Abstract
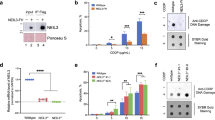
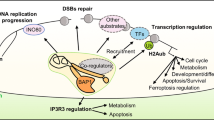
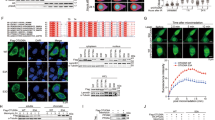
BRCA1-associated protein 1 (BAP1) has emerged as a major tumor suppressor gene in diverse cancer types, notably in malignant pleural mesothelioma (DPM), and has also been identified as a germline cancer predisposition gene for DPM and other select cancers. However, its role in the response to DNA damage has remained unclear. Here, we show that BAP1 inactivation is associated with increased DNA damage both in Met-5A human mesothelial cells and human DPM cell lines. Through proteomic analyses, we identified PRKDC as an interaction partner of BAP1 protein complexes in DPM cells and 293 T human embryonic kidney cells. PRKDC encodes the catalytic subunit of DNA protein kinase (DNA-PKcs) which functions in the nonhomologous end-joining (NHEJ) pathway of DNA repair. Double-stranded DNA damage resulted in prominent nuclear expression of BAP1 in DPM cells and phosphorylation of BAP1 at serine 395. A plasmid-based NHEJ assay confirmed a significant effect of BAP1 knockdown on cellular NHEJ activity. Combination treatment with X-ray irradiation and gemcitabine (as a radiosensitizer) strongly suppressed the growth of BAP1-deficient cells. Our results suggest reciprocal positive interactions between BAP1 and DNA-PKcs, based on phosphorylation of BAP1 by the latter and deubiquitination of DNA-PKcs by BAP1. Thus, functional interaction of BAP1 with DNA-PKcs supports a role for BAP1 in NHEJ DNA repair and may provide the basis for new therapeutic strategies and new insights into its role as a tumor suppressor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Febres-Aldana CA, Fanaroff R, Offin M, Zauderer MG, Sauter JL, Yang SR, et al. Diffuse pleural mesothelioma: advances in molecular pathogenesis, diagnosis and treatment. Ann Rev Pathol. 2023. https://doi.org/10.1146/annurev-pathol-042420-092719.
Bott M, Brevet M, Taylor BS, Shimizu S, Ito T, Wang L, et al. The nuclear deubiquitinase BAP1 is commonly inactivated by somatic mutations and 3p21.1 losses in malignant pleural mesothelioma. Nat Genet. 2011;43:668–72.
Hmeljak J, Sanchez-Vega F, Hoadley KA, Shih J, Stewart C, Heiman D, et al. Integrative molecular characterization of malignant pleural mesothelioma. Cancer Discov. 2018;812:1548–65.
Harbour JW, Onken MD, Roberson ED, Duan S, Cao L, Worley LA, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science. 2010;330:1410–3.
Jensen DE, Proctor M, Marquis ST, Gardner HP, Ha SI, Chodosh LA, et al. BAP1: a novel ubiquitin hydrolase which binds to the BRCA1 RING finger and enhances BRCA1-mediated cell growth suppression. Oncogene. 1998;16:1097–112.
Testa JR, Cheung M, Pei J, Below JE, Tan Y, Sementino E, et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet. 2011;43:1022–5.
Wiesner T, Obenauf AC, Murali R, Fried I, Griewank KG, Ulz P, et al. Germline mutations in BAP1 predispose to melanocytic tumors. Nat Genet. 2011;43:1018–21.
Chau C, van Doorn R, van Poppelen NM, van der Stoep N, Mensenkamp AR, Sijmons RH, et al. Families with BAP1-Tumor predisposition syndrome in the netherlands: path to identification and a proposal for genetic screening guidelines. Cancers. 2019;11:1114.
Kadariya Y, Cheung M, Xu J, Pei J, Sementino E, Menges CW, et al. Bap1 Is a bona fide tumor suppressor: genetic evidence from mouse models carrying heterozygous germline Bap1 mutations. Cancer Res. 2016;76:2836–44.
Hartlerode AJ, Scully R. Mechanisms of double-strand break repair in somatic mammalian cells. Biochem J. 2009;423:157–68.
Cary RB, Peterson SR, Wang J, Bear DG, Bradbury EM, Chen DJ. DNA looping by Ku and the DNA-dependent protein kinase. Proc Natl Acad Sci USA. 1997;94:4267–72.
Walker JR, Corpina RA, Goldberg J. Structure of the Ku heterodimer bound to DNA and its implications for double-strand break repair. Nature. 2001;412:607–14.
Yaneva M, Kowalewski T, Lieber MR. Interaction of DNA-dependent protein kinase with DNA and with Ku: biochemical and atomic-force microscopy studies. EMBO J. 1997;16:5098–112.
Mahaney BL, Meek K, Lees-Miller SP. Repair of ionizing radiation-induced DNA double-strand breaks by non-homologous end-joining. Biochem J. 2009;417:639–50.
Blunt T, Finnie NJ, Taccioli GE, Smith GC, Demengeot J, Gottlieb TM, et al. Defective DNA-dependent protein kinase activity is linked to V(D)J recombination and DNA repair defects associated with the murine scid mutation. Cell. 1995;80:813–23.
Douglas P, Sapkota GP, Morrice N, Yu Y, Goodarzi AA, Merkle D, et al. Identification of in vitro and in vivo phosphorylation sites in the catalytic subunit of the DNA-dependent protein kinase. Biochem J. 2002;368:243–51.
Ismail IH, Davidson R, Gagne JP, Xu ZZ, Poirier GG, Hendzel MJ. Germline mutations in BAP1 impair its function in DNA double-strand break repair. Cancer Res. 2014;74:4282–94.
Kwon J, Lee D, Lee SA. BAP1 as a guardian of genome stability: implications in human cancer. Exp Mol Med. 2023;55:745–54.
Yu H, Pak H, Hammond-Martel I, Ghram M, Rodrigue A, Daou S, et al. Tumor suppressor and deubiquitinase BAP1 promotes DNA double-strand break repair. Proc Natl Acad Sci USA. 2014;111:285–90.
Rathkey D, Khanal M, Murai J, Zhang J, Sengupta M, Jiang Q, et al. Sensitivity of mesothelioma cells to PARP inhibitors is not dependent on BAP1 but Is enhanced by temozolomide in cells with high-schlafen 11 and Low-O6-methylguanine-DNA methyltransferase expression. J Thorac Oncol. 2020;15:843–59.
Yang H, Xu D, Gao Y, Schmid RA, Peng RW. The association of BAP1 loss-of-function with the defect in homologous recombination repair and sensitivity to PARP-targeted therapy. J Thorac Oncol. 2020;15:e88–e90.
Fennell DA, King A, Mohammed S, Branson A, Brookes C, Darlison L, et al. Rucaparib in patients with BAP1-deficient or BRCA1-deficient mesothelioma (MiST1): an open-label, single-arm, phase 2a clinical trial. Lancet Respir Med. 2021;9:593–600.
Ghafoor A, Mian I, Wagner C, Mallory Y, Agra MG, Morrow B, et al. Phase 2 study of olaparib in malignant mesothelioma and correlation of efficacy with germline or somatic mutations in BAP1 gene. JTO Clin Res Rep. 2021;2:100231.
Passiglia F, Righi L, Bironzo P, Listì A, Farinea G, Capelletto E, et al. Niraparib plus dostarlimab in pleural mesothelioma or non-small cell lung cancer harboring HRR mutations: Interim results of the UNITO-001 phase 2 prospective trial. Clin Cancer Res. 2023. https://doi.org/10.1158/1078-0432.CCR-23-2431.
Westphalen CB, Fine AD, Andre F, Ganesan S, Heinemann V, Rouleau E, et al. Pan-cancer analysis of homologous recombination repair-associated gene alterations and genome-wide loss-of-heterozygosity score. Clin Cancer Res. 2022;28:1412–21.
Baas R, JvdW F, Bleijerveld OB, van Attikum H, Sixma TK. Proteomic analysis identifies novel binding partners of BAP1. PLoS ONE. 2021;16:e0257688.
Singh A, Busacca S, Gaba A, Sheaff M, Poile C, Nakas A, et al. BAP1 loss induces mitotic defects in mesothelioma cells through BRCA1-dependent and independent mechanisms. Oncogene. 2023;42:572–85.
Masoomian B, Shields JA, Shields CL. Overview of BAP1 cancer predisposition syndrome and the relationship to uveal melanoma. J Curr Ophthalmol. 2018;30:102–9.
Eletr ZM, Yin L, Wilkinson KD. BAP1 is phosphorylated at serine 592 in S-phase following DNA damage. FEBS Lett. 2013;587:3906–11.
Moll U, Lau R, Sypes MA, Gupta MM, Anderson CW. DNA-PK, the DNA-activated protein kinase, is differentially expressed in normal and malignant human tissues. Oncogene. 1999;18:3114–26.
Iliakis G, Rosidi B, Wang M, Wang H. Plasmid-based assays for DNA end-joining in vitro. Methods Mol Biol. 2006;314:123–31.
Ma Y, Lieber MR. In vitro nonhomologous DNA end joining system. Methods Enzymol. 2006;408:502–10.
Zenke FT, Zimmermann A, Sirrenberg C, Dahmen H, Kirkin V, Pehl U, et al. Pharmacologic inhibitor of DNA-PK, M3814, potentiates radiotherapy and regresses human tumors in mouse models. Mol Cancer Ther. 2020;19:1091–101.
Bueno R, Stawiski EW, Goldstein LD, Durinck S, De Rienzo A, Modrusan Z, et al. Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat Genet. 2016;48:407–16.
Cantin GT, Yi W, Lu B, Park SK, Xu T, Lee JD, et al. Combining protein-based IMAC, peptide-based IMAC, and MudPIT for efficient phosphoproteomic analysis. J Proteome Res. 2008;7:1346–51.
Olsen JV, Blagoev B, Gnad F, Macek B, Kumar C, Mortensen P, et al. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell. 2006;127:635–48.
Serakinci N, Christensen R, Graakjaer J, Cairney CJ, Keith WN, Alsner J, et al. Ectopically hTERT expressing adult human mesenchymal stem cells are less radiosensitive than their telomerase negative counterpart. Exp Cell Res. 2007;313:1056–67.
Guo G, Chmielecki J, Goparaju C, Heguy A, Dolgalev I, Carbone M, et al. Whole-exome sequencing reveals frequent genetic alterations in BAP1, NF2, CDKN2A, and CUL1 in malignant pleural mesothelioma. Cancer Res. 2015;75:264–9.
Zauderer MG, Bott M, McMillan R, Sima CS, Rusch V, Krug LM, et al. Clinical characteristics of patients with malignant pleural mesothelioma harboring somatic BAP1 mutations. J Thorac Oncol. 2013;8:1430–3.
Dogrusoz M, Ruschel Trasel A, Cao J, Ҫolak S, van Pelt SI, Kroes WGM, et al. Differential expression of DNA repair genes in prognostically-favorable versus unfavorable uveal melanoma. Cancers. 2019;11:1104.
Kuznetsoff JN, Owens DA, Lopez A, Rodriguez DA, Chee NT, Kurtenbach S, et al. Dual screen for efficacy and toxicity identifies HDAC inhibitor with distinctive activity spectrum for BAP1-mutant uveal melanoma. Mol Cancer Res. 2021;19:215–22.
Liu Z, Lin D, Zhou Y, Zhang L, Yang C, Guo B, et al. Exploring synthetic lethal network for the precision treatment of clear cell renal cell carcinoma. Sci Rep. 2022;12:13222.
Krug LM, Pass HI, Rusch VW, Kindler HL, Sugarbaker DJ, Rosenzweig KE, et al. Multicenter phase II trial of neoadjuvant pemetrexed plus cisplatin followed by extrapleural pneumonectomy and radiation for malignant pleural mesothelioma. J Clin Oncol. 2009;27:3007–13.
Minatel E, Trovo M, Polesel J, Baresic T, Bearz A, Franchin G, et al. Radical pleurectomy/decortication followed by high dose of radiation therapy for malignant pleural mesothelioma. Final results with long-term follow-up. Lung Cancer. 2014;83:78–82.
Boons CC, Vant MW, Burgers JA, Beckeringh JJ, Wagner C, Hugtenburg JG. The value of pemetrexed for the treatment of malignant pleural mesothelioma: a comprehensive review. Anticancer Res. 2013;33:3553–61.
Ewald B, Sampath D, Plunkett W. H2AX phosphorylation marks gemcitabine-induced stalled replication forks and their collapse upon S-phase checkpoint abrogation. Mol Cancer Ther. 2007;6:1239–48.
Lawrence TS, Eisbruch A, McGinn CJ, Fields MT, Shewach DS. Radiosensitization by gemcitabine. Oncology. 1999;13:55–60.
Wachters FM, van Putten JW, Maring JG, Zdzienicka MZ, Groen HJ, Kampinga HH. Selective targeting of homologous DNA recombination repair by gemcitabine. Int J Radiat Oncol Biol Phys. 2003;57:553–62.
Blackford AN, Jackson SP. ATM, ATR, and DNA-PK: the trinity at the heart of the DNA damage response. Mol Cell. 2017;66:801–17.
Yap TA, Aerts JG, Popat S, Fennell DA. Novel insights into mesothelioma biology and implications for therapy. Nat Rev Cancer. 2017;17:475–88.
Li H, Vogel H, Holcomb VB, Gu Y, Hasty P. Deletion of Ku70, Ku80, or both causes early aging without substantially increased cancer. Mol Cell Biol. 2007;27:8205–14.
Righi L, Duregon E, Vatrano S, Izzo S, Giorcelli J, Rondon-Lagos M, et al. BRCA1-Associated Protein 1 (BAP1) immunohistochemical expression as a diagnostic tool in malignant pleural mesothelioma classification: a large retrospective study. J Thorac Oncol. 2016;11:2006–17.
Sebastiaan Winkler G, Lacomis L, Philip J, Erdjument-Bromage H, Svejstrup JQ, Tempst P. Isolation and mass spectrometry of transcription factor complexes. Methods. 2002;26:260–9.
Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem. 2003;75:4646–58.
Acknowledgements
We are grateful to Ronald C. Hendrickson (Proteomics & Microchemistry, Memorial Sloan Kettering Cancer Center), Sho Fujisawa (Molecular Cytology, Memorial Sloan Kettering Cancer Center) and Toshiki Terao, Rina Nishiyama and Reiko Kondo (Okayama University, Medical School) for providing technical assistance and opportunities to discuss this work.
Author information
Authors and Affiliations
Contributions
TI and ML conceptualized the project. TI designed the experiments. TI and TH performed experiments. HEB helped with proteomics studies. MJB provided data. SK and ST provided advice and guidance. MGZ provided clinical context. HS and ML finalized the manuscript. ML provided overall supervision of the project.
Corresponding author
Ethics declarations
Financial support
DOD CMDRP grant W81XWH 15 1 0210 (Zauderer) and NCI grant P30 CA0087448.
Competing interests
The authors declare no potential conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sato, H., Ito, T., Hayashi, T. et al. The BAP1 nuclear deubiquitinase is involved in the nonhomologous end-joining pathway of double-strand DNA repair through interaction with DNA-PK. Oncogene 43, 1087–1097 (2024). https://doi.org/10.1038/s41388-024-02966-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-024-02966-w