Abstract
Background
Neonates with serum creatinine (SCr) rise ≥0.3 mg/dL and/or ≥50% SCr rise are more likely to die, even when controlling for confounders. These thresholds have not been tested in newborns. We hypothesized that different gestational age (GA) groups require different SCr thresholds.
Methods
Neonates in Assessment of Worldwide Acute Kidney Epidemiology in Neonates (AWAKEN) with ≥1 SCr on postnatal days 1–2 and ≥1 SCr on postnatal days 3–8 were assessed. We compared the mortality predictability of SCr absolute (≥0.3 mg/dL) vs percent (≥50%) rise. Next, we determine usefulness of combining absolute with percent rise. Finally, we determined the optimal absolute, percent, and maximum SCr thresholds that provide the highest mortality area under curve (AUC) and specificity for different GA groups.
Results
The ≥0.3 mg/dL rise outperformed ≥50% SCr rise. Addition of percent rise did not improve mortality predictability. The optimal SCr thresholds to predict AUC and specificity were ≥0.3 and ≥0.6 mg/dL for ≤29 weeks GA, and ≥0.1 and ≥0.3 mg/dL for >29 week GA. The maximum SCr value provides great specificity.
Conclusion
Unique SCr rise cutoffs for different GA improves outcome prediction. Percent SCr rise does not add value to the neonatal AKI definition.
Similar content being viewed by others
Introduction
Research on acute kidney injury (AKI) in critically ill neonates has lagged, relative to research in pediatric and adult populations. Recently, several small, single-center studies in select neonatal groups (congenital heart disease,1,2,3,4 sepsis,5 hypoxic injury,6,7,8,9 receiving extracorporeal membrane oxygenation,10,11,12 and very-low birthweight infants13,14,15,16,17,18,19) suggest that AKI is common and that neonates with AKI have higher mortality. We, the Neonatal Kidney Collaborative, recently published on the epidemiology of neonatal AKI (nAKI) using a 24-center retrospective cohort of 2022 infants across the gestational age (GA) spectrum in the Assessment of Worldwide Acute Kidney Epidemiology in Neonates (AWAKEN) database.20,21 Using the Kidney Disease: Improving Global Outcomes (KDIGO) AKI definition which defines AKI as a rise in SCr ≥0.3 mg/dl and/or a ≥50% rise in SCr from baseline, we found that 30% of the cohort had nAKI. As shown in older children and adults, nAKI was associated with higher mortality and longer length of stay (LOS), even after controlling for multiple confounders.
Despite finding a strong association between nAKI using the KDIGO definition with outcomes, this definition was adapted from an adult patient-driven definition and may not be optimal for use in neonates. In 2013, an NIH-NIDDK Neonatal AKI definition working group22 concluded that the KDIGO definition was the best currently available approach to define nAKI, as it allows for consistency across studies and neonatal populations. They strongly suggested that this definition (and other approaches) should be critically interrogated against meaningful clinical endpoints, in large neonatal cohorts. However, they identified several major potential problems in validity for using the KDIGO definition in neonates. One issue was that the definition does not consider the changes which occur normally in SCr concentrations in the first postnatal week of life. This trajectory varies by GA for, many reasons, including: infants SCr starts as maternal SCr,quantity and stage of nephron developmental. Given the variable physiologic trajectory of SCr across GA groups, the same change in SCr for ascertaining AKI in all neonates, may not be appropriate. Furthermore, the extent to which using the criterion of percent change in SCr in neonates adds to the use of an absolute change in SCr is unknown. Finally, the cutoffs which will achieve the most valid and parsimonious definition for nAKI need to be determined.
Tailoring a nAKI definition which addresses neonatal-specific physiology can only have a positive effect on a more accurate description of nAKI epidemiology, a better understanding of the risk factors associated with AKI, the ability to prognosticate outcomes and the ability to develop and implement specific therapies designed to improve AKI outcomes. Using data from our international AWAKEN database of patients admitted to the neonatal intensive care unit (NICU), we explored our hypothesis the SCr thresholds will differ by GA groups. To address this hypothesis, we (a) compared the performance of the KDIGO absolute and percent SCr rise thresholds to predict mortality for 3 GA groups, (b) determined the optimal absolute SCr rise, percent SCr rise, and maximum SCr thresholds to maximize AUC and specificity of mortality for each of 3 GA categories, (c) compared the performance of these newly derived SCr thresholds to predict mortality against each other and against the KDIGO definition, and (d) evaluated whether the addition of a percentage rise in SCr provides any added value to an absolute rise in SCr.
Methods
Patient population
Complete description of the formation of the NKC, and methods for developing the AWAKEN database have been published elsewhere.16 The University of Alabama at Birmingham Institutional Review Board (IRB) approved this collaborative study, and each center received approval from their respective IRBs. The study was registered at ClinicalTrials.gov NCT02443389.
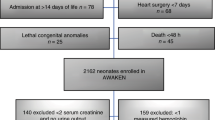
The main criteria for AWAKEN study inclusion was admission to NICU within the first 14 days of life and receipt of intravenous fluid for at least 2 days of hospitalization. Exclusion criteria included: (1) admission at ≥ 14 days of life, (2) congenital heart disease requiring surgical repair at < 7 days of life, (3) lethal chromosomal anomaly, (4) death within 48 h of NICU admission, and (5) severe congenital kidney and urinary tract abnormalities. For the current analysis, infants needed to meet 2 additional criteria: (a) infants had to have at least 1 SCr drawn on day 1 or 2 (day of birth = day 1) which needed to be at least 0.5 mg/dl, and (b) infants had to have at least 1 SCr drawn on days 3–8 (so that we could ascertain changes in SCr). Fig. 1 shows the reasons for exclusion of the AWAKEN cohort for this analysis. The analysis is performed on 3 GA groups, ≤ 29 weeks, 29–36 weeks, and ≥36 weeks. This GA classification was used in previous AWAKEN publications, aimed at highlighting differences across GA, while preserving sample size in the lowest GA group.
The KDIGO definition of nAKI
All clinically measured SCr values available from the medical record were used. As previously described, KDIGO defines AKI using a rise ≥0.3 mg/dL and/or by ≥50% in SCr from baseline SCr. For the purposes of this study, when describing the KDIGO methods, we needed to have only one baseline and one peak to define an absolute and a percent SCr rise. We used the lowest SCr on days 1 or 2 as the baseline SCr and the highest SCr on postnatal days 3–8 as the peak. The median number of SCr during days 1–2 was 2 (IQR = 2–3). The median number of SCr on days 3–7 was 3 (IQR = 2–5). Notably, we do not address how UOP cutoffs alone or in combination with SCr cutoffs are associated with mortality because there was a very large number of patients who did not have quantifiable UOP documented in the medical record.
Statistical analysis
Categorical variables were compared between groups using the Chi-square test or Fisher exact test (where appropriate). Continuous variables were tested for normality using the Shapiro–Wilk Test. For normally distributed continuous variables the mean ± standard deviation (SD) were reported and compared between groups using a Student t-test. For non-normally distributed variables, the median and interquartile range (IQR) were reported, and groups were compared using the Wilcoxon Signed Ranks test.
The primary outcome was hospital mortality. Nonparametric tests were used to compare the areas under the receiver operating curves as previously described by DeLong et.al.23 Comparing the differences between the specificity was performed using the McNemar’s test as previously described.24 SAS 9.4 (Cary, North Carolina) was used for all analyses.
Results
Patient population
First, we evaluated differences in demographics by GA groups (Table 1). Second, we compared demographics from a sensitivity analysis between subjects enrolled in AWAKEN that were included vs. subjects that were excluded based on additional criteria (Table 2). Third, we calculated KDIGO nAKI incidence and mortality rates separately for 3 different GA categories ( ≤ 29 weeks, 29–36 weeks, and ≥36 weeks). The sensitivity, specificity, negative predictive value (NPV), positive predictive value (PPV) for absolute, and percent SCr rise cutoffs to predict mortality were calculated. The positive likelihood ratio (LR + ), the negative likelihood ratio (LR−) and the receiver area under the curve (AUC) for predicting mortality were then calculated using the sensitivity and specificity values. We compared differences between absolute SCr rise (reference), percent SCr rise, and absolute and/or percent SCr rise for all three GA groups for the AUC and specificity to predict mortality (Table 3).
Fourth, we derived the optimal thresholds that would yield the highest possible AUC for mortality using the absolute SCr rise from baseline, the percent SCr rise from baseline, and the maximum SCr value during the first postnatal week for each of the three GA groups. Using these optimized thresholds we report the incidence, mortality, sensitivity, specificity, NPV, PPV, LR + , LR−, and AUC to predict mortality. We compared differences between absolute SCr rise (reference), percent SCr rise, and absolute and/or percent SCr rise and max SCr for all three GA groups for AUC (Table 4).
Fifth, we derived absolute SCr rise, percent SCr rise, and maximum SCr value thresholds that yield the highest specificity without significantly affecting the overall AUC for each of the three GA groups. Using these optimized thresholds we report the incidence, mortality, sensitivity, specificity, NPV, PPV, LR + , LR−, and AUC to predict mortality. We compare differences between the specificity to predict mortality using absolute SCr change (reference), percent SCr change, absolute and/or percent SCr rise, and Max SCr change for all 3 GA groups (Table 5)
Figure 1 shows that 990/2162 (45.8%) infant enrolled in AWAKEN were included in this study. Of the 1172 who were excluded, 423 did not have a SCr on postnatal days 1 or 2 and 847 did not have at least one SCr measured on days 3–8. The specific neonatal and maternal demographics, reasons for hospital admission, birth complications, and maternal medications and disposition are shown in Table 1 stratified by GA categories.
Differences between included and excluded subjects
Table 2 compares the differences in the AWAKEN subjects included vs. excluded in the current analysis. There were more infants ≤ 29 weeks and less infants in the ≥36 week GA group in the included patients. Mortality rates of the included group was higher than the excluded group [49/990 (5%) vs. 34/1172 (2.9%); p < 0.01). Compared to those excluded, included infants were more often Hispanic, born in-center, had lower Apgar scores, admission of prematurity, respiratory failure, HIE, omphalocele and born of a mother with pre-eclampsia/eclampsia, maternal steroids, anti-hypertensive, and steroids. Included infants were less likely large for GA, admitted for sepsis, hypoglycemia, hyperbillirubinemia, and necrotizing enterocolitis.
Counts and mortality rates by absolute and percent SCr rise thresholds
The mortality rate for the cohort was 49/990 (5.0%). The median (IQR) day of death was on postnatal day 21 (10, 47). The mortality rate for the ≤ 29 weeks GA was 14.1%; for the 29–36 week GA cohort was 2.8% and for the ≥ 36 week cohort was 3.4%. Fig. 2 shows the number of subjects attaining the absolute and percent change SCr threshold, and associated mortality rates. Approximately half (46%) the cohort had either no rise or a decrease in SCr from baseline; those subjects had a mortality rate of 1.3%. As expected, as the absolute or percent SCr rise thresholds increase, so do the mortality rates.
Performance of KDIGO thresholds for absolute SCr rise, percent SCr rise, and combination to predict mortality
Table 3 shows the performance of different components of the KDIGO thresholds for the 3 GA groups. p-values are shown for differences in specificity and AUC using the ≥0.3 mg/dl thresholds as reference. The absolute SCr rise threshold for KDIGO of ≥0.3 mg/dl provides excellent sensitivity (0.92) but poor specificity (0.5) to predict mortality in those ≤ 29 weeks. Alternatively, for the 29–36 week groups and the ≥36 weeks, the ≥0.3 mg/dl cutoff provided poor sensitivity but excellent specificity. Similar trends were seen when using the percent SCr rise threshold of ≥50%; but the overall the performance was worse than using absolute SCr rise threshold. When either one or both of the KDIGO criteria were explored (≥0.3 mg/dl or ≥50% percent rise), the performance did not improve, in fact, compared to the the specificity component for the 29–36 week groups and the ≥0.3 mg/dl alone, the specificity was worse for the 29–36 week and the ≥36 weeks group, and the AUC was significantly worse for the ≤ 29 weeks group.
Association of AUC-driven optimal thresholds with mortality, using absolute and percent SCr rise and maximal (max) SCr concentration
The optimal absolute SCr rise thresholds (which maximized AUC to predict mortality) were ≥ 0.3, ≥ 0.1, and ≥ 0.1 mg/dl rise in the three increasing GA groups, respectively; the optimal percent SCr thresholds were ≥ 37%, ≥ 14% and ≥ 8%, respectively; and the optimal max SCr thresholds were > 1.13, 0.98, and 0.91 mg/dl, respectively (Table 4). In the 29–36 weeks GA group only, the AUC to predict mortality was significantly higher using the optimal percent SCr rise compared to the optimal absolute SCr rise method (AUC = 0.73 vs. 0.75; p-value = 0.002, Table 4). There was no statistically significant difference AUC values of the optimal absolute SCr rise vs. the optimal max SCr value methods (all p > 0.05, Table 4). The AUC’s for predicting mortality were not significantly higher when using the optimal absolute and/or percent SCr rise methods vs. using only the optimal absolute SCr rise threshold method (Table 4), in any GA group. Rather, in the ≤ 29 weeks GA group, adding optimal percent SCr to the optimal absolute SCr rise method was associated with a significantly lower AUC vs. using only the optimal absolute SCr rise (AUC = 0.68 vs. 0.71; p = 0.004).
Association of specificity-driven optimal thresholds with mortality, using absolute and percent SCr rise and maximal (max) SCr concentration
The optimal absolute SCr rise thresholds (which maximized specificity for mortality) were ≥ 0.6, ≥ 0.3, and ≥ 0.3 mg/dl rise in the three increasing GA groups, respectively; the optimal percent SCr thresholds were ≥ 93, 66, and 40%, respectively; and the optimal max SCr thresholds were ≥ 1.59, 1.24, and 1.51 mg/dl, respectively (Table 5).
Compared to the absolute SCr rise threshold, the percent SCr threshold gave a lower specificity for the ≥ 36 week GA group (p < 0.001), and the combination of both the absolute and/or percent SCr group provided lower specificity for all the GA groups (all p < 0.001). The max SCr value gave a higher specificity for the ≤ 29 week cohort and the ≥36 week cohort (p < 0.001), but no significant difference was evident in the 29–36 week GA cohort.
Differences between KDIGO and optimal SCr thresholds
Several insights are made when comparing the KDIGO (Table 3) and the optimized absolute SCr rise thresholds (Tables 4 and 5). For the ≤ 29 weeks GA, the optimized absolute SCr rise to maximized AUC was identical to the cutoff used in KDIGO while the optimized absolute rise thresholds was lower for both the 29–36 week and ≥36 weeks GA groups. Alternatively, for the ≤ 29 weeks GA the optimized absolute SCr rise to maximized specificity was ≥0.6 mg/dl, while for the optimized absolute SCr rise to maximize specificity happen to be the same threshold used in KDIGO for both the 29–36 week and ≥36 weeks GA groups.
Comparing the KDIGO nAKI definition thresholds to determined optimal thresholds
Several insights are made when comparing the KDIGO (Table 3) and the optimized absolute SCr rise thresholds (Tables 4 and 5). In the ≤ 29 weeks GA group, the AUC-driven optimal absolute SCr rise was the same as the threshold used in the KDIGO definition (i.e., ≥0.3 mg/dl), and a higher threshold ( ≥ 0.6 mg/dl) was found to optimize the specificity. For the 29–36 week and ≥36 weeks GA groups, the specificity-driven optimal absolute SCr rise thresholds was the same as the KDIGO cutoff, while optimization of AUC required a lower SCr threshold of ≥ 0.1 mg/dl for both groups.
Discussion
The ability to diagnose AKI using SCr-based definitions during the first postnatal week is complex and challenging. Using a meaningful clinical outcome (mortality) we tested the hypothesis that the ideal cutoffs for SCr changes in the first week of life differ by GA groups. Indeed, we found that the optimal absolute and percent SCr rise cutoffs are higher in ≤ 29 week GA neonates. We found that overall, the absolute SCr change has better ability to predict mortality than percent SCr changes, and we did not find a major benefit to incorporate both a percent and absolute change. In fact incorporating the percent SCr change made the ability to maximize AUC and specificity worse, suggesting that the use of percent SCr rise adds little value and increases complexity. Interestingly, the max SCr outperformed was as good as a SCr rise at predicting mortality AUC, and was better at predicting mortality specificity in some of the cohort.
Despite its limitations, SCr changes continue to be used in neonatal cohorts as a metric to diagnose nAKI. We have previously shown that the KDIGO nAKI definition is independently associated with meaningful outcomes across the GA spectrum.21 Despite the use of by us and others to diagnose AKI in neonates, it is important to recognize that this empiric definition was adapted from the adult literature25 and has not been validated in neonates. In 2013, an expert working group from the NIH-sponsored Neonatal AKI Workshop recommended that researchers use the KDIGO definition to define nAKI because it would allow comparison between studies. However, this working group emphasized that in order to have true meaning, the KDIGO empiric definition or a novel nAKI definition would need to be tested against hard meaningful endpoints.22 Without this critical step, the field of nAKI could waste decades using an incorrect definition in describing the natural history of kidney disease and could lead to failed intervention nAKI studies.
The use of SCr to define nAKI is particularly challenging due to the obligate cardiovascular and renal adaptations during the transition into the extra-uterine environment. At birth, neonatal SCr reflects maternal SCr. Over the next days, the neonate establishes their own SCr steady-state which is primarily determined by innate kidney function. As kidney function varies greatly by GA, so will the SCr trajectories and steady-state levels.26 In this context, it may be normal for an extremely premature infant without nAKI to have a slight rise in SCr after birth. This was shown nicely in a study of extremely low birthweight infants that showed that 89% of infants born < 26 weeks GA had a rise in SCr ≥0.3 mg/dL.14 Alternatively, a healthy neonate with normal kidney function, should have a steady decline in SCr after birth. The absence of this normal trajectory could signify a substantial injury. This was recently shown in a study of term asphyxiated neonates by Gupta et.al which compared clinical parameters i.e., receipt of hemodynamic support) and kidney injury biomarkers between 3 groups of infants: (1) infants with nAKI (SCr ≥0.3 mg/dL), (2) infants whose SCr trajectories did not drop as expected but did not meet nAKI criteria, and (3) infants with normal SCr trajectories. They clearly showed that infants with abnormal SCr trajectories were more similar to those with nAKI than to those who had normal SCr trajectory. This suggests that term infants without the normal expected SCr decline may have a significant kidney injury.27 For these reasons, it would not be surprising that the nAKI thresholds differ by GA.
Our analysis only explores the SCr criteria to define nAKI during the first postnatal week. Similar studies to determine the optimal SCr cutoffs values after the first postnatal week will need to be performed. We do not explore the KDIGO UOP criteria’s ability to predict mortality. We do not explore which cutoffs are most predictive of mortality. Furthermore, determination of the SCr trends, the impact that fluid adjustment of SCr has on SCr cutoffs and combinations of SCr and UOP thresholds are not explored in this manuscript. We acknowledge that the associations we make between SCr thresholds and mortality do not infer a causal relationship, but instead allow for a better understanding of the associations between SCr changes and mortality. These limitations should be addressed in large multi-center studies that have adequate data for their analysis. The strengths of our study includes a large sample size of infants which allows us to test how different SCr metrics (absolute rise vs. percent rise vs. max value) predict mortality in different GA groups. Despite these strengths, we acknowledge several important limitations. The main limitation of the study is our ability to fully generalize across all infants. The main inclusion/exclusion criteria for AWAKEN was that NICU infants must have received intravenous fluids for at least 48 h. One of the main reasons for this decision was to capture infants that were more likely to have ample SCr values to decipher SCr-AKI status. Furthermore, in order to gain as much internal validity as possible for this analysis, we excluded infants who did not have at least one SCr in the first two postnatal days and at least one on postnatal days 3–8. These criteria selected infants that tended to be more ill (see Table 2). Studies that incorporate large cohorts with systematically collected SCr data on all NICU infants are needed to corroborate our findings and allow for generalizability to the entire NICU. We also acknowledge that although we chose patients who had at least 1 SCr in the early part and 1 in the later part of the week (and most had multiple values at both of these timepoints) not all infants had daily SCr values. In addition, choosing to delineate infants across only 3 GA could influence our findings, and we acknowledge that for example, infants who are 24 weeks GA could have different optimal SCr thresholds than infants born at 28 week GA. Finally, we did not adjust for fluid status, and it is possible that fluid balance could alter the SCr values and the performance of the changes in SCr,28 independent of kidney functional changes.
In conclusion, this study provides insights into on one of the most elusive, yet fundamental questions in neonatal nephrology—how to best delineate a significant change in kidney function during the first week of life. This analysis suggests that optimal SCr thresholds that predict meaningful outcomes differ across GA groups, that absolute SCr outperformed percent SCr rise, and that incorporation of both metrics does not provide much added value. Maximum SCr value can provide very good mortality specificity. In infants ≤29 weeks GA The absolute SCr threshold ≥0.3 mg/dL predicts mortality with great sensitivity (but bad specificity); alternatively, in infants ≥ 29 weeks GA, this threshold predicts mortality with high specificity (but poor sensitivity).
We caution investigators and researchers to change the KDIGO nAKI definition based on just this study. Instead, we encourage similar evaluation of other cohorts, at different timepoints. Studies designed to understand how UOP cutoffs, and fluid adjusted corrections can be used to better define neonatal AKI are greatly needed. Furthermore, a multi-center prospective studies that captures SCr and UOP systematically and evaluates other outcome metrics (i.e., CKD, and hypertension during childhood) is needed to corroborate and advance these findings. Similar studies on neonates that develop AKI after the first week of age are also greatly needed. Once these data is available, expert panels should be developed to cohesively make evidence-based recommendation on the most meaningful nAKI definition for clinicians and researchers to use. Finally, although the term “AKI” has gained wide acceptance to be synonymous with changes in SCr, patients can have functional changes in kidney function without true injury (i.e., dehydration), while others may have true tubular injury without a functional SCr change. Studies that also incorporate tubular injury biomarkers are needed to better understand how specific etiologies (acute tubular injury with an acute decrease in kidney function vs. acute decrease in kidney function without tubular injury) are greatly needed.
References
Wong, J. H. et al. Severe acute kidney injury following stage 1 Norwood palliation: effect on outcomes and risk of severe acute kidney injury at subsequent surgical stages. Pediatr. Crit. Care. Med. 17, 615–623 (2016).
Taylor, M. L. et al. Mild postoperative acute kidney injury and outcomes after surgery for congenital heart disease. J. Thorac. Cardiovasc. Surg. 146, 146–152 (2013).
dos Santos El Halal, M. G. & Carvalho, P. R. Acute kidney injury according to pediatric RIFLE criteria is associated with negative outcomes after heart surgery in children. Pediatr. Nephrol. 28, 1307–1314 (2013).
Aydin, S. I. et al. Acute kidney injury after surgery for congenital heart disease. Ann. Thorac. Surg. 94, 1589–1595 (2012).
Mathur, N. B., Agarwal, H. S. & Maria, A. Acute renal failure in neonatal sepsis. Indian J. Pediatr. 73, 499–502 (2006).
Sarkar, S. et al. Relationship between acute kidney injury and brain MRI findings in asphyxiated newborns after therapeutic hypothermia. Pediatr. Res. 75, 431–435 (2014).
Alaro, D., Bashir, A., Musoke, R. & Wanaiana, L. Prevalence and outcomes of acute kidney injury in term neonates with perinatal asphyxia. Afr. Health Sci. 14, 682–688 (2014).
Selewski, D. T., Jordan, B. K., Askenazi, D. J., Dechert, R. E. & Sarkar, S. Acute kidney injury in asphyxiated newborns treated with therapeutic hypothermia. J. Pediatr. 162, 725–729 e721 (2013).
Askenazi, D. J. et al. Fluid overload and mortality are associated with acute kidney injury in sick near-term/term neonate. Pediatr. Nephrol. 28, 661–666 (2013).
Fleming, G. M. et al. The incidence of acute kidney injury and its effect on neonatal and pediatric extracorporeal membrane oxygenation outcomes: a multicenter report from the kidney intervention during extracorporeal membrane oxygenation study group. Pediatr. Crit. Care. Med. 17, 1157–1169 (2016).
Gadepalli, S. K., Selewski, D. T., Drongowski, R. A. & Mychaliska, G. B. Acute kidney injury in congenital diaphragmatic hernia requiring extracorporeal life support: an insidious problem. J. Pediatr. Surg. 46, 630–635 (2011).
Askenazi, D. J. et al. Acute kidney injury and renal replacement therapy independently predict mortality in neonatal and pediatric noncardiac patients on extracorporeal membrane oxygenation. Pediatr. Crit. Care. Med. 12, e1–e6 (2011).
Askenazi, D. J., Griffin, R., McGwin, G., Carlo, W. & Ambalavanan, N. Acute kidney injury is independently associated with mortality in very low birthweight infants: a matched case-control analysis. Pediatr. Nephrol. 24, 991–997 (2009).
Carmody, J. B., Swanson, J. R., Rhone, E. T. & Charlton, J. R. Recognition and reporting of AKI in very low birth weight infants. Clin. J. Am. Soc. Nephrol. 9, 2036–2043 (2014).
Daga, A., Dapaah-Siakwan, F., Rajbhandari, S., Arevalo, C. & Salvador, A. Diagnosis and risk factors of acute kidney injury in very low birth weight infants. Pediatr. Neonatol. 58, 258–263 (2017).
Koralkar, R. et al. Acute kidney injury reduces survival in very low birth weight infants. Pediatr. Res. 69, 354–358 (2011).
Nagaraj, N., Berwal, P. K., Srinivas, A. & Berwal, A. A study of acute kidney injury in hospitalized preterm neonates in NICU. J. Neonatal Perinat. Med 9, 417–421 (2016).
Stojanovic, V., Barisic, N., Milanovic, B. & Doronjski, A. Acute kidney injury in preterm infants admitted to a neonatal intensive care unit. Pediatr. Nephrol. 29, 2213–2220 (2014).
Viswanathan, S., Manyam, B., Azhibekov, T. & Mhanna, M. J. Risk factors associated with acute kidney injury in extremely low birth weight (ELBW) infants. Pediatr. Nephrol. 27, 303–311 (2012).
Jetton, J. G. et al. Assessment of Worldwide Acute Kidney Injury Epidemiology in Neonates: design of a retrospective cohort study. Front. Pediatr. 4, 68 (2016).
Jetton, J. G. et al. Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet.: Child Adolesc. Health 1, 184–194 (2017).
Zappitelli, M. et al. Developing a neonatal acute kidney injury research definition: a report from the NIDDK neonatal AKI workshop. Pediatr. Res. 82, 569–573 (2017).
DeLong, E. R., DeLong, D. M. & Clarke-Pearson, D. L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44, 837–845 (1988).
Trajman, A. & Luiz, R. R. McNemar chi2 test revisited: comparing sensitivity and specificity of diagnostic examinations. Scand. J. Clin. Lab. Invest. 68, 77–80 (2008).
Kellum, J. A., . & Lameire, N. KDIGO AKI Guideline Work Group Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1).Crit. Care 17, 204 (2013).
Askenazi, D. J., Ambalavanan, N. & Goldstein, S. L. Acute kidney injury in critically ill newborns: what do we know? What do we need to learn? Pediatr. Nephrol. 24, 265–274 (2009).
Gupta, C., Massaro, A. N. & Ray, P. E. A new approach to define acute kidney injury in term newborns with hypoxic ischemic encephalopathy. Pediatr. Nephrol. 31, 1167–1178 (2016).
Askenazi, D., Saeidi, B., Koralkar, R., Ambalavanan, N. & Griffin, R. L. Acute changes in fluid status affect the incidence, associative clinical outcomes, and urine biomarker performance in premature infants with acute kidney injury. Pediatr. Nephrol. 31, 843–851 (2016).
Acknowledgements
The authors would like to thank Krysta Smith (Department of Pediatrics, University of Alabama at Birmingham) for help with technical editing and proofreading of this manuscript. The authors would also like to thank the outstanding work of the following clinical research personnel and colleagues for their involvement in AWAKEN: Ariana Aimani, Samantha Kronish, Ana Palijan, MD, Michael Pizzi—Montreal Children’s Hospital, McGill University Health Centre, Montreal, Quebec, Canada; Laila Ajour, BS, Julia Wrona, BS—University of Colorado, Children’s Hospital Colorado, Aurora, Colorado, USA; Melissa Bowman, RN—University of Rochester, Rochester, New York, USA; Teresa Cano, RN, Marta G. Galarza, MD, Wendy Glaberson, MD, Aura Arenas Morales, MD, Denisse Cristina Pareja Valarezo, MD—Holtz Children’s Hospital, University of Miami, Miami, Florida, USA; Sarah Cashman, BS, Madeleine Stead, BS—University of Iowa Children’s Hospital, Iowa City, Iowa, USA; Jonathan Davis, MD, Julie Nicoletta, MD—Floating Hospital for Children at Tufts Medical Center, Tufts University School of Medicine, Boston, Massachusetts, USA; Alanna DeMello—British Columbia Children’s Hospital, Vancouver, British Columbia, Canada; Lynn Dill, RN—University of Alabama at Birmingham, Birmingham, Alabama, USA; Ellen Guthrie, RN—MetroHealth Medical Center, Case Western Reserve University, Cleveland, Ohio, USA, Nicholas L. Harris, BS, Susan M. Hieber, MSQM—C.S. Mott Children’s Hospital, University of Michigan, Ann Arbor, Michigan, USA; Katherine Huang, Rosa Waters—University of Virginia Children’s Hospital, Charlottesville, Virginia, USA; Judd Jacobs, Ryan Knox, BS, Hilary Pitner, MS, Tara Terrell—Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio, USA; Nilima Jawale, MD—Maimonides Medical Center, Brooklyn, New York, USA; Emily Kane—Australian National University, Canberra, Australia; Vijay Kher, DM, Puneet Sodhi, MBBS—Medanta Kidney Institute, The Medicity Hospital, Gurgaon, Haryana, India; Grace Mele—New York College of Osteopathic Medicine, Westbury, New York, USA; Patricia Mele, DNP—Stony Brook Children’s Hospital, Stony Brook, New York, USA; Charity Njoku, Tennille Paulsen, Sadia Zubair—Texas Children’s Hospital, Baylor College of Medicine, Houston, Texas, USA; Emily Pao—University of Washington, Seattle Children’s Hospital, Seattle, Washington, USA; Becky Selman RN, Michele Spear, CCRC—University of New Mexico Health Sciences Center Albuquerque, New Mexico, USA; Melissa Vega, PA-C—The Children’s Hospital at Montefiore, Bronx, New York, USA); and Leslie Walther RN—Washington University, St. Louis, Missouri, USA.
Neonatal Kidney Collaborative Contributors:
The following individuals served as collaborators and site investigators for the AWAKEN study. They collaborated in protocol development and review, local IRB submission, data collection, and participated in drafting or review of the manuscript, and their names should be citable by PubMed.
Neonatal Kidney Collaborative
David T. Selewski, MD10, Subrata Sarkar, MD10, Alison Kent, MD11, Jeffery Fletcher, PhD11, Shahnaz Duara, MD2, Jennifer R. Charlton, MD12, Jonathan R. Swanson MD12, Ronnie Guillet, MD8, Carl D’Angio, MD8, Ayesa Mian, MD8, Deepak Kumar, MD4, Jennifer G. Jetton, MD13, Patrick D. Brophy, MD13, Tarah T. Colaizy, MD13, Jonathan M. Klein, MD13, Ayse Akcan Arikan, MD14, Christopher J. Rhee, MD14, Stuart L. Goldstein, MD15, Amy T. Nathan, MD15, Juan C. Kupferman, MD16, Alok Bhutada, MD16, Shantanu Rastogi, MD16, Elizabeth Bonachea, MD17, John Mahan, MD17, Alexandra Smith, MD18, Mamta Fuloria, MD19, Kimberly Reidy, MD19, Frederick J. Kaskel, MD19, Danielle E. Soranno, MD20, Jason Gien, MD20, Katja M. Gist, DO20, Aftab S. Chishti, MD21, Mina H. Hanna, MD21, Sangeeta Hingorani, MD22, Michelle Starr, MD22, Sunny Juul MD22, Craig S. Wong, MD23, Catherine Joseph, MD23, Tara DuPont, MD23, Robin Ohls, MD23, Amy Staples, MD23, Surender Khokhar, MD24, Mary Revenis, MD7, Sidharth K. Sethi, MD25, Smriri Rohatgi, MD25, Cherry Mammen, MD26, Anne Synnes, MDCM26, Sanjay Wazir, MD27, Pia Wintermark, MD28, Robert Woroniecki, MD29, Shanty Sridhar, MD29, Susan Ingraham, MD30, Arwa Nada, MD31
Funding
Cincinnati Children’s Hospital Center for Acute Care Nephrology provided funding to create and maintain the AWAKEN Medidata Rave electronic database. The Pediatric and Infant Center for Acute Nephrology (PICAN) provided support for web meetings, for the NKC steering committee annual meeting at the University of Alabama at Birmingham (UAB), as well as support for some of the AWAKEN investigators at UAB (L.B.J., R.J.G.). PICAN is part of the Department of Pediatrics at the University of Alabama at Birmingham (UAB), and is funded by Children’s of Alabama Hospital, the Department of Pediatrics, UAB School of Medicine, and UAB’s Center for Clinical and Translational Sciences (CCTS, NIH grant UL1TR001417). Finally, the AWAKEN study at the University of New Mexico was supported by the Clinical and Translational Science Center (CTSC, NIH grant UL1TR001449) and by the University of Iowa Institute for Clinical and Translational Science (U54TR001356). Funding sources for this study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Author information
Authors and Affiliations
Consortia
Contributions
D.A. contributed to the conceptualization and design of the study, collected data, aided the data analysis, and drafted the initial manuscript. C.A., M.M., and M.Z. contributed to the conceptualization and design of the study, collected data, aided the data analysis, and revised the manuscript for critically important intellectual content. L.B. and R.G. completed the data analysis and interpretation, contributed to the drafting, and revised the manuscript for critically important intellectual content. R.R., J.D., T.K.D., P.R., S.P., .M.D., L.M., N.A., F.S.C., and E.R. contributed to the study design, collecting or supervising the collection of data, and revising the manuscript for critically important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work
Corresponding author
Ethics declarations
Ethics Committee Approval
The University of Alabama at Birmingham Institutional Review Board (IRB) approved this collaborative study, and each center received approval from their respective IRBs.
Competing interests
All authors declare no real or perceived conflicts of interest that could affect the study design, collection, analysis and interpretation of data, writing of the report, or the decision to submit for publication. For full disclosure, we provide here an additional list of other author’s commitments and funding sources that are not directly related to this study: David J Askenazi serves on the speaker board for Baxter (Baxter, USA), and the Acute Kidney Injury (AKI) Foundation (Cincinnati, OH, USA); he also receives grant funding for studies not related to this manuscript from National Institutes of Health—National Institutes of Diabetes and Digestive and Kidney Diseases (NIH-NIDDK, R01 DK103608 and NIH-FDA, R01 FD005092). Jennifer G Jetton is supported by the University of Iowa Institute for Clinical and Translational Sciences NIH U54TR001356. Juan C. Kupferman is on the speaker’s Bureau and consultant for Alexion Pharmaceuticals. Robert Woroniecki is supported by the Department of Pediatrics at Stony Brook Children’s hospital (NY, USA). AWAKEN investigators at the Canberra Hospital were supported by the Canberra Hospital Private Practice fund, and investigators at University of Virginia Children’s Hospital were supported by a 100 Women Who Care Grant (100 Women Charitable Foundation, CA, USA).
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Askenazi, D., Abitbol, C., Boohaker, L. et al. Optimizing the AKI definition during first postnatal week using Assessment of Worldwide Acute Kidney Injury Epidemiology in Neonates (AWAKEN) cohort. Pediatr Res 85, 329–338 (2019). https://doi.org/10.1038/s41390-018-0249-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-018-0249-8
This article is cited by
-
Acute kidney injury during the first week of life: time for an update?
Pediatric Nephrology (2024)
-
Management of Acute Kidney Injury in Critically Ill Children
Indian Journal of Pediatrics (2023)
-
Clinical course of neonatal acute kidney injury: multi-center prospective cohort study
BMC Pediatrics (2022)
-
Improving the identification of acute kidney injury in the neonatal ICU: three centers’ experiences
Journal of Perinatology (2022)
-
Survival of infants treated with CKRT: comparing adapted adult platforms with the Carpediem™
Pediatric Nephrology (2022)