Abstract
Background
Acute kidney injury (AKI) in preterm neonates is associated with poor outcomes that may worsen in the setting of recurrent episodes of AKI. This study defines and studies the incidence, risk factors, and outcomes of recurrent AKI (rAKI).
Methods
Retrospective chart review of the neonates born at a gestational age of ≤28 weeks admitted to the neonatal intensive care unit (NICU) between January 2014 and December 2018. We identified AKI based on the serum creatinine (Scr) concentrations using the Kidney Disease: Improving Global Outcomes (KDIGO) criteria. rAKI was defined as the occurrence of AKI after Scr from the prior AKI had returned to baseline.
Results
Forty-nine of the 205 (24%) preterm neonates developed rAKI. An earlier diagnosis (<7 days old) and a higher KDIGO stage (stage 3) at the initial episode of AKI was associated with rAKI (p = 0.03). Preterm neonates with rAKI had higher mortality as compared to those with a single episode of AKI (sAKI) (adjusted odds ratio (aOR) 4.55, 95% confidence interval (CI), 1.12–18.51). Length of stay (LOS) was longer among neonates with rAKI as compared to those with sAKI by 36 days (95% CI 24.9–47.1).
Conclusions
Recurrent AKI in preterm neonates was associated with earlier episodes and higher KDIGO stage of the initial AKI episode. Neonates with rAKI had higher mortality and longer LOS compared to those with sAKI.
Impact
-
Definition and study of the incidence of rAKI and its associated outcomes among preterm neonates.
-
Recurrent AKI is common among preterm neonates and may contribute to worse outcomes for premature neonates in the NICU.
-
Early recognition of the risk factors for AKI, and effective management of initial AKI and early phase of recurrent AKI may improve outcomes of these preterm neonates.
Similar content being viewed by others
Introduction
Acute kidney injury (AKI) is common in the neonatal intensive care unit (NICU) and has been shown to be an independent predictor of poor short- and long-term outcomes.1,2 In a large multicenter cohort study, ~30% of all sick neonates admitted to NICU developed AKI, with a higher occurrence observed in neonates ≤29 weeks gestational age (GA).3 Moreover, neonates are predisposed to recurrent episodes of AKI. In a single-center study of very low birth weight neonates (VLBW), 17% had more than one episode of AKI.4 Although recurrent AKI (rAKI) has been mentioned in a few studies, none have defined the parameters that define rAKI.4,5,6
Neonates at risk of AKI may also be at risk of rAKI, especially if the risk factors associated with initial AKI persist. Common persistent and repeated risk factors related to a single episode of AKI (sAKI) in preterm neonates during their NICU stay include the need for intubation, surgical interventions, exposure to diuretics, vasopressors and nonsteroidal anti-inflammatory drug (NSAIDs), presence of patent ductus arteriosus (PDA), necrotizing enterocolitis (NEC), intraventricular hemorrhage (IVH), bronchopulmonary dysplasia (BPD), neonatal hypertension, and fluid imbalance.5,7,8,9,10,11,12,13,14 To address these gaps in knowledge, this study aims to propose a definition of rAKI and to assess its incidence, risk factors, and outcomes among preterm neonates who developed rAKI. We hypothesized that the rAKI is common among preterm neonates and is associated with worse outcomes as compared to neonates with an sAKI.
Methods
Study population
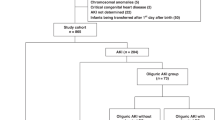
This retrospective cohort study was conducted at the Maimonides Medical Center NICU and included neonates born at ≤28 weeks GA admitted between January 2014 and December 2018. The study was approved by the Institutional Review Board. We excluded all preterm neonates who died within 48 h of life, those with congenital heart disease requiring surgical repair at <7 days of life, severe congenital kidney abnormalities, and neonates transferred from outside hospitals for further care.
Data collection
The clinical data were obtained from Neodata (Isoprime Corp., Lisle, IL). We ran a query to extract medical record numbers of preterm neonates that met the criteria and conducted data abstraction and data management with the Microsoft Access database. Data were recorded from birth to the end of the NICU stay. Data collection was validated by a second abstracter before it was submitted for analysis.
Definition of AKI
Serum creatinine (Scr) for all study preterm neonates was monitored until discharge. Among preterm neonates born at ≤28 weeks GA, routine care in our NICU included measuring Scr every 24 h for the first week, 2–3 times per week while the neonate was on parenteral nutrition, and once a week once the neonate was on full enteral feeds. AKI was defined using the modified Kidney Disease: Improving Global Outcomes (KDIGO) criteria3 as summarized in Table 1. While Scr decreases after birth in most neonates, a few extremely preterm neonates may have an increase in Scr soon after birth. We addressed this in our definition and classified AKI only when Scr increased after a downward trend. Urine output was not included in the definition because of the inaccuracy in its measurement and variations associated with fluid balance in the preterm neonates.
Definition of rAKI
rAKI was defined using the modified KDIGO criteria when AKI occurred after Scr value had returned with a minimum reduction of ≥0.3 mg/dl from peak Scr value towards baseline from initial episode of AKI. The baseline was the lowest Scr before which initial AKI was diagnosed. Recurrent AKI was further classified into complete and incomplete based on the return to the baseline value of the Scr before the initial AKI. Complete rAKI was defined as ≥75% return to the baseline Scr value and incomplete rAKI as 25−74% return to the baseline of SCr before it increased again to be defined as a new episode of AKI. Preterm neonates with <25% return to baseline were classified as having a continuation of the initial episode of AKI and grouped with an sAKI. For example, for a baseline Scr of 0.8 mg/dl that increased to 1.6 mg/dl during the first episode of AKI, if it decreased by ≥0.6 mg/dl, which is ≥75% of the increase (0.8 mg/dl) from baseline, and then increased again, it would be classified as a complete rAKI. However, if it decreased by >0.3–0.6 mg/dl (>25–74% of the increase (0.8 mg/dl) with a minimum decrease of 0.3 mg/dl) and then increased again, it would be classified as an incomplete rAKI. If the decrease in Scr was 0.2 mg/dl (<25% of the increase (0.8 mg/dl) prior to an increase, then it would be classified as sAKI. This classification was necessary to assess for possible relationships among the subgroups of rAKI and their outcomes and to distinguish a real AKI recurrence when the return to baseline of Scr is 25% or more before another episode of AKI from the continuation of the initial AKI when the return to baseline of Scr is <25%.
Clinical characteristics and outcomes
The clinical characteristics and outcomes that were studied included GA, birth weight (BW), multiple births, ethnicity, sepsis (culture positive and/or antibiotics use for more than 48 h), hemodynamically significant PDA (diagnosed by echocardiographic examination), IVH (any grade diagnosed by head ultrasound read by a pediatric radiologist), retinopathy of prematurity (ROP) (any stage diagnosed by a pediatric ophthalmologist), BPD (requirement of positive end-expiratory pressure or oxygen for >36 weeks of corrected GA), NEC (diagnosed by the presence of pneumatosis intestinalis or free air in the abdomen as diagnosed by a pediatric radiologist), and need for mechanical ventilation, defined as a requirement of ventilation more than continuous positive airway pressure, including noninvasive and invasive mechanical ventilation and oscillator ventilation. Growth failure was defined as a decrease in weight of more than 2 standard deviations (SDs) from BW to discharge weight. Length of stay (LOS) was measured as days from admission to the NICU until discharge. We classified antibiotic use as gentamicin administration for >48 h (as almost all neonates of <28 weeks GA received gentamicin for 48 h) and any administration of vancomycin, since these were the two most used nephrotoxic antibiotics used in the NICU. Vasopressor use was classified as any administration of dopamine, dobutamine, and epinephrine, and diuretic use as any administration of furosemide or hydrochlorothiazide. We did not study the use of caffeine because it was administered to all the neonates in the study since it is routinely started in neonates 28 weeks GA within 48 h of birth.
Data analysis
A sample size of 205 participants provided sufficient power (>80%) to determine the estimated difference of 25% in odds of the primary outcome of mortality. Summary statistics were used to report proportions of key clinical characteristics and outcomes categorized by AKI subgroups. For continuous variables that satisfied the Shapiro–Wilk test for normality, summary statistics were reported using means and SDs, while for variables that were not normally distributed, we employed medians and interquartile ranges for descriptive statistics. We conducted χ2 test or Fisher’s exact test for comparison of proportions. For the test of associations, we utilized the Student’s t test, Wilcoxon’s rank-sum test, Kruskal–Wallis analysis of variance (ANOVA), and ANOVA as indicated. Binary outcomes, such as mortality and growth failure, were reported using crude odds ratio (OR) and 95% confidence intervals (CIs) calculated through logistic regression. Linear regression models were run for crude parameter estimates for continuous variables, such as LOS with associated 95% CI reported. To control for potential confounders, we ran robust multivariable logistic and linear regression models for each outcome, reporting adjusted ORs and parameter estimates with 95% CIs. STATA v15 (Stata Corp LLC, College Station, TX) was used for all analyses.
Results
Patient characteristics
Two hundred and twenty-five preterm neonates admitted to the NICU during the study period met the inclusion criteria. Of these, 20 neonates were excluded from the study. (Fig. 1). Of the remaining 205 neonates included in the study, 106 (52%) were males. African Americans and Asians were the most common races, each accounting for 23% of the preterm neonates. Mean BW for the entire cohort was 890 ± 251 g, with extremely low BW (<1000 g) preterm neonates accounting for 138 (65%) of participants and 179 (87%) being appropriate for GA. Most (69%, n = 139) were delivered by cesarean section and 91% (n = 187) receiving antenatal steroids. Table 2 summarizes the patient characteristics of neonates classified into no AKI (nAKI), sAKI, and rAKI groups.
sAKI and rAKI: incidence and contributing factors
Fifty-eight percent (n = 116) of preterm neonates had at least one episode of AKI during their stay in the NICU. Of these, 42% (n = 49) had more than one episode of AKI or rAKI. Of these preterm neonates with rAKI, 34 (69.4%) had ≤3 episodes, 9 (18.4%) had 4–6 episodes, and 6 (12.2%) had ≥7 episodes of AKI with a median number of complete rAKI episodes of 2 and of incomplete rAKI of 3. Of the 42% with rAKI, 57% (n = 28) had complete rAKI (return of Scr to ≥75% of the Scr baseline preceding the initial AKI event), while 43% (n = 21) had incomplete rAKI (return of Scr to ≥25–74% of the Scr baseline preceding the initial AKI event) (Fig. 1).
Recurrent AKI was associated with smaller GA, lower BW, and lower APGAR at 1 min, corresponding to neonates who were more likely to require resuscitation. Recurrent AKI was also associated with more days on mechanical ventilation and TPN use, and increased use of pressors, diuretics, and potentially nephrotoxic medication like vancomycin and gentamicin (Tables 2 and 3).
Relationship between the initial episode of AKI and rAKI
There was a higher incidence of rAKI among preterm neonates who had developed AKI early (<7 days of life) and those who had more severe AKI (stage 3 AKI) (Table 3).
Comparison of outcomes associated with sAKI and rAKI with nAKI (Table 4)
Preterm neonates with sAKI had higher odds of death and NEC as compared to those with nAKI (Table 4). After multivariable analysis, the odds of NEC (OR 2.42, 95% CI 1.07–5.49) remained higher in preterm neonates with sAKI. rAKI was associated with a higher incidence of all the studied adverse outcomes. After multivariable analysis, the odds of death (OR 8.34, 95% CI 1.89–36.87), NEC (OR 4.99, 95% CI 2.08–11.93), sepsis (OR 14.04, 95% CI 3.05–64.73), and growth failure (OR 3.18, 95% CI 1.12–8.98) remained higher among neonates with rAKI as compared to nAKI. LOS was also longer among neonates with rAKI as compared to those without AKI (43 days, 95% CI 31.9–54.1 days).
Moreover, we observed a graded increase in mortality when AKI was stratified as sAKI, incomplete rAKI, and complete rAKI. Compared to preterm neonates without AKI, neonates with complete rAKI had the highest odds of death (aOR 8.66, 95% CI 1.68–44.66), followed by preterm neonates with incomplete rAKI (aOR 7.86, 95% CI 1.23–50.11), followed by preterm neonates with sAKI (aOR 1.83, 95% CI 0.47–7.09), which did not differ statistically from neonates without AKI.
Comparison of outcomes between sAKI and rAKI (Table 5)
As we observed differences in clinical outcomes between the sAKI and rAKI when compared to nAKI, we further compared the outcomes between sAKI and all rAKI, incomplete and complete rAKI (Table 5). Recurrent AKI had higher adjusted odds of all adverse outcomes except growth failure as compared to sAKI. When compared to sAKI, neonates with complete rAKI as well as incomplete rAKI had higher odds of adverse outcomes including LOS, BPD, and PVL. Those with complete rAKI also had higher odds of death, grade 3 and 4 IVH, sepsis, and growth failure, while those with incomplete rAKI had higher odds of NEC as compared to sAKI. Odds of death did not differ between incomplete rAKI and sAKI.
Discussion
We found that rAKI is common among preterm neonates and occurs in a large proportion of those who develop AKI. Recurrent AKI occurred more among preterm neonates who were on longer time on mechanical ventilation and on total parenteral nutrition had higher use of diuretics, vasopressors, and nephrotoxic antibiotics. The risk of rAKI was highest among neonates that had an early and more severe episode of initial AKI. Preterm neonates with rAKI have higher mortality and longer LOS compared to neonates with nAKI or those with sAKI. Preterm neonates with rAKI also had higher odds of comorbidities, including BPD, NEC, IVH, and sepsis as compared to those with nAKI and sAKI.
Our findings validate a prior single-center study4 and confirm that the occurrence of rAKI is common among VLBW neonates. In addition, our study proposes a definition for rAKI and supports its validity by demonstrating its association with worse outcomes as compared to neonates with nAKI and sAKI, a detail that was not explored in the earlier study.4
Factors that have been previously identified (3) as risk factors for AKI were found to be significant contributors to rAKI as well as early-onset (<7 days of life) and severe (KDIGO stage 3) initial episodes of AKI in preterm neonates in our study. Continuation or repeated occurrence of an initial or a new insult associated with AKI may result in rAKI, which is of particular importance in extremely preterm neonates as their 12–16 weeks long stay in the NICU makes them more susceptible to rAKI as compared to neonates hospitalized for other reasons. Therefore, studies looking at aggressive efforts to mitigate the occurrence of new insults as well as initial AKI episodes in the NICU are needed to understand how this may reduce the burden of rAKI and associated outcomes, as exemplified by the careful use of nephrotoxic medications as described in the nephrotoxic injury negated by just-in-time action (NINJA) study, which may contribute to the reduction in rAKI after an initial episode of AKI.15
Approximately 60% of nephrons are formed between 20 and 36 weeks GA.16,17 Given the nascent stage of nephrogenesis in very premature neonates, a sAKI may be a risk factor for rAKI. Furthermore, continuation or repeated occurrence of an initial or a new insult associated with AKI may result in rAKI. This is of particular importance in extremely preterm neonates because their stay in the NICU can be 12–16 weeks long. Although AKI has been described among neonates with Congenital Anomalies of Kidneys and Urinary Tract (CAKUT), because these anomalies are associated with a lower number of nephrons,6 by excluding preterm neonates with CAKUT, we highlight that rAKI can occur in preterm neonates without any kidney malformations. Our observations suggest that the factors that underlie rAKI in neonates with CAKUT, such as perinatal asphyxia, maternal obstetric disease, prematurity, low BW, length of ventilation days, and sepsis6 are also important for rAKI among preterm neonates without CAKUT. The inclusion of an objective definition of rAKI in our study also addresses this limitation of the prior study that linked rAKI with CAKUT.6
Recurrent AKI was associated with increased odds of mortality and LOS compared to sAKI and those with nAKI. In our cohort, neonates with rAKI stayed on an average a little more than 5 weeks longer in the NICU as compared to neonates with sAKI, validating the findings by Carmody et al., who reported longer hospital stay and higher mortality among VLBW neonates with rAKI as compared to one episode of AKI.4 This longer LOS is relevant for many reasons, including the higher cost of care, higher risk for nosocomial infections, and a longer period of separation from parents. While more studies are needed to elucidate the long-term effects of a longer NICU stay on neurodevelopmental outcomes, our observed higher incidence of adverse outcomes, such as BPD, NEC, IVH, and sepsis in rAKI compared to sAKI, may have also contributed to the poor longer-term effects on the preterm neonates, including poor neurodevelopmental outcomes.
Our study further examined the new definition of rAKI, which is based on the extent to which Scr returns to baseline after the initial AKI. We used the extent of return to the baseline to define complete recovery, incomplete recovery, and continuation of the initial AKI episode. Neonates with complete rAKI had an increased odds of adverse outcomes including increased LOS and higher mortality compared to those without AKI and those with sAKI. Neonates with incomplete rAKI also had longer LOS, but their mortality did not significantly differ from those without AKI or with sAKI. This differentiation between complete and incomplete rAKI has important clinical relevance because if the recurrence (increase in Scr after returning towards baseline) is recognized earlier and the factors associated with worsening rAKI are reversed, it would prevent progression of incomplete to complete rAKI and thereby prevent subsequent adverse outcomes, especially death. This clinical relevance is also supported by the graded increase in the odds of mortality among those with nAKI to those with sAKI to incomplete rAKI and to complete rAKI.
There are inherent limitations to our study associated with the retrospective data analysis including restraints of sample size. A smaller sample size may result in larger CIs, and, theoretically, difficulty in detecting smaller differences in comparison groups. We did not have the number of times creatinine was measured in each neonate. Similarly, due to the variability of measuring urine output, we did not include it in the defining AKI. As with other retrospective studies, we recommend a cautious interpretation of results. Although there are robust associations between the risk factors and the occurrence of rAKI, causality cannot be established based on our findings. Despite these limitations, our study is important as it explores a novel and unique question in the field of neonatal AKI and proposes a definition and classification of rAKI. Further, since all the neonates were recruited from the same NICU with the same set of providers, there was minimal variation in the standard of care for all the neonates including protocols on ventilatory support, caffeine use, hourly urine output measurement, fluid balance management, measurement of SCr among the study population, and discharging criteria during our study period.
Conclusion
Our study demonstrated that rAKI is common among preterm neonates in the NICU and is an independent risk factor for adverse outcomes. Early recognition and mitigation of risk factors may prevent complete rAKI and its associated poor outcomes. Our results provide a basis for further exploration of the definition of rAKI and its classification. Furthermore, we identified possible risk factors for rAKI and discussed the need for proactive steps to mitigate rAKI and prevent its associated poor outcomes.
References
Askenazi, D. J., Feig, D., Graham, N., Hui-Stickle, S. & Goldstein, S. 3–5-year longitudinal follow-up of pediatric patients after acute renal failure. Kidney Int. 69, 184–189 (2006).
Askenazi, D. J. AWAKEN-ING a new frontier in neonatal nephrology. Front. Pediatr. 8, 21 (2020).
Jetton, J. G. et al. Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicenter, multinational, observational cohort study. Lancet Child Adolesc. Health 1, 184–194 (2017).
Carmody, J. B., Swanson, J. R., Rhone, E. T. & Charlton, J. R. Recognition and reporting of AKI in very low birth weight infants. Clin. J. Am. Soc. Nephrol. 9, 2036–2043 (2014).
Charlton, J. R. et al. Late onset neonatal acute kidney injury: results from the AWAKEN Study. Pediatr. Res. 85, 339–348 (2019).
Cleper, R. et al. Neonatal acute kidney injury: recording rate, course, and outcome: one center experience. J. Matern. Fetal Neonatal Med. 32, 3379–3385 (2019).
Bauer, A. S. & Harer, M. W. Acute kidney injury in the preterm neonate. Curr. Treat. Options Pediatr. 4, 373–385 (2018).
Kupferman, J. C., Yitayew, M. & Rastogi, S. Acute kidney injury in term neonates. Curr. Treat. Options Pediatr. 4, 386–403 (2018).
Starr, M. C. et al. Acute kidney injury and bronchopulmonary dysplasia in premature neonates born less than 32 weeks’ gestation. Am. J. Perinatol. 37, 341–348 (2020).
Kraut, E. J., on behalf of the Neonatal Kidney Collaborative (NKC), Boohaker, L. J., Askenazi, D. J., Fletcher, J. & Kent, A. L. Incidence of neonatal hypertension from a large multicenter study [Assessment of Worldwide Acute Kidney Injury Epidemiology in Neonates—AWAKEN]. Pediatr. Res. 84, 279–289 (2018).
Selewski, D. T. et al. The impact of fluid balance on outcomes in premature neonates: a report from the AWAKEN study group. Pediatr. Res. 87, 550–557 (2020).
Stoops, C. et al. The association of intraventricular hemorrhage and acute kidney injury in premature infants from the Assessment of the Worldwide Acute Kidney Injury Epidemiology in Neonates (AWAKEN) study. Neonatology 116, 321–330 (2019).
Harer, M. W. et al. Association between early caffeine citrate administration and risk of acute kidney injury in preterm neonates: results from the AWAKEN study. JAMA Pediatr. 172, e180322 (2018).
Charlton, J. R. et al. Incidence and risk factors of early onset neonatal AKI. Clin. J. Am. Soc. Nephrol. 14, 184–195 (2019).
Stoops, C. et al. Baby NINJA (Nephrotoxic Injury Negated by Just-in-time Action): reduction of nephrotoxic medication-associated acute kidney injury in the neonatal intensive care unit. J. Pediatr. 215, 223–228.e6 (2019).
Ryan, D. et al. Development of the human fetal kidney from mid to late gestation in male and female infants. EBioMedicine 27, 275–283 (2018).
Hinchliffe, S. A., Sargent, P. H., Howard, C. V., Chan, Y. F. & van Velzen, D. Human intrauterine renal growth expressed in absolute number of glomeruli assessed by the disector method and Cavalieri principle. Lab Invest. 64, 777–784 (1991).
Author information
Authors and Affiliations
Contributions
O.O.A. and S.R. made the following contributions: conception and design, acquisition of data, or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; and final approval of the version to be published. Y.S. made the following contributions: acquisition of data and interpretation of data and drafting the article or revising it critically for important intellectual content. J.C.K. and A.B. made the following contributions: drafting the article or revising it critically for important intellectual content and final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Adegboyega, O.O., Singh, Y., Bhutada, A. et al. Recurrent acute kidney injury in preterm neonates is common and associated with worse outcomes and higher mortality. Pediatr Res 92, 284–290 (2022). https://doi.org/10.1038/s41390-021-01740-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01740-y
This article is cited by
-
Recurrent neonatal acute kidney injury: incidence, predictors, and outcomes in the neonatal intensive care unit
Journal of Perinatology (2024)
-
Perinatal risk factors associated with acute kidney injury severity and duration among infants born extremely preterm
Pediatric Research (2024)
-
Recent Advances in Acute Kidney Injury in Preterm Infants
Current Pediatrics Reports (2022)