Abstract
Background
Bilirubin is produced by the breakdown of hemoglobin and is normally catabolized and excreted. Neurotoxic accumulation of serum bilirubin often occurs in premature infants. The homozygous Gunn rat lacks uridine diphosphate glucuronosyltransferase 1A1 (UGT1A1), the enzyme needed to biotransform bilirubin. This rodent model of hyperbilirubinemia emulates many aspects of bilirubin toxicity observed in the human infant. We demonstrate that choline supplementation in early postnatal development is neuroprotective in the choline-restricted Gunn rat, when hyperbilirubinemia is induced on postnatal day 5.
Methods
We first compared behaviors and cerebellar weight of pups born to dams consuming regular rat chow to those of dams consuming choline-restricted diets. Second, we measured behaviors and cerebellar weights of pups born to choline-restricted dams, reared on a choline-restricted diet, supplemented with or without choline, and treated with or without sulfadimethoxine (SDMX).
Results
A choline-restricted diet did not change the behavioral outcomes, but cerebellar weight was reduced in the choline-restricted group regardless of genotype or SDMX administration. SDMX induced behavioral deficits in jj pups, and choline supplementation improved most behavioral effects and cerebellar weight in SDMX-treated jj rats.
Conclusions
These results suggest that choline may be used as a safe and effective neuroprotective intervention against hyperbilirubinemia in the choline-deficient premature infant.
Impact
-
This article investigates the effect of neonatal jaundice/bilirubin neurotoxicity on cerebellar-mediated behaviors.
-
This article explores the potential use of choline as an intervention capable of ameliorating the effect of bilirubin on the choline-restricted developing brain.
-
This article opens the door for future studies on the action of choline in the presence of hyperbilirubinemia, especially in preterm neonates.
Similar content being viewed by others
Introduction
Bilirubin is produced by the breakdown of hemoglobin, which is normally catabolized further and excreted. Accumulation of bilirubin in blood to concentrations associated with neurotoxicity occurs in the vast majority of preterm infants <35 weeks gestation.1,2 Premature infants are sensitive to bilirubin neurotoxicity in the first week of life even when levels of bilirubin are much lower than is known to be toxic for term infants.3,4 Unconjugated bilirubin, that is dissociated from albumin (free bilirubin (Bf)), can readily cross the barrier into the brain and accumulate there. The effects of bilirubin in the developing brain include disruption of lipid membranes, glutamate excitotoxicity, cell cycle arrest, and inflammation.5,6,7,8 The breadth of these mechanisms provides insight into the observed vulnerability of specific regions in the developing brain. Neurons of the globus pallidus exhibit high tonic rates of activity, rendering this region particularly vulnerable to glutamatergic excitotoxicity,9 while brain regions like the cerebellum may be at heightened risk because many of the principle cells, such as granule cells, migrate and proliferate in late gestation and early postnatal development.10 Granule cells in the cerebellum of premature infants can likely experience cell cycle arrest. Bf exhibits high affinity for lipid membranes and disrupts both cell membrane dynamics and the integrity of organelle membranes, compromising energy production, calcium buffering, and signal transduction.6
Drastic increases in cerebellar volume occur during the third trimester of gestation.11 Gestational weeks 20–40 in the human parallel the first postnatal week of cerebellar development in the rat, each undergoing robust granule cell proliferation and migration inward from the external granule cell layer at this time.10,12,13,14 Purkinje cells begin to form dendritic trees around postnatal day (P) 6 in the rat, and stabilization and refinement of contacts between Purkinje and granule cells continues into the third postnatal week.15,16 The prolonged development of the cerebellum renders it extremely vulnerable to perinatal insults.12 The sensitivity of the developing cerebellum to the neurotoxic effects of bilirubin has been demonstrated in the Gunn rat.17,18,19 Homozygous Gunn rats (jj) possess a genetic mutation that leads to the loss of the activity of uridine diphosphate glucuronosyltransferase 1A1 (UGT1A1), and as a consequence, concentrations of total serum bilirubin are elevated.20 This elevation peaks between P15 and P19,17,20 The Gunn rat cerebellum exhibits ectopic Purkinje cells, loss of volume in the granule and molecular layer, and loss of normal synapse development and refinement.5,18,21 UGT1A1 is only reduced in heterozygous littermates (Nj) (~50% of wild type), which does not lead to hyperbilirubinemia.22
Our laboratory recently began utilizing the Gunn rat, treating them on P5 with sulfadimethoxine (SDMX) to model preterm hyperbilirubinemia.19 SDMX displaces bilirubin from albumin, acutely increasing levels of Bf.17 The administration of SDMX on P5 did not elicit overt signs of bilirubin-induced toxicity but disrupted expression of ultrasonic vocalizations (USVs) and motor behavior at later ages.19
Bilirubin, like ethanol, is a lipophilic molecule with exposed hydrophilic regions; both have adverse effects on cell membrane dynamics.23,24,25 Choline has been shown to ameliorate the effects of ethanol exposure on cerebellar-mediated behavior in mice.26 Dietary choline can modulate the three known pathways of choline action in the brain: (1) phosphorylation to phosphocholine produces the precursor for phosphatidylcholine and sphingomyelin, key components of the plasma membrane27,28; (2) choline can be oxidized by choline dehydrogenase to betaine, a precursor to methionine and a 1-carbon donor for methylation29,30,31; and (3) finally, choline can be acetylated by choline acetyltransferase to acetylcholine, a major neurotransmitter in the forebrain and hippocampus.32
Here we report our findings on the impact of choline supplementation on the bilirubin-induced behavioral alterations exhibited by Gunn rats treated with SDMX on P5.19 To mimic the common human condition of choline deficiency,29,30,31 and to control for the amount of choline in the diets of the dams, we tested Nj and jj pups from dams placed on choline-restricted diets on gestational day 5 (G5). We first determined whether there are behavioral differences in behavior between the pups of dams who were on regular rat chow and those whose dams were on choline-restricted diets. Next, we determined the effect of choline supplementation for pups whose dams were on choline-restricted diets treated with SDMX compared to those treated with saline.
Methods
Gunn rats were obtained from the Rat Resource & Research Center and a breeding colony was established at the University of Maryland. All breeding and experimental procedures were approved by the University of Maryland Institutional Animal Care and Use Committee. Litters were generated by pairing jj males mated with Nj females. Females were checked daily for the presence of a mucus plug. The day a plug was observed was designated as G0. Dams were placed on a choline-restricted diet (518753, Dyets, Bethlehem, PA) on G5. On P1, pups were genotyped to determine Nj and jj status. Each pup was assigned to receive either saline or choline by block randomization. Pups were injected subcutaneously with either choline [100 mg/kg body weight, based on our previous work with ethanol26] or an equivalent volume of saline daily from P1 to P20. On P5, pups were further randomly assigned to be injected intraperitoneally with either SDMX (200 mg/kg) or an equivalent volume of saline. This resulted in four treatment groups for both Nj and jj pups: Saline from P1 to P20 and Saline on P5 (Saline/Saline), Saline from P1 to P20 and SDMX on P5 (Saline/SDMX), Choline from P1 to P20 and Saline on P5 (Choline/Saline), and Choline from P1 to P20 and SDMX on P5 (Choline/SDMX). Following weaning, pups were maintained on the choline-restricted diet until euthanized on P30.
Ultrasonic vocalization
On P6, each pup was briefly removed from the dam and put in the right corner of a test cage for a 5-min test session. A microphone capable of capturing ultrasonic frequencies was mounted 20 cm above the test cage, and output was recorded using Avisoft (Avisoft Bioacoustics, Berlin, Germany). Both the latency to emit the first vocalization and the total number of 40 kHz vocalizations were measured. Each pup was tested in three 5-min sessions, with a 20-min interval between tests. Pups were returned to the dam between tests. The average of the sessions was generated for analysis.
Negative geotaxis
On P10, pups were placed on a 45 degree inclined surface covered with mesh to permit traction. Pups were placed with their heads toward the lowest part of the incline. The time required to turn their head to face the higher part of the incline was recorded. Pups were tested three times with 20-min intervals between tests. Pups remained with the dam between tests. The average of the three tests was calculated for analysis.
Rotarod
Performance on an accelerating rotarod was assessed on P28–30. Pups were placed on a horizontal cylinder (Roto-Rod series 8, IITC Life Science Inc., CA) rotating at 5 rpm. The rotor steadily accelerated to 45 rpm over 100 s. The latency to fall was recorded for each daily testing session. The average time was calculated and used for analysis.
Cerebellar weights
On P30, pups were euthanized. The cerebellum was carefully dissected free from the brain stem and cerebrum and weighed.
Statistical analysis
We first sought to determine the effects of a defined, choline-restricted diet and a standard diet. To do this, we compared pups Nj and jj pups from our previous publication19 that were given a standard laboratory chow19 with pups whose dams were on a choline-restricted diet and not treated with choline from the current data set. Mixed factor analysis of variance (ANOVA) with Diet2 and Genotype2 and SDMX treatment2 as between-group factors were conducted for each dependent variable. Analyses were then conducted to determine the effects of choline supplementation in our model of preterm hyperbilirubinemia. ANOVA with Genotype2 and SDMX treatment2 and choline/saline treatment2 was conducted for each dependent variable: (1) number of USVs, (2) negative geotaxis performance; (3) rotarod performance; and (4) cerebellar weight.
Results
Preliminary analysis of each dependent variable confirmed the absence of sex differences, largest F (1,112) = 2.166. Therefore, we analyzed males and females together to simplify the experimental design. See Table 1 for the number of pups for each group.
Effect of choline-restricted diet
A main effect of diet was not detected on any behavioral measures (Fig. 1a–c), largest Fs <1. However, a main effect of diet was evident on cerebellar weight, F(1,84) = 24.58, p < 0.0001 (Fig. 1d). The main effect of genotype was significant for all measures, smallest F(1,84) = 52.25, p < 0.001, and the main effect of SDMX treatment was also significant for all measures, smallest F(1,84) = 20.26, p < 0.001. The diet by genotype interaction was significant for negative geotaxis, driven by a slightly different pattern across the two genotypes and treatment groups F(1,84) = 5.03, p < 0.02. A significant interaction of diet and genotype was also significant for cerebellar weight, F(1,84) = 11.18, p < 0.001, driven by the lack of an effect of diet in jj pups treated with SDMX, while the effect of the choline-restricted diet was evident in all other conditions. As expected, the interaction of genotype and SDMX treatment was significant for all measures, smallest F(1,84) = 15.46, p < 0.001. No other interactions were significant.
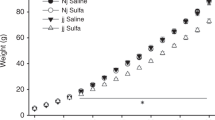
a Number of ultrasonic vocalizations measured on P6. b Negative geotaxis measured on P10. c Rotarod measured on P28–30. d Cerebellar weights measured on P30. Bars represent group means ± SEM. Choline-restricted diet was given from G5 to P20. Nj heterozygous Gunn rat pups, jj homozygous Gunn rat pups, SDMX sulfadimethoxine. Asterisk (*) indicates significant interaction between genotype and SDMX administration; gamma (γ) indicates significant interaction between genotype and diet; hash (#) indicates a main effect of diet.
Effect of choline supplementation
Ultrasonic vocalizations
A main effect of genotype was detected [F(1,120) = 154.52, p < 0.0001], as well as a main effect of SDMX [F(1,120) = 115.06, p < 0.0001], but not a main effect of choline [F < 1]. Treatment of jj pups with SDMX drastically reduced the number of USVs, and choline did not increase the number in jj Gunn rats (Fig. 2a). The genotype × SDMX interaction was significant [F(1,120) = 113.45, p < 0.0001], indicating that USVs were reduced only in jj GR rats treated with SDMX. Choline did not reverse the drastic reduction in vocalizations observed in jj rats induced by SDMX administration.
a Ultrasonic vocalization measured on P6. b Negative geotaxis measured on P10. c Rotarod measured on P28-–30. d Cerebellar weights measured on P30. Choline-restricted diet was given from G5 to P20, and choline was administered from P1 to 20. Bars represent group means ± SEM. Nj heterozygous Gunn rat pup, jj homozygous Gunn rat pups, SDMX sulfadimethoxine. Asterisk (*) indicates significant interaction between genotype and SDMX administration; hat (^) indicates significant three-way interaction between genotype, SDMX, and choline; hash (#) indicates significant difference between jj saline SDMX and jj saline choline.
Negative geotaxis
A significant main effect of genotype was detected [F(1,120) = 43.40, p < 0.0001], as well as a main effect of SDMX [F(1,120) = 32.68, p < 0.0001], and a main effect of choline [F(1,120) = 16.02, p < 0.0001]. The interaction of genotype × SDMX × choline was significant as well [F(1,120) = 14.92, p < 0.0001]. Significance is driven by the robust increase in time required to point the snout forward only in one genotype (i.e., jj pups) and only jj pups treated with SDMX. Choline reduced the observed increased latency to turn in jj pups treated with SDMX and did not change behavior in Nj pups (see Fig. 2b).
Rotarod
A main effect of genotype was confirmed by ANOVA [F(1,120) = 75.65, p < 0.0001], as well as a main effect of treatment with SDMX [F(1,120) = 36.56, p < 0.0001] and choline [F(1,120) = 7.15, p < 0.009]. The interaction of genotype × SDMX × choline was significant [F(1,120) = 13.284, p < 0.0001], indicating that SDMX and choline had effects unique to the jj rat. This is due to the different pattern of responses between Nj and jj rats to SDMX and choline. A lower latency to fall was observed only in the jj rat treated with SDMX, and only this group exhibited a change in latency when treated with choline (see Fig. 2c). Neither the SDMX nor choline treatments affected performance of the Nj pups.
Cerebellar weight
The main effect of genotype was again detected [F(1,120) = 10.85, p < 0.001], as well as a main effect of treatment with SDMX [F(1,120) = 9.84, p < 0.002] and choline [F(1,120) = 5.24, p < 0.024]. The genotype × SDMX interaction was significant [F(1,120) = 8.80, p < 0.004], indicating that SDMX affected cerebellar weight in only one genotype (i.e., jj pups, see Fig. 2d). Bonferroni post hoc analysis was used to determine the effect of choline in jj pups treated with SDMX. jj pups treated with saline and SDMX were different from all other groups, p < 0.001. Choline significantly increased cerebellar weight in jj pups treated with SDMX and were no different than any Nj group, p > 0.1.
Discussion
The major findings from this study are: (1) A choline-restricted alone diet did not exacerbate poor behavioral outcomes measured here in response to hyperbilirubinemia. Cerebellar weight was reduced in choline-restricted animals compared to pups of dams fed regular rat chow, demonstrating that choline restriction impedes cerebellar growth. Though behavior was largely unaffected, more sensitive tests might detect effects of a choline-restricted diet, as the entire developing brain is likely affected; (2) Choline supplementation had no effect on the Nj Gunn rat, further indicating that choline restriction did not impair behavior in the Nj; and (3) Choline supplementation improved most behavioral effects and increased cerebellar weight in jj rats treated with SDMX.
We consistently observe a reduction in the number of USVs within 24 h of SDMX in jj pups.19 Expression of 40 kHz vocalizations in pups separated from the dam peaks between P5 and P9, presumably to encourage maternal return to the nest. The number of USVs is sensitive to the pup’s physiological status.33 Cholinergic systems support normal expression of USVs in neonatal rat pups, and expression of USVs indicates distress in adult rats.34,35 Inflammation reduces USVs when induced at P5 despite no other observable signs of illness.36 Similarly, we do not observe other indices of illness or neurotoxicity in the pups at this timepoint. Choline supplementation did not restore the number of vocal emissions, however. Whether expression of USVs returns to normal is not known. It is possible that choline facilitates recovery at later timepoints, as with other behaviors.
Cholinergic systems play a trophic role in brain development and are directly modulated by choline administration.37,38 The mechanisms of choline-mediated neuroprotection are likely diverse, supporting structure and circuit function. In both typically developing rats and those exposed to perinatal insults, choline supplementation during sensitive developmental periods can improve cognitive function throughout the lifespan.38 We found that choline increased the latency to fall in SDMX-treated jj rats. Rotarod performance is particularly sensitive to perturbations of cerebellar development and injury.39,40 The cerebellum must interact with cortico-basal ganglia circuits for normal motor coordination and successful rotarod performance.41 Choline might protect brain function through different mechanisms in the cerebellum than in the basal ganglia. The basal ganglia are extremely sensitive to bilirubin toxicity.25 The primary input structure of the basal ganglia are cholinergic interneurons of the striatum, which synthesize and co-release acetylcholine and glutamate.42 As a precursor to acetylcholine, choline might improve the rapid feedback mechanisms under cholinergic direction and regulate striatal function and behavior.43 Further study of this potential mechanism is needed.
The developing brain requires unique cell membrane dynamics to support cell genesis and growth. Choline supports cell membrane integrity as a precursor for critical components of the plasma membrane.27,28 Lipid membrane microdomains, known as lipid rafts, directly mediate cerebellar granule cell neurite extension.24,44 Choline has been shown to protect lipid rafts from the adverse effects of ethanol exposure.23,45 Bilirubin can disrupt membrane dynamics and signal transduction as well.9 We have previously shown that ethanol, another neurotoxic lipophilic agent, alters the protein composition of lipid rafts, which are vital for cell signaling and many more processes. Obstructing a key internal messaging vehicle such as lipid rafts, in developing neurons, can be detrimental to the overall maturation of the brain. Ethanol has been shown to alter protein composition in lipid rafts, and choline was shown to ameliorate this effect.23,45 In addition, choline reduces the harmful effects of ethanol on behavior, which corroborates a relationship between choline and lipid rafts in the cerebellum.26 In this paper, we have demonstrated that bilirubin has similar effects as ethanol on cerebellar-mediated behavior and that choline ameliorates those effects on behavior. Two other mechanisms may underlie the effect of choline. One is that choline is a precursor to the methyl donor S-adenosyl methionine and may exert its effects through methylation of proteins, DNA, RNA, or lipids.30,31 The second mechanism may be that choline alters the biotransformation rate of bilirubin, essentially reducing the Bf in serum. Both of these mechanisms will be investigated in future studies.
One limitation to this study is that we did not measure the effect of choline supplementation on the behavior of pups whose dams were on normal diets, as opposed to choline-restricted diets. Our methodology is meant to mimic the common human condition of choline deficiency;29 however, we cannot conclude that the effect of choline supplementation would be the same in subjects with normal choline intake. It has been shown that the adequate intake of choline for very low birth weight (VLBW) infants is actually higher than for normal infants, and furthermore, <2% of VLBW infants receive the adequate amount of choline in their diet.46
Another limitation to this study is that we did not measure the total bilirubin or Bf in the blood or the brain. We also did not measure the time course of bilirubin concentrations, though it is known that, in the absence of SDMX, total serum bilirubin naturally peaks at approximately P16 in the jj Gunn rat, before the pup can biotransform the compound effectively.22 Regardless of the bilirubin concentration in either the brain or the blood, we observed significant motor dysfunction at much later timepoints following SDMX administration, without overt signs of distress at earlier timepoints. If the concentration of bilirubin in the brain or blood was higher than would be found in the neonatal intensive care unit, we still observe that choline provides significant amelioration of bilirubin’s detrimental effects, and does so safely. The dosage of choline used was based on our previous studies with ethanol; however, it is higher than is currently used for humans. The required dose in preterm infants is not clear. In one study, choline was supplemented to baseline choline intake at 30 mg/kg/day, and it was found to improve lipid homeostasis in human preterm infants.47 The dose of choline varies as a function of growth rate. As the growth rate in rats is higher than humans, higher choline doses may be required in rat pups than in humans.
We used the Gunn rat model to investigate the effects of preterm neonatal jaundice, though future studies might investigate the effects of choline supplementation on Gunn rats that are given SDMX on P16, the natural peak of bilirubin in the blood in the Gunn rat pup. Future studies are needed to measure the concentrations of both bilirubin and choline in the serum and correlate these with concentrations in the brain of experimental animals. This data should be verified clinically in the blood of newborns. Complicating the diagnosis and implementation of therapeutic intervention in premature infants is the fact that measuring levels of total bilirubin in the blood does not reliably predict the likelihood of bilirubin-induced neurotoxicity.3 Acute bilirubin neurotoxicity may occur without obvious clinical symptoms.2 Over time, the condition can progress to severe symptoms such as motor incoordination, learning disabilities, and balance disorders.48 Developing safe and effective treatments, and interventions that do not require excessively invasive procedures, is ideal, particularly in the context of the fragile neonate. Future studies should investigate the impact of bilirubin and choline on other vulnerable areas of the developing brain, such as the globus pallidus, brainstem auditory nuclei, and hippocampus, using physiological and biochemical experiments, with behavioral tests to corroborate. Developmental supplementation of choline may protect the nervous system from injury and promote long-term cognitive benefit in typically developing rodents.49 Our results strengthen the rationale for choline supplementation as a fundamental part of neonatal care.
References
van der Schoor, L. W. et al. Unconjugated free bilirubin in preterm infants. Early Hum. Dev. 106–107, 25–32 (2017).
Bhutani, V. K., Wong, R. J. & Stevenson, D. K. Hyperbilirubinemia in preterm neonates. Clin. Perinatol. 43, 215–232 (2016).
Amin, S. B. & Wang, H. Bilirubin albumin binding and unbound unconjugated hyperbilirubinemia in premature infants. J. Pediatr. 192, 47–52 (2018).
Watchko, J. F. & Jeffrey Maisels, M. The enigma of low bilirubin kernicterus in premature infants: why does it still occur, and is it preventable? Semin. Perinatol. 38, 397–406 (2014).
Lin, S. et al. Minocycline blocks bilirubin neurotoxicity and prevents hyperbilirubinemia-induced cerebellar hypoplasia in the Gunn rat. Eur. J. Neurosci. 22, 21–27 (2005).
Robert, M. C. et al. Alterations in the cell cycle in the cerebellum of hyperbilirubinemic Gunn rat: a possible link with apoptosis? PLoS ONE 8, e79073 (2013).
Rodrigues, C. M. P. et al. Perturbation of membrane dynamics in nerve cells as an early event during bilirubin-induced apoptosis. J. Lipid Res 43, 885–894 (2002).
Rodrigues, C. M. P., Solá, S., Brito, M. A., Brites, D. & Moura, J. J. G. Bilirubin directly disrupts membrane lipid polarity and fluidity, protein order, and redox status in rat mitochondria. J. Hepatol. 36, 335–341 (2002).
Watchko, J. F. Kernicterus and the molecular mechanisms of bilirubin-induced CNS injury in newborns. Neuromolecular Med. 8, 513–529 (2006).
Altman, J. Morphological development of rat cerebellum and some of its mechanisms. Exp. Brain Res. (Suppl. 6) 8–49 (1982).
Chang, C. H., Chang, F. M., Yu, C. H., Ko, H. C. & Chen, H. Y. Assessment of fetal cerebellar volume using three-dimensional ultrasound. Ultrasound Med. Biol. 26, 981–988 (2000).
Volpe, J. J. Cerebellum of the premature infant: rapidly developing, vulnerable, clinically important. J. Child Neurol. 24, 1085–1104 (2009).
Rakic, P. & Sidman, R. L. Histogenesis of cortical layers in human cerebellum, particularly the lamina dissecans. J. Comp. Neurol. 139, 473–500 (1970).
Sidman, R. L. & Rakic, P. Neuronal migration, with special reference to developing human brain: a review. Brain Res. 62, 1–35 (1973).
Hashimoto, K., Ichikawa, R., Kitamura, K., Watanabe, M. & Kano, M. Translocation of a “winner” climbing fiber to the Purkinje cell dendrite and subsequent elimination of “losers” from the soma in developing cerebellum. Neuron 63, 106–118 (2009).
Watanabe, M. & Kano, M. Climbing fiber synapse elimination in cerebellar Purkinje cells. Eur. J. Neurosci. 34, 1697–1710 (2011).
Cannon, C., Daood, M. J., O’Day, T. L. & Watchko, J. F. Sex-specific regional brain bilirubin content in hyperbilirubinemic Gunn rat pups. Biol. Neonate 90, 40–45 (2006).
Conlee, J. W. & Shapiro, S. M. Development of cerebellar hypoplasia in jaundiced Gunn rats: a quantitative light microscopic analysis. Acta Neuropathol. 93, 450–460 (1997).
Waddell, J., He, M., Tang, N., Rizzuto, C. & Bearer, C. F. A Gunn rat model of preterm hyperbilirubinemia. Pediatr. Res. 87, 480–484 (2020).
Johnson, L., Sarmiento, F., Blanc, W. A. & Day, R. Kernicterus in rats with an inherited deficiency of glucuronyl transferase. Am. J. Dis. Child. 97, 591–608 (1959).
Yamamura, H. & Takagishi, Y. Cerebellar hypoplasia in the hyperbilirubinemic Gunn rat: morphological aspects. Nagoya J. Med. Sci. 55, 11–21 (1993).
Schutta, H. S. & Johnson, L. Clinical signs and morphologic abnormalities in Gunn rats treated with sulfadimethoxine. J. Pediatr. 75, 1070–1079 (1969).
Davis, N. L., Tang, N., He, M., Lee, D. & Bearer, C. F. Choline ameliorates ethanol induced alterations in tyrosine phosphorylation and distribution in detergent-resistant membrane microdomains of L1 cell adhesion molecule in vivo. Birth Defects Res. 112, 480–489 (2020).
Tang, N. et al. Ethanol causes the redistribution of L1 cell adhesion molecule in lipid rafts. J. Neurochem. 119, 859–867 (2011).
Watchko, J. F. Bilirubin-induced neurotoxicity in the preterm neonate. Clin. Perinatol. 43, 297–311 (2016).
Bearer, C. F. F., Wellmann, K. A. A., Tang, N., He, M. & Mooney, S. M. M. Choline ameliorates deficits in balance caused by acute neonatal ethanol exposure. Cerebellum 14, 413–420 (2015).
Gallego-Ortega, D. et al. Differential role of human choline kinase alpha and beta enzymes in lipid metabolism: implications in cancer onset and treatment. PLoS ONE 4, e7819 (2009).
Wu, G. & Vance, D. E. Choline kinase and its function. Biochem. Cell Biol. 88, 559–564 (2010).
Zeisel, S. H. & da Costa, K. A. Choline: an essential nutrient for public health. Nutr. Rev. 67, 615–623 (2009).
Niculescu, M. D. & Zeisel, S. H. Diet, methyl donors and DNA methylation: interactions between dietary folate, methionine and choline. J. Nutr. 132, 2333S–2335S (2002).
Mehedint, M. G., Niculescu, M. D., Craciunescu, C. N. & Zeisel, S. H. Choline deficiency alters global histone methylation and epigenetic marking at the Re1 site of the calbindin 1 gene. FASEB J. 24, 184–195 (2010).
Zeisel, S. H. Nutritional genomics: defining the dietary requirement and effects of choline. J. Nutr. 141, 531–534 (2011).
Branchi, I., Santucci, D. & Alleva, E. Ultrasonic vocalisation emitted by infant rodents: a tool for assessment of neurobehavioural development. Behav. Brain Res. 125, 49–56 (2001).
Krüger, H. S., Brockmann, M. D., Salamon, J., Ittrich, H. & Hanganu-Opatz, I. L. Neonatal hippocampal lesion alters the functional maturation of the prefrontal cortex and the early cognitive development in pre-juvenile rats. Neurobiol. Learn. Mem. 97, 470–481 (2012).
Silkstone, M. & Brudzynski, S. M. Dissimilar interaction between dopaminergic and cholinergic systems in the initiation of emission of 50-kHz and 22-kHz vocalizations. Pharmacol. Biochem Behav. 188, 172815 (2020).
Nascimento, A. F. et al. Lipopolysaccharide-induced sickness behavior in lactating rats decreases Ultrasonic Vocalizations and exacerbates immune system activity in male offspring. Neuroimmunomodulation 22, 213–221 (2015).
Ulus, I. H., Wurtman, R. J., Mauron, C. & Blusztajn, J. K. Choline increases acetylcholine release and protects against the stimulation-induced decrease in phosphatide levels within membranes of rat corpus striatum. Brain Res. 484, 217–227 (1989).
Meck, W. H. & Williams, C. L. Characterization of the facilitative effects of perinatal choline supplementation on timing and temporal memory. Neuroreport 8, 2831–2835 (1997).
Goodlett, C. R., Thomas, J. D. & West, J. R. Long-term deficits in cerebellar growth and rotarod performance of rats following “binge-like” alcohol exposure during the neonatal brain growth spurt. Neurotoxicol. Teratol. 13, 69–74 (1991).
Hamm, R. J., Pike, B. R., O’dell, D. M., Lyeth, B. G. & Jenkins, L. W. The rotarod test: an evaluation of its effectiveness in assessing motor deficits following traumatic brain injury. J. Neurotrauma 11, 187–196 (1994).
Sakayori, N. et al. Motor skills mediated through cerebellothalamic tracts projecting to the central lateral nucleus. Mol. Brain 12, 13 (2019).
Soreq, H. Checks and balances on cholinergic signaling in brain and body function. Trends Neurosci. 38, 448–458 (2015).
Kljakic, O., Janickova, H., Prado, V. F. & Prado, M. A. M. Cholinergic/glutamatergic co-transmission in striatal cholinergic interneurons: new mechanisms regulating striatal computation. J. Neurochem. 142, 90–102 (2017).
Nakai, Y. & Kamiguchi, H. Migration of nerve growth cones requires detergent-resistant membranes in a spatially defined and substrate-dependent manner. J. Cell Biol. 159, 1097–1108 (2002).
Tang, N. et al. Choline partially prevents the impact of ethanol on the lipid raft dependent functions of l1 cell adhesion molecule. Alcohol Clin. Exp. Res. 38, 2722–2730 (2014).
Bernhard, W. et al. Choline supply of preterm infants: assessment of dietary intake and pathophysiological considerations. Eur. J. Nutr. 52, 1269–1278 (2013).
Bernhard, W. et al. Combined choline and DHA supplementation: a randomized controlled trial. Eur. J. Nutr. 59, 729–739 (2020).
Das, S. & van Landeghem, F. K. H. Clinicopathological spectrum of bilirubin encephalopathy/kernicterus. Diagnostics 9, 24 (2019).
Meck. W. H. & Williams, C. L. Metabolic imprinting of choline by its availability during gestation: implications for memory and attentional processing across the lifespan. Neurosci. Biobehav. Rev. 27, 385–399 (2003).
Acknowledgements
This work was supported by the NIH/NICHD R21HD085061.
Author information
Authors and Affiliations
Contributions
J.W.: substantial contributions to analysis and interpretation of data, drafting and revising article critically for important intellectual content, and final approval of the version to be published; N.C.R.: drafting the article or revising it critically for important intellectual content; M.H.: substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; N.T.: substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; C.F.B.: substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; and final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Waddell, J., Rickman, N.C., He, M. et al. Choline supplementation prevents the effects of bilirubin on cerebellar-mediated behavior in choline-restricted Gunn rat pups. Pediatr Res 89, 1414–1419 (2021). https://doi.org/10.1038/s41390-020-01187-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-01187-7
This article is cited by
-
Choline supplementation mitigates effects of bilirubin in cerebellar granule neurons in vitro
Pediatric Research (2024)
-
Models of bilirubin neurological damage: lessons learned and new challenges
Pediatric Research (2023)