 105, 242–247 (2019).
Darnall, R. et al. Early postnatal exposure to intermittent hypoxia results in significant alterations in white matter integrity in a rat pup model of apnea of prematurity. FASEB J. 30, 983.3–983.3 (2016).
Cai, J., Tuong, C. M. & Gozal, D. A neonatal mouse model of intermittent hypoxia associated with features of apnea in premature infants. Respir. Physiol. Neurobiol. 178, 210–217 (2011).
Martin, R. J. et al. Intermittent hypoxic episodes in preterm infants: do they matter? Neonatology 100, 303–310 (2011).
Germon, T. J. et al. Cerebral near infrared spectroscopy: emitter-detector separation must be increased. Br. J. Anaesth. 82, 831–837 (1999).
Funding
This study was funded by the NIH grant K23 NS111086, the Washington University Institute of Clinical and Translational Sciences KL2 Training Program (NIH/NCATS KL2 TR000450), The Barnes-Jewish Hospital Foundation and the Washington University ICTS Clinical and Translational Funding Program (NIH/NCATS UL1 TR000448), and the Cerebral Palsy Alliance Research Foundation.
Author information
Authors and Affiliations
Contributions
Conception, data collection, and analysis (Z.A.V.); drafting of the manuscript and critical revision (Z.A.V., H.V.W., S.M.M., and A.M.M.); approval of submitted final manuscript (Z.A.V., H.V.W., S.M.M., and A.M.M.).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent statement
Informed written consent was obtained for all study patients under a protocol approved by the local IRB.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Vesoulis, Z.A., Whitehead, H.V., Liao, S.M. et al. The hidden consequence of intraventricular hemorrhage: persistent cerebral desaturation after IVH in preterm infants. Pediatr Res 89, 869–877 (2021). https://doi.org/10.1038/s41390-020-01189-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-01189-5
This article is cited by
-
Neuromonitoring in neonatal critical care part II: extremely premature infants and critically ill neonates
Pediatric Research (2023)
-
Degree of ventriculomegaly predicts school-aged functional outcomes in preterm infants with intraventricular hemorrhage
Pediatric Research (2022)
-
Association of early cerebral oxygen saturation and brain injury in extremely preterm infants
Journal of Perinatology (2022)
-
A neonatal neuroNICU collaborative approach to neuromonitoring of posthemorrhagic ventricular dilation in preterm infants
Pediatric Research (2022)
-
Cerebral saturation reflects anterior cerebral artery flow parameters by Doppler ultrasound in the extremely premature newborn
Journal of Perinatology (2022)