Abstract
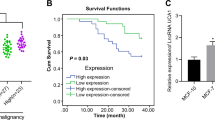
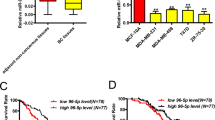
MicroRNAs play significant roles in various malignancies, with breast cancer (BC) being no exception. Consequently, we explored the functional mechanism of miR-135 in the progression of BC. In total, 55 pairs of BC and matched adjacent normal tissues were clinically collected from patients, followed by quantification of miR-135 and zinc finger protein 217 (ZNF217) expression patterns in BC tissues and cells. Accordingly, high ZNF217 expression and low miR-135 expression levels were identified in BC tissues and cells. Subsequently, the expressions of miR-135 and ZNF217 were altered to evaluate their effects on BC cell migration, invasion and EMT initiation. It was found that when ZNF217 was silenced or miR-135 was elevated, BC cell malignant behaviors were significantly inhibited, which was reproduced in nude mice for in vivo evidence. Furthermore, dual-luciferase reporter gene assay revealed the presence of direct binding between miR-135 and ZNF217. Subsequent co-immunoprecipitation, methylated-RNA binding protein immunoprecipitation and photoactivatable ribonucleoside enhanced-crosslinking and immunoprecipitation assays further revealed that ZNF217 could upregulate NANOG by reducing N6-methyladenosine levels via methyltransferase-like 13 (METTL3). Collectively, our findings highlighted the role of the miR-135/ZNF217/METTL3/NANOG axis in the progression of BC, emphasizing potential therapeutic targets ZNF217 silencing and miR-135 upregulation in preventing or treating BC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Material.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Akram M, Iqbal M, Daniyal M, Khan AU. Awareness and current knowledge of breast cancer. Biol Res. 2017;50:33.
Sun YS, Zhao Z, Yang ZN, Xu F, Lu HJ, Zhu ZY, et al. Risk factors and preventions of breast cancer. Int J Biol Sci. 2017;13:1387–97.
Vendrell JA, Thollet A, Nguyen NT, Ghayad SE, Vinot S, Bieche I, et al. ZNF217 is a marker of poor prognosis in breast cancer that drives epithelial-mesenchymal transition and invasion. Cancer Res. 2012;72:3593–606.
Zhang C, Zhi WI, Lu H, Samanta D, Chen I, Gabrielson E, et al. Hypoxia-inducible factors regulate pluripotency factor expression by ZNF217- and ALKBH5-mediated modulation of RNA methylation in breast cancer cells. Oncotarget. 2016;7:64527–42.
Aguilo F, Zhang F, Sancho A, Fidalgo M, Di Cecilia S, Vashisht A, et al. Coordination of m(6)A mRNA methylation and gene transcription by ZFP217 regulates pluripotency and reprogramming. Cell Stem Cell. 2015;17:689–704.
Qin S, Li Y, Cao X, Du J, Huang X. NANOG regulates epithelial-mesenchymal transition and chemoresistance in ovarian cancer. Biosci Rep. 2017;37:BSR20160247.
Yeung KT, Yang J. Epithelial-mesenchymal transition in tumor metastasis. Mol Oncol. 2017;11:28–39.
Zhao M, Ang L, Huang J, Wang J. MicroRNAs regulate the epithelial-mesenchymal transition and influence breast cancer invasion and metastasis. Tumour Biol. 2017;39:1010428317691682.
McGuire A, Brown JA, Kerin MJ. Metastatic breast cancer: the potential of miRNA for diagnosis and treatment monitoring. Cancer Metastasis Rev. 2015;34:145–55.
Hamam R, Hamam D, Alsaleh KA, Kassem M, Zaher W, Alfayez M, et al. Circulating microRNAs in breast cancer: novel diagnostic and prognostic biomarkers. Cell Death Dis. 2017;8:e3045.
Yang Y, Ishak Gabra MB, Hanse EA, Lowman XH, Tran TQ, Li H, et al. MiR-135 suppresses glycolysis and promotes pancreatic cancer cell adaptation to metabolic stress by targeting phosphofructokinase-1. Nat Commun. 2019;10:809.
Yamada Y, Hidaka H, Seki N, Yoshino H, Yamasaki T, Itesako T, et al. Tumor-suppressive microRNA-135a inhibits cancer cell proliferation by targeting the c-MYC oncogene in renal cell carcinoma. Cancer Sci. 2013;104:304–12.
Taipaleenmaki H, Browne G, Akech J, Zustin J, van Wijnen AJ, Stein JL, et al. Targeting of Runx2 by miR-135 and miR-203 impairs progression of breast cancer and metastatic bone disease. Cancer Res. 2015;75:1433–44.
Jiang D, Zhou B, Xiong Y, Cai H. miR-135 regulated breast cancer proliferation and epithelial-mesenchymal transition acts by the Wnt/beta-catenin signaling pathway. Int J Mol Med. 2019;43:1623–34.
Goh JN, Loo SY, Datta A, Siveen KS, Yap WN, Cai W, et al. microRNAs in breast cancer: regulatory roles governing the hallmarks of cancer. Biol Rev Camb Philos Soc. 2016;91:409–28.
Jiang X, Zhang C, Qi S, Guo S, Chen Y, Du E, et al. Elevated expression of ZNF217 promotes prostate cancer growth by restraining ferroportin-conducted iron egress. Oncotarget. 2016;7:84893–906.
Li Z, Du L, Dong Z, Yang Y, Zhang X, Wang L, et al. MiR-203 suppresses ZNF217 upregulation in colorectal cancer and its oncogenicity. PLoS One. 2015;10:e0116170.
Cohen PA, Donini CF, Nguyen NT, Lincet H, Vendrell JA. The dark side of ZNF217, a key regulator of tumorigenesis with powerful biomarker value. Oncotarget. 2015;6:41566–81.
Kocak A, Heselmeyer-Haddad K, Lischka A, Hirsch D, Fiedler D, Hu Y, et al. High levels of chromosomal copy number alterations and TP53 mutations correlate with poor outcome in younger breast cancer patients. Am J Pathol. 2020;190:1643–56.
Horne HN, Oh H, Sherman ME, Palakal M, Hewitt SM, Schmidt MK, et al. E-cadherin breast tumor expression, risk factors and survival: pooled analysis of 5,933 cases from 12 studies in the Breast Cancer Association Consortium. Sci Rep. 2018;8:6574.
Qiu Y, Liu Y, Li WH, Zhang HQ, Tian XX, Fang WG. P2Y2 receptor promotes the migration and invasion of breast cancer cells via EMT-related genes Snail and E-cadherin. Oncol Rep. 2018;39:138–50.
Smith BN, Burton LJ, Henderson V, Randle DD, Morton DJ, Smith BA, et al. Snail promotes epithelial mesenchymal transition in breast cancer cells in part via activation of nuclear ERK2. PLoS One. 2014;9:e104987.
Satelli A, Li S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell Mol Life Sci. 2011;68:3033–46.
Rezaei M, Friedrich K, Wielockx B, Kuzmanov A, Kettelhake A, Labelle M, et al. Interplay between neural-cadherin and vascular endothelial-cadherin in breast cancer progression. Breast Cancer Res. 2012;14:R154.
Wang X, Zhou X, Zeng F, Wu X, Li H. miR-485-5p inhibits the progression of breast cancer cells by negatively regulating MUC1. Breast Cancer. 2020;27:765–75.
Lin C, Gao B, Yan X, Lei Z, Chen K, Li Y, et al. MicroRNA 628 suppresses migration and invasion of breast cancer stem cells through targeting SOS1. Onco Targets Ther. 2018;11:5419–28.
Littlepage LE, Adler AS, Kouros-Mehr H, Huang G, Chou J, Krig SR, et al. The transcription factor ZNF217 is a prognostic biomarker and therapeutic target during breast cancer progression. Cancer Disco. 2012;2:638–51.
Bellanger A, Donini CF, Vendrell JA, Lavaud J, Machuca-Gayet I, Ruel M, et al. The critical role of the ZNF217 oncogene in promoting breast cancer metastasis to the bone. J Pathol. 2017;242:73–89.
Batista PJ, Molinie B, Wang J, Qu K, Zhang J, Li L, et al. m(6)A RNA modification controls cell fate transition in mammalian embryonic stem cells. Cell Stem Cell. 2014;15:707–19.
Gibadulinova A, Bullova P, Strnad H, Pohlodek K, Jurkovicova D, Takacova M, et al. CAIX-mediated control of LIN28/let-7 axis contributes to metabolic adaptation of breast cancer cells to hypoxia. Int J Mol Sci. 2020;21:4299.
Hu C, Xu L, Liang S, Zhang Z, Zhang Y, Zhang F. Lentivirus-mediated shRNA targeting Nanog inhibits cell proliferation and attenuates cancer stem cell activities in breast cancer. J Drug Target. 2016;24:422–32.
Wen S, Qin Y, Wang R, Yang L, Zeng H, Zhu P, et al. A novel Lnc408 maintains breast cancer stem cell stemness by recruiting SP3 to suppress CBY1 transcription and increasing nuclear beta-catenin levels. Cell Death Dis. 2021;12:437.
Li F, Xu Y, Xu X, Ge S, Zhang F, Zhang H, et al. lncRNA HotairM1 depletion promotes self-renewal of cancer stem cells through HOXA1-Nanog regulation loop. Mol Ther Nucleic Acids. 2020;22:456–70.
Wang L, Choi HS, Lee B, Choi JH, Jang YS, Seo JW. 13R,20-Dihydroxydocosahexaenoic acid, a novel dihydroxy-DHA derivative, inhibits breast cancer stemness through regulation of the Stat3/IL-6 signaling pathway by inducing ROS production. Antioxidants (Basel). 2021;10:457.
Suarez-Sanchez FJ, Lequerica-Fernandez P, Rodrigo JP, Hermida-Prado F, Suarez-Canto J, Rodriguez-Santamarta T, et al. Tumor-infiltrating CD20(+) B lymphocytes: significance and prognostic implications in oral cancer microenvironment. Cancers (Basel). 2021;13:395.
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47.
Ayuk SM, Abrahamse H, Houreld NN. The role of photobiomodulation on gene expression of cell adhesion molecules in diabetic wounded fibroblasts in vitro. J Photochem Photobio B. 2016;161:368–74.
Yang D, Qiao J, Wang G, Lan Y, Li G, Guo X, et al. N6-Methyladenosine modification of lincRNA 1281 is critically required for mESC differentiation potential. Nucleic Acids Res. 2018;46:3906–20.
Vu LP, Pickering BF, Cheng Y, Zaccara S, Nguyen D, Minuesa G, et al. The N(6)-methyladenosine (m(6)A)-forming enzyme METTL3 controls myeloid differentiation of normal hematopoietic and leukemia cells. Nat Med. 2017;23:1369–76.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81502656 and No. 81501140).
Author information
Authors and Affiliations
Contributions
Conception and design of research: L-MX, JZ, YM, PW; performed experiments: YM, Y-JY, HY, L-MX; analyzed data: LZ, JZ, Y-JY, HY; interpreted results of experiments: YM, Y-JY, PW; prepared figures: HY, JW, X-CC; drafted manuscript: HY, JW, X-CC; edited and revised manuscript: L-MX, JZ, LZ; approved final version of manuscript: L-MX, JZ, YM, Y-JY, HY, JW, X-CC, LZ, PW.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Xu, LM., Zhang, J., Ma, Y. et al. MicroRNA-135 inhibits initiation of epithelial-mesenchymal transition in breast cancer by targeting ZNF217 and promoting m6A modification of NANOG. Oncogene 41, 1742–1751 (2022). https://doi.org/10.1038/s41388-022-02211-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-022-02211-2
This article is cited by
-
Methyltransferase-like proteins in cancer biology and potential therapeutic targeting
Journal of Hematology & Oncology (2023)
-
miR-135 protects against atrial fibrillation by suppressing intracellular calcium-mediated NLRP3 inflammasome activation
Journal of Cell Communication and Signaling (2023)
-
Helicobacter pylori infection induces abnormal expression of pro-angiogenic gene ANGPT2 and miR-203a in AGS gastric cell line
Brazilian Journal of Microbiology (2023)