Abstract
Background
Preterm infants are at enhanced risk of brain injury due to altered cerebral haemodynamics during postnatal transition. This observational study aimed to assess the clinical determinants of transitional cerebrovascular reactivity and its association with intraventricular haemorrhage (IVH).
Methods
Preterm infants <32 weeks underwent continuous monitoring of cerebral oxygenation and heart rate over the first 72 h after birth. Serial cranial and cardiac ultrasound assessments were performed to evaluate the ductal status and to diagnose IVH onset. The moving correlation coefficient between cerebral oxygenation and heart rate (TOHRx) was calculated. Linear mixed-effect models were used to analyse the impact of relevant clinical variables on TOHRx. The association between TOHRx and IVH development was also assessed.
Results
Seventy-seven infants were included. A haemodynamically significant patent ductus arteriosus (hsPDA) (β = 0.044, 95% CI: 0.007–0.081) and ongoing dopamine treatment (β = 0.096, 95% CI: 0.032–0.159) were associated with increasing TOHRx, indicating impaired cerebrovascular reactivity. A significant association between TOHRx, mean arterial blood pressure (β = −0.004, 95% CI: −0.007, −0.001) and CRIB-II score (β = 0.007, 95% CI: 0.001–0.015) was also observed. TOHRx was significantly higher in infants developing high-grade IVH compared to those without IVH.
Conclusions
Dopamine treatment, low blood pressure, hsPDA and high CRIB-II are associated with impaired cerebrovascular reactivity during postnatal transition, with potential implications on IVH development.
Impact
-
The correlation coefficient between cerebral oxygenation and heart rate (TOHRx) provides a non-invasive estimation of cerebrovascular reactivity, whose failure has a potential pathogenic role in the development of IVH in preterm infants.
-
This study shows that cerebrovascular reactivity during the transitional period improves over time and is affected by specific clinical and therapeutic factors, whose knowledge could support the development of individualized neuroprotective strategies in at-risk preterm infants.
-
The evidence of increased TOHRx in infants developing high-grade compared to low-grade or no IVH during the transitional period further supports the role of impaired cerebrovascular reactivity in IVH pathophysiology.
Similar content being viewed by others
Introduction
The first 72 h after premature birth represent a critical phase of haemodynamic adaptation to extrauterine life and is characterized by an enhanced risk of brain injury.1,2,3 The autoregulation of cerebral blood flow (CBF) is a physiological mechanism aimed at maintaining stable cerebral perfusion despite fluctuating blood pressure.4 The failure of the preterm cerebral vasculature to maintain uniform CBF over a range of systemic blood pressure results in a pressure-passive circulation,4,5 which has been implicated in the development of intraventricular haemorrhage (IVH).6,7 A deeper understanding of cerebrovascular determinants within the transitional phase may support the development of individualized approaches aimed at preventing harmful fluctuations of CBF in this vulnerable population.
Cerebral oxygenation (cTOI), measured by near-infrared spectroscopy, has been largely used as a surrogate for dynamic CBF monitoring in the neonatal population.8 The correlation between mean arterial blood pressure (MABP) and cTOI is primarily used to investigate cerebral autoregulation;8,9 this method, however, requires invasive arterial blood pressure monitoring, which may not be feasible or practical in several neonatal care settings. Due to their immature myocardial function, preterm infants are more dependent on increasing their heart rate (HR) in order to increase cardiac output and blood pressure; hence, the correlation coefficient between cTOI and HR (TOHRx) has been assessed as a non-invasive measure of cerebrovascular reactivity.3,10,11,12
This study aimed to assess the main determinants of cerebrovascular reactivity, measured non-invasively in very preterm infants during postnatal transition, and to evaluate its patterns in relation to IVH development.
Methods
Study population and ethics
Infants <32 weeks’ gestation admitted to the Neonatal Intensive Care Unit of Sant’Orsola-Malpighi Hospital (Bologna, Italy) between February 2018 and January 2022 were consecutively enrolled in this observational prospective study. The main exclusion criteria were genetic abnormalities, major congenital malformations and conditions that may influence cTOI, such as severe anaemia (defined as haematocrit <30%) or persistent pulmonary hypertension requiring inhaled nitric oxide.
This study was conducted in conformity with principles and regulations of the Helsinki Declaration. The study protocol was approved by the Ethics Committee of Sant’Orsola-Malpighi Hospital, Bologna, Italy. Written informed consent was obtained from the infants’ parents/legal guardians.
Cerebrovascular reactivity monitoring
Continuous monitoring of cTOI was performed using a NIRO-200NX oximeter (Hamamatsu Phototonics, Japan) sampling at 1-Hz, with disposable neonatal sensors placed on the forehead. HR was simultaneously monitored with a pulse oximeter Masimo Radical-7 (Masimo Corporation, Irvine, CA), whose averaging time was set at 2 s.
Monitoring started within 12 h after birth and continued up to 72 h after birth. Using an RS232 cable, the monitoring devices were connected to a laptop running ICM+® (https://icmplus.neurosurg.cam.ac.uk, Cambridge Enterprise, UK), a software tool that allows real-time synchronized recording of cTOI and HR for the whole monitoring period and includes a calculation engine for TOHRx computation.13 After the recording, the ICM+ traces were visually inspected; time periods showing signal noise or interruptions or periods of handling, care or invasive procedures were considered as likely artefactual and excluded from the computation. Artefact removal was blinded to IVH status. TOHRx was calculated as the moving correlation coefficient between 10-s averaged cTOI and HR values using 5-min time windows (Fig. 1), as previously described.11 Since TOHRx indicates the correlation gradient between slow waves of HR and cTOI, it is assumed that positive TOHRx values indicate impaired cerebrovascular reactivity, whereas zero or negative values reflect a reactive circulation.3,11 During the monitoring period, systolic, diastolic and mean blood pressure was measured at regular intervals (i.e. from every 30-min to 6 hourly) by the oscillometric technique. Both TOHRx and MABP measurements were averaged over each day of life.
Ultrasound assessments
Ultrasound assessments were performed using a portable ultrasound machine (Cx50, Philips Healthcare, Eindhoven, Netherlands); the operators performing ultrasound scans were blinded to TOHRx results. Cranial ultrasound scans (CrUSS) were obtained with a convex 8–5 MHz transducer through the anterior and mastoid fontanelles to exclude major brain abnormalities and to detect IVH development as well as its severity, classified according to Volpe’s grading.14 CrUSS were performed at enrolment and repeated every 6–12 h if an incipient IVH was noted, otherwise it was performed 12–24 hourly.
Echocardiograms were performed using a linear 12-MHz probe, with the aim of excluding congenital defects and evaluating cardiac function, left ventricular output (LVO) and the ductal status. The first assessment was performed at the enrolment and repeated every 6–12 h in the presence of a patent ductus arteriosus (PDA), or 12–24 hourly if there was no evidence of PDA.
LVO was calculated according to the formula [(left ventricular outflow velocity time integral [VTI]) × (HR) × (left ventricular outflow cross-sectional area)] and indexed to body weight.15 The left ventricular outflow diameter was measured from the parasternal long axis view using the leading-edge technique, whereas VTI was estimated sampling the left ventricular outflow tract from an apical five-chamber view with pulse-waved Doppler, applying the angle correction as appropriate to optimize LVO calculation.
The PDA diameter was measured from the high parasternal view at the point of maximum constriction, caring to avoid colour-Doppler interference outside the vessel wall. The transductal flow pattern was evaluated from the parasternal short axis using continuous-wave Doppler and, based on the ratio of end-diastolic to peak-systolic velocity, defined as pulsatile (≥0.5) or restrictive (<0.5).16 The left atrium-to-aortic root (LA:Ao) ratio was measured on M-Mode scans from the parasternal long-axis view. In the presence of a PDA, the flow velocity in the anterior cerebral artery (ACA) and in the descending aorta was measured using pulsed-wave Doppler from the median sagittal view and from the low subcostal sagittal view, respectively. Based on these echocardiographic features, the PDA was defined as haemodynamically significant (hsPDA) in the presence of pulsatile shunt pattern, LA:Ao ratio ≥1.5, PDA diameter ≥1.5 mm/kg and/or evidence of reversed end-diastolic flow in the descending aorta and/or ACA.17
Clinical and therapeutic data collection
For each infant, the following data were collected:
-
Antenatal and perinatal: corticosteroids administration (complete course vs. incomplete course or not given); gestational age (GA); Apgar score at 1 and 5 min; birth weight <10th percentile (small for gestational age (SGA)); Clinical Risk Index for Babies II (CRIB-II) score.18
-
Postnatal (0–72 h): daily MABP and haemoglobin; daily ductal status (hsPDA vs. restrictive or closed duct); daily ongoing respiratory support (invasive vs. non-invasive ventilation or self-ventilating in air); daily ongoing cardiovascular drugs (dopamine and/or dobutamine), related dosage and treatment duration; occurrence of prematurity-related complications (i.e. IVH or other brain lesions, sepsis, necrotizing enterocolitis, etc.).
Statistical analysis
Data distribution was evaluated using the Shapiro–Wilk test. Continuous variables were expressed as median (interquartile range [IQR]) or mean (standard deviation) as appropriate; categorical variables were summarized as frequencies and percentages.
Since TOHRx values followed a normal distribution, differences between day 1, 2 and 3 were evaluated using repeated-measures analysis of variance with Bonferroni adjustment for multiple comparisons.
The univariate effect on TOHRx values of GA, antenatal steroids administration, SGA, CRIB-II score, ductal and ventilatory status, ongoing dopamine or dobutamine and MABP was evaluated using repeated-measures linear mixed-effects models (LMM), which accounted for the trend of TOHRx values over time (day 1, day 2 and day 3) for each subject. The effects investigated were the time trend of TOHRx values regardless of the independent variables and the mean effect of the independent variables. The independent variables were chosen due to their known physiological effects on neonatal haemodynamics, autonomic or cerebrovascular reactivity.11,19,20,21 Those variables whose status changed over the study period (e.g. MABP, administration of cardiovascular drugs, ductal status, mode of respiratory support) were handled as time-dependent covariates; this means that the daily status of each of these variables was included in the LMM and therefore associated with the related daily-averaged TOHRx values.
Statistically significant variables from univariate analyses were included in a multiple repeated-measures LMM; the variance inflation factor (VIF) was used to assess multicollinearity between the model terms. A VIF <5 indicates a low correlation of that predictor with other predictors, a value between 5 and 10 indicates a moderate correlation, while VIF values >10 are a sign for high, not tolerable correlation of model predictors. Since no assumptions were made on within‐subject variability, the unstructured variance–covariance matrix was chosen to allow unequal variances and unequal correlations between response variables among time points for all patients. In LMMs, this matrix controls for the type 1 error rate for multiple comparisons, regardless of the true covariance structure.22 A sensitivity analysis excluding infants with a GA ≥ 28 weeks was also performed.
Due to the small size of the low- and high-grade IVH groups, the TOHRx distribution in relation to IVH severity on each day of life was evaluated using Kruskal–Wallis test, while median of differences between TOHRx values before and after IVH detection were compared within the IVH group using Wilcoxon signed-rank test.
Statistical analyses were performed using the SPSS software, version 26 (IBM Corp. Released 2019. IBM SPSS Statistics for Windows, Armonk, NY: IBM Corp). Significance level was set at p < 0.05.
Results
A total of 77 infants were included in the study (Fig. 2); the neonatal characteristics of this population are detailed in Table 1. During the study period, 16 out of 72 infants developed IVH, of which 11 were of low grade (grade I, n = 6; grade II, n = 5) and 5 were of high grade (grade III, n = 4; grade III with periventricular venous infarction, n = 1); the median age at IVH detection was 36 (IQR 24–50) hours. None of the infants died or developed sepsis or necrotizing enterocolitis during the first 72 h after birth.
Daily clinical and haemodynamic features of the study infants are shown in Table 2. Dobutamine was administered in the presence of hypotension (defined as a MABP < GA associated with any of the following: steady tachycardia, capillary refill time >3 s, decreased urine output or metabolic acidosis)23 and/or echocardiographic evidence of impaired cardiac contractility, whereas dopamine was commenced in hypotensive infants without evidence of impaired cardiac contractility or who did not respond to dobutamine and/or fluid expansion. The starting dosage of dopamine and dobutamine was 5 mcg/kg/min; two of the treated infants required higher dopamine or dobutamine doses (6 and 7 mcg/kg/min, respectively). Based on the therapeutic response, dopamine was tapered to 3 mcg/kg/min before discontinuation. No infant received other cardiovascular drugs or hydrocortisone during the study period.
Periods with artefactual or interrupted cTOI or HR signals that have been excluded from TOHRx computation were <10% of the total monitoring duration for all the infants included.
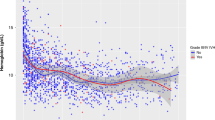
TOHRx significantly decreased over the first 72 h, with a mean difference of 0.093 (p < 0.001) between day 1 and day 3 (Fig. 3). This decreasing trend was confirmed significant even after the adjustment for time-dependent covariates at the multiple LMM (see Table 3).
Univariate analysis revealed significantly higher TOHRx values in the presence of a hsPDA (β = 0.098 [95% CI 0.062; 0.133], p < 0.001) compared to a restrictive or closed PDA, in invasively ventilated infants (β = 0.134 [95% CI 0.083; 0.185], p < 0.001) and during treatment with dopamine (β = 0.164 [95% CI 0.103; 0.225], p < 0.001) and dobutamine (β = 0.123 [95% CI 0.072; 0.175], p < 0.001). A significant, positive correlation between TOHRx and CRIB-II score (β = 0.017, [95% CI 0.012; 0.023], p < 0.001) was also observed, while an inverse correlation was noted with GA (weeks, β = −0.026 [95% CI −0.035; −0.016], p < 0.001) and MABP (mmHg, β = −0.010 [95% CI −0.012; −0.006], p < 0.001). No association was found between antenatal steroids, SGA and TOHRx.
The results of the multiple LMM, which included statistically significant variables from the univariate analyses, are detailed in Table 3. Given the high collinearity between CRIB-II score and GA, which itself is part of the score computation, this latter variable was excluded from the model; no multicollinearity issues were found among the other covariates since all VIF values were <5. Ongoing dopamine treatment (β = 0.096, p = 0.003) and the presence of a hsPDA (β = 0.043, p = 0.021) were independently associated with increased TOHRx values. The relationship between TOHRx and MABP (mmHg, β = −0.004, p = 0.047) and with CRIB-II score (β = 0.007, p = 0.044) were also confirmed significant.
Except for the correlation with MABP, all the above results were confirmed at the sensitivity analysis on extremely preterm infants (GA < 28 weeks, n = 34), which showed even stronger associations between increasing TOHRx, CRIB-II, hsPDA and dopamine treatment (see Supplementary Table).
Daily TOHRx values in relation to IVH development were also evaluated. As shown in Fig. 4, TOHRx values were significantly higher in high-grade IVH infants compared to controls on day 1 (Kruskal–Wallis’ H = 31.95, p = 0.005), day 2 (H = 31.33, p = 0.008) and day 3 (H = 31.54, p = 0.007). A significant difference between the low- and high-grade IVH groups, with higher TOHRx values in the latter group, was also observed on day 3 (H = 30.90, p = 0.031). In infants who developed IVH during the monitoring period, the median TOHRx values preceding IVH detection were significantly higher than those following IVH detection (Wilcoxon signed-rank test = 21.0, p = 0.015).
The central line in the boxplot is the median, the box margins are the 25th and 75th percentiles, and the whiskers represent the range. The black triangle indicates the median age at IVH detection. Significant pairwise comparisons are highlighted as follows: **p = 0.005; †p = 0.008; ‡p = 0.007; *p = 0.031.
Discussion
The results of this study support the feasibility and the potential of continuous non-invasive measurement of cerebrovascular reactivity in preterm infants in the first 72 h after birth and have identified a pool of antenatal, perinatal and postnatal exposures that are associated with an altered cerebrovascular reactivity and may play an important role in the development of several prematurity-related complications;24 the present study builds on this ‘exposome’ concept by evaluating the impact of specific factors and treatments on transitional cerebrovascular reactivity in preterm infants.
Based on the dependency of cardiac output upon HR, TOHRx provides a non-invasive estimation of cerebrovascular reactivity, with more positive values reflecting an increased correlation between HR and cTOI and as such suggesting defective mechanism of CBF stabilization. This parameter has been previously investigated in preterm neonates, showing increased values in infants with worse CRIB-II scores, during arterial hypotension or who developed IVH.10,11,25 TOHRx has also been used to define MABP levels at which cerebrovascular reactivity was most preserved (i.e. associated with the most negative TOHRx values) during the transitional period.10,12
We observed a significant TOHRx shift towards more negative values during the first 72 h of life, indicating a progressive improvement of cerebrovascular reactivity. This finding, which was confirmed after the adjustment for several clinical covariates and appeared even more marked at the sensitivity analysis performed on the extremely preterm subgroup, is consistent with previous evidence obtained using MABP–cTOI correlation indexes26 and may reflect the gradual haemodynamic stabilization that accompanies the completion of postnatal cardiovascular transition.
Our results have shown that the presence of a hsPDA was associated with a greater impairment of cerebrovascular reactivity. Notably, this association was even more significant when evaluated in the subgroup of infants <28 weeks’ gestation, which are at highest risk for hsPDA persistence and for the development of prematurity-related neurological complications. The haemodynamic consequences of a hsPDA are mainly due to the transductal diastolic run-off of systemic blood flow towards the lower pulmonary vascular resistance circuit. A substantial increase in cardiac output is subsequently required to guarantee adequate end-organ perfusion.27 The functional immaturity of the preterm myocardium, however, limits the ability to compensate for the hypoperfusion of major systemic vessels, including the cerebral arteries.28,29,30 Our finding suggests that the PDA-related haemodynamic disturbances may reduce cerebral perfusion pressure below the lower autoregulation limit, thus increasing the risk of cerebral pressure passivity.
Dopamine is often used in the management of hypotensive neonates; its vasopressor effects lie in a dose-dependent stimulation of the alpha-adrenergic, beta-adrenergic and dopaminergic receptors and in its serotoninergic actions.31 The role of dopamine on neonatal cerebral autoregulation is still controversial: while dysfunctional autoregulation had been previously reported in treated preterm infants,32,33,34 conflicting results have been observed in animal models,35 and recent data on small neonatal subgroups has not clarified this point further.36,37 We observed an independent positive association between ongoing dopamine treatment and TOHRx; although this finding requires further validation on larger samples and with higher titrations, it suggests that the vascular effects of dopamine may contribute to alter CBF regulation. Since the indication for dopamine treatment is hypotension itself, establishing the individual role of these two factors on cerebrovascular reactivity remains a challenging issue; in the present study, however, the impact of dopamine was confirmed even after the adjustment for daily-averaged MABP, which, in turn, showed an inverse, independent relationship with TOHRx. Since low blood pressure may cause a decrease in cerebral perfusion pressure below the autoregulation range, this finding reflects the expected physiology and is consistent with previous data based on continuous MABP monitoring,9 indicating that in preterm infants the baseline MABP may lie closer to the lower limit of cerebral autoregulation. It has been estimated that up to 20% of very-low-birth-weight infants experience phases of low blood pressure during the first 48 h of life, as a consequence of circulatory maladaptation.23 Given the inverse correlation between MABP and TOHRx, we speculate that the improvement in cerebrovascular reactivity may mirror the progressive MABP increase observed during this period.
CRIB-II score is a weighted average of GA, birth weight, base excess at birth and admission temperature.18 This score, which is a strong early predictor for the mortality risk in preterm infants, was positively associated with cerebrovascular reactivity at both univariate and multivariate analysis. Similar results, based either on TOHRx11 or on advanced cross-spectral analyses correlating MABP and cTOI,38,39 have been previously reported, suggesting that an increased cerebral pressure passivity may contribute to the poorer clinical outcomes associated with increasing scores.
The pathogenic potential of impaired cerebral autoregulation on IVH development has been previously investigated using different correlation indices, leading to heterogeneous results.3,6,12,25,26 In this study, we observed significantly increased TOHRx values in infants who developed high-grade IVH compared to no IVH throughout the whole transitional period. Moreover, within the IVH population, we also observed significantly higher TOHRx prior to the bleeding onset, consistently with previous data comparing IVH infants with time-matched controls.25 With the limitation of the small sample sizes of the IVH groups, these results are consistent with previous evidence supporting the association between impaired cerebrovascular reactivity and IVH pathophysiology.6,26,40 However, given the limited number of IVH infants and especially of those with severe IVH, the present results need further investigation on larger samples to better clarify the relationship between TOHRx, its clinical determinants and IVH and to investigate whether TOHRx changes in relation to IVH occurrence may differ upon IVH severity.
The unavailability of a simultaneous invasive blood pressure monitoring also needs to be acknowledged among the study limitations, as it would have allowed a comparison of the observed TOHRx values against indices of cerebral pressure-passivity derived from continuous MABP monitoring.8 Although arterial catheterization is the gold-standard technique for blood pressure measurement, it is not exempt from complications and a tendence towards less invasive approaches, including a decreased use of arterial lines, is being increasingly adopted in neonatal settings.41,42 Hence, one of the most important advantages in using TOHRx as a surrogate metric for cerebrovascular reactivity is that it can be determined in a strictly non-invasive manner and using largely available devices.
Daily TOHRx averages, matched with concomitant clinical data, were used for statistical analysis. The averaged values were calculated from several thousand data obtained over 24-h epochs, thus minimizing a possible ceiling effect. On the other hand, the adopted averaging periods may have smoothed the physiological variability of cerebrovascular reactivity. However, since TOHRx cut-off values to define an impaired reactivity have not been validated on large neonatal cohorts or against invasive autoregulatory indices, we chose to handle TOHRx as a continuous variable rather than adopting predefined thresholds to calculate the magnitude or the duration of the impairment.
It is possible that some of the conditions significantly associated with an increased TOHRx may primarily shift the HR baseline towards higher ranges (e.g. hsPDA) or decrease cTOI levels (e.g. mechanical ventilation), with possible effects on the observed results. However, TOHRx is a moving correlation coefficient between HR and cTOI and, as such, is not influenced by the absolute baseline values of these parameters but rather reflects the mutual direction of their changes.
Our data highlight the feasibility of non-invasive, continuous monitoring of cerebrovascular reactivity in the preterm population and indicate that lowered blood pressure, the presence of a hsPDA and dopamine treatment may be associated with an impairment of this mechanism during the transitional period. These findings support the potential of TOHRx as a marker for the haemodynamic relationship existing between systemic blood flow and CBF, consistently with the physiological rationale underlying this parameter.
The validation of TOHRx as a non-invasive marker of cerebrovascular reactivity in further larger trials and in specific high-risk conditions, such as extremely low GAs, may aid promoting its use to identify neonates at high neurological risk and to develop individualized neuroprotective strategies.
Data availability
The datasets analysed during the current study are available from the corresponding author upon reasonable request.
References
Noori, S., McCoy, M., Anderson, M. P., Ramji, F. & Seri, I. Changes in cardiac function and cerebral blood flow in relation to peri/intraventricular hemorrhage in extremely preterm infants. J. Pediatr. 164, 264.e1–270.e1 (2014).
Soul, J. S. et al. Fluctuating pressure-passivity is common in the cerebral circulation of sick premature infants. Pediatr. Res. 61, 467–473 (2007).
Sortica da Costa, C. et al. Changes in hemodynamics, cerebral oxygenation and cerebrovascular reactivity during the early transitional circulation in preterm infants. Pediatr. Res. 86, 247–253 (2019).
Panerai, R. B., Kelsall, A. W., Rennie, J. M. & Evans, D. H. Cerebral autoregulation dynamics in premature newborns. Stroke 26, 74–80 (1995).
Vesoulis, Z. A. & Mathur, A. M. Cerebral autoregulation, brain injury, and the transitioning premature infant. Front. Pediatr. 5, 64 (2017).
Alderliesten, T. et al. Cerebral oxygenation, extraction, and autoregulation in very preterm infants who develop peri-intraventricular hemorrhage. J. Pediatr. 162, 698.e2–704.e2 (2013).
O’Leary, H. et al. Elevated cerebral pressure passivity is associated with prematurity-related intracranial hemorrhage. Pediatrics 124, 302–309 (2009).
Thewissen, L. et al. Measuring near-infrared spectroscopy derived cerebral autoregulation in neonates: from research tool toward bedside multimodal monitoring. Front. Pediatr. 6, 117 (2018).
MM, G. et al. Relationship between cerebrovascular dysautoregulation and arterial blood pressure in the premature infant. J. Perinatol. 31, 722–729 (2011).
da Costa, C. S. et al. Monitoring of cerebrovascular reactivity for determination of optimal blood pressure in preterm infants. J. Pediatr. 167, 86–91 (2015).
Mitra, S. et al. Heart rate passivity of cerebral tissue oxygenation is associated with predictors of poor outcome in preterm infants. Acta Paediatr. 103, e374–e382 (2014).
da Costa, C. S., Czosnyka, M., Smielewski, P. & Austin, T. Optimal mean arterial blood pressure in extremely preterm infants within the first 24 h of life. J. Pediatr. 203, 242–248 (2018).
Smielewski, P. et al. ICM+: software for on-line analysis of bedside monitoring data after severe head trauma. Acta Neurochir. Suppl. 95, 43–49 (2005).
Volpe, J. J. Neurology of the Newborn 5th edn (Saunders Elsevier, 2008).
de Boode, W. P., Kluckow, M., McNamara, P. J. & Gupta, S. Role of neonatologist-performed echocardiography in the assessment and management of patent ductus arteriosus physiology in the newborn. Semin. Fetal Neonatal Med. 23, 292–297 (2018).
Jain, A. & Shah, P. S. Diagnosis, evaluation, and management of patent ductus arteriosus in preterm neonates. JAMA Pediatr. 169, 863–872 (2015).
van Laere, D. et al. Application of NPE in the assessment of a patent ductus arteriosus. Pediatr. Res. 84, 46–56 (2018).
Parry, G., Tucker, J. & Tarnow-Mordi, W. & UK Neonatal Staffing Study Collaborative Group. CRIB II: an update of the clinical risk index for babies score. Lancet 361, 1789–1791 (2003).
Rhee, C. J. et al. Neonatal cerebrovascular autoregulation. Pediatr. Res. 84, 602–610 (2018).
Wu, T. W., Azhibekov, T. & Seri, I. Transitional hemodynamics in preterm neonates: clinical relevance. Pediatr. Neonatol. 57, 7–18 (2016).
Weiss, S. J. & Niemann, S. Effects of antenatal corticosteroids on cortisol and heart rate reactivity of preterm infants. Biol. Res. Nurs. 17, 487–494 (2015).
Mallinckrodt, C. H., Kaiser, C. J., Watkin, J. G., Molenberghs, G. & Carroll, R. J. The effect of correlation structure on treatment contrasts estimated from incomplete clinical trial data with likelihood-based repeated measures compared with last observation carried forward Anova. Clin. Trials 1, 477–489 (2004).
Dasgupta, S. J. & Gill, A. B. Hypotension in the very low birthweight infant: the old, the new, and the uncertain. Arch. Dis. Child. Fetal Neonatal Ed. 88, F450–F454 (2003).
Nayeri, U. A., Buhimschi, C. S., Zhao, G., Buhimschi, I. A. & Bhandari, V. Components of the antepartum, intrapartum, and postpartum exposome impact on distinct short-term adverse neonatal outcomes of premature infants: a prospective cohort study. PLoS ONE 13, e0207298 (2018).
Cimatti, A. G. et al. Cerebral oxygenation and autoregulation in very preterm infants developing IVH during the transitional period: a pilot study. Front Pediatr. 8, 381 (2020).
Hoffman, S. B., Cheng, Y. J., Magder, L. S., Shet, N. & Viscardi, R. M. Cerebral autoregulation in premature infants during the first 96 h of life and relationship to adverse outcomes. Arch. Dis. Child. Fetal Neonatal Ed. 104, F473–F479 (2019).
Deshpande, P., Baczynski, M., McNamara, P. J. & Jain, A. Patent ductus arteriosus: the physiology of transition. Semin. Fetal Neonatal Med. 23, 225–231 (2018).
Baumgartner, S. et al. Left ventricular pumping during the transition-adaptation sequence in preterm infants: impact of the patent ductus arteriosus. Pediatr. Res. 83, 1016–1023 (2018).
Noori, S. & Seri, I. Hemodynamic antecedents of peri/intraventricular hemorrhage in very preterm neonates. Semin. Fetal Neonatal Med. 20, 232–237 (2015).
Hsu, K. H., Nguyen, J., Dekom, S., Ramanathan, R. & Noori, S. Effects of patent ductus arteriosus on organ blood flow in infants born very preterm: a prospective study with serial echocardiography. J. Pediatr. 216, 95.e2–100.e2 (2020).
Noori, S. & Seri, I. Neonatal blood pressure support: the use of inotropes, lusitropes, and other vasopressor agents. Clin. Perinatol. 39, 221–238 (2012).
Eriksen, V. R., Hahn, G. H. & Greisen, G. Dopamine therapy is associated with impaired cerebral autoregulation in preterm infants. Acta Paediatr. 103, 1221–1226 (2014).
Solanki, N. S. & Hoffman, S. B. Association between dopamine and cerebral autoregulation in preterm neonates. Pediatr. Res. 88, 618–622 (2020).
Chock, V. Y., Ramamoorthy, C. & Van Meurs, K. P. Cerebral autoregulation in neonates with a hemodynamically significant patent ductus arteriosus. J. Pediatr. 160, 936–942 (2012).
Eriksen, V. R., Rasmussen, M. B., Hahn, G. H. & Greisen, G. Dopamine therapy does not affect cerebral autoregulation during hypotension in newborn piglets. PLoS ONE 12, e0170738 (2017).
Thewissen, L. et al. Cerebral oxygen saturation and autoregulation during hypotension in extremely preterm infants. Pediatr. Res. 90, 373–380 (2021).
Dempsey, E. M. et al. Hypotension in preterm infants (HIP) randomised trial. Arch. Dis. Child. Fetal Neonatal Ed. 106, 398–403 (2021).
Wong, F. Y. et al. Impaired autoregulation in preterm infants identified by using spatially resolved spectroscopy. Pediatrics 121, e604–e611 (2008).
Zhang, Y. et al. Spectral analysis of systemic and cerebral cardiovascular variabilities in preterm infants: relationship with clinical risk index for babies (CRIB). Physiol. Meas. 32, 1913–1928 (2011).
Tsuji, M. et al. Cerebral intravascular oxygenation correlates with mean arterial pressure in critically ill premature infants. Pediatrics 106, 625–632 (2000).
Sekar, K. C. Iatrogenic complications in the neonatal intensive care unit. J. Perinatol. 30(Suppl), S51–S56 (2010).
Stoll, B. J. et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA 314, 1039–1051 (2015).
Acknowledgements
We gratefully thank Giulia Frabboni, Monica Gessaroli and Roberta Parladori for their assistance in data acquisition.
Funding
This study was supported by a research grant (Young Investigator START-UP Award 2019) from the European Society for Paediatric Research (ESPR), provided to S.M. in 2019.
Author information
Authors and Affiliations
Contributions
S.M., T.A. and L.C. designed the study. S.M. enrolled the patients and acquired the data. F.V. and F.C. contributed to data acquisition. Data analysis was performed by M.I. and S.M. M.C. and P.S. contributed to methodology, data acquisition and interpretation. T.A., L.C., S.G. and V.P. contributed to the interpretation of data. S.M. wrote the first draft of the manuscript. M.C., P.S., M.I., S.G., F.V., V.P., F.C., L.C. and T.A. critically revised the manuscript for important intellectual content. All the authors have approved the approved the final version of the manuscript submitted for publication and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Corresponding author
Ethics declarations
Competing interests
P.S. and M.C. have a financial interest in a fraction of the licensing fees for the software ICM+ (through Cambridge Enterprise Ltd, Cambridge, UK), used in this research project. The other authors have no conflict of interest to declare.
Ethics approval and consent to participate
The study protocol was approved by the S. Orsola University Hospital Ethics Committee, Bologna, Italy (protocol no. 328/2017/O/Oss), and written informed consent for study participation was obtained from the parents/legal guardians of each patient.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Martini, S., Czosnyka, M., Smielewski, P. et al. Clinical determinants of cerebrovascular reactivity in very preterm infants during the transitional period. Pediatr Res 92, 135–141 (2022). https://doi.org/10.1038/s41390-022-02090-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02090-z
This article is cited by
-
Near-infrared spectroscopy monitoring of neonatal cerebrovascular reactivity: where are we now?
Pediatric Research (2023)