Abstract
Complete blockade of the HER2 protein itself and HER signaling network is critical to achieving effective HER2-targeted therapies. Despite the success of HER2-targeted therapies, the diseases will relapse in a significant fraction of patients with HER2+ breast cancers. How to improve the therapeutic efficacy of existing HER2-targeted agents remains an unmet clinical need. Here, we uncover a role of Melatonin in diminishing HER2-mediated signaling by destruction of HER2 protein. Mechanistically, Melatonin treatment attenuated the protective effect of the HSP90 chaperone complex on its client protein HER2, triggering ubiquitylation and subsequent endocytic lysosomal degradation of HER2. The inhibitory effect of Melatonin on HER2 signaling substantially enhanced the cytotoxic effects of the pan-HER inhibitor Neratinib in HER2+ breast cancer cells. Lastly, we demonstrate that dual inhibition of HER2 by combined use of Melatonin and Neratinib effectively blocked the growth of HER2+ breast tumor xenografts in vivo. Our findings shed light on the potential use of Melatonin in a novel dual HER2 blockade strategy for HER2+ breast cancer treatment.
Similar content being viewed by others
Introduction
Amplification/overexpression of human epidermal growth factor HER2/ERBB2 is present in 20% of breast cancer. To date, HER2 positivity remains the only biomarker with demonstrated clinical utility in anti-HER2 targeted therapies [1]. Several classes of HER2-targeted agents have been developed for the treatment of HER2-positive (HER2+) breast cancer, including monoclonal antibodies (Trastuzumab and Pertuzumab), small molecule tyrosine kinase inhibitors (Lapatinib and Neratinib) and antibody-drug conjugates (trastuzumab emtansine T-DM1) [1,2,3,4]. While the clinical application of HER2-targeted agents has greatly improved the prognosis of HER2+ breast cancer, the diseases will inevitably relapse. The effectiveness of HER2-targeted treatments relies on more complete blockade of the HER2 protein itself and HER signaling network [1, 4]. Combinatorial HER2 blockade by combining different classes of HER2-targeted agents has demonstrated superior anti-tumor activity to anti-HER2 monotherapy in the preclinical and clinical settings [2,3,4]. However, how to improve the efficacy of existing therapeutics and identify more effective combinatorial strategies remains a pressing need for HER2+ breast cancer.
Neratinib, a next-generation irreversible small molecule inhibitor of receptor tyrosine kinases (HER1/EGFR, HER2, and HER4), has been recently approved for the treatment of HER2+ breast cancer [3,4,5,6,7,8]. Neratinib is a more potent inhibitor than the dual EGFR/HER2 tyrosine kinase inhibitor (TKI) Lapatinib, potentiating the cytotoxic effect of trastuzumab in HER2+ breast cancer cell lines [3, 9]. It has been reported that Neratinib has the ability to overcome Lapatinib resistance caused by incomplete inhibition of HER kinases [9, 10]. However, several mechanisms of resistance to Neratinib have been reported including dysregulated BCL2 family member expression and increased activity of Neratinib metabolizing enzyme cytochrome P450 CYP3A4 [11, 12]. In addition, somatic activating HER2 mutations have been detected in HER2+ breast cancer patients subjected to ongoing SUMMIT “basket” trial of Neratinib (NCT01953926), suggesting hyperactivation of HER family kinases may confer resistance to Neratinib [13]. There is thus an urgent need to identify novel targeted therapeutic combinations to improve the efficacy of Neratinib by abrogating the aberrant expression or activation of HER2 in the treatment of HER2+ or HER2-mutated breast cancer.
Melatonin is a natural hormone secreted by the pineal gland of human and mammals. Numerous studies including our recent work have reported Melatonin exhibits antitumor properties through diverse mechanisms of action [14,15,16,17,18,19,20]. In the current study, we investigated the potential effect of Melatonin as single agent and in combination with Neratinib in the treatment of HER2+ breast cancer cell lines. Interestingly, our study reveals an unexpected role of Melatonin in dysregulating HER2 protein stability and potentiating the cytotoxic effect of Neratinib in HER2+ breast cancer.
Results
Melatonin treatment recapitulates the effects of HER2-targeted agents
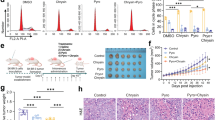
We first examined the effect of Melatonin on cell survival in three HER2+ breast cancer cell lines that carry oncogenic PIK3CA mutations, including HCC1954 (HER2 amplification, PIK3CA H1047R) and MDA-MB-361 (HER2 amplification, PIK3CA E545K), and MCF7 (PIK3CA E545K) with ectopic overexpression of HER2 (MCF7/HER2). Melatonin treatment resulted in significantly increased cell death in a time- and dose-dependent manner (Fig. 1A and Supplementary Fig. 1A). To understand the cytotoxic effect of Melatonin on HER2+ breast cancer cells, we recently performed transcriptome profiling of the HCC1954 cells treated with or without Melatonin by RNA sequencing (RNA-Seq, GSE175906). The gene set enrichment analysis (GSEA) revealed that Melatonin treatment was significantly inversely associated with enrichment of gene sets including EMT, KRAS, SRC, NF-κB, and IL6-JAK-STAT3 (Fig. 1B, C, Supplementary Fig. 1B), all of which have been previously linked to the therapeutic effects of HER2-targeted therapies [2, 21,22,23,24]. Consistent with these findings, Melatonin treatment led to markedly decreased phospho-AKT, phospho-ERK, phospho-SRC signals, and phospho-NF-κB signals, as well as attenuated EMT signature (reduced N-cadherin and increased E-cadherin expression) in HCC1954 cells (Fig. 1D). The observation that Melatonin treatment recapitulates many effects caused by HER2-targeted agents prompted us to investigate whether Melatonin may influence the expression and/or activity of HER2 itself.
A Cell death in the HER2+ breast cancer cells treated with Melatonin was determined by PI staining and FACS analysis. Drug treatment conditions were shown as indicated. Quantitation for three independent experiments is shown. Data are shown as Mean ± S.D. B Gene Set Enrichment Analysis (GSEA) of gene sets associated with Melatonin treatment in HCC1954 cells. Melatonin, 2 mM, 24 h. Normalized Enrichment Score (NES), p value and FDR q values of the correlation are shown. C The quantification of signatures downstream of HER2 as shown in B. D Western blot analysis of HER2 downstream signaling proteins in HCC1954 cells treated with Melatonin. Drug treatment conditions were shown as indicated. Vinculin was used as a loading control. The quantification of protein abundance is shown. Data are representative of three independent experiments. n. s., not significant. *p < 0.05, **p < 0.01, ***p < 0.001 (Student’s t test).
Melatonin decreases HER2 protein stability
We next investigated whether Melatonin regulates HER2 protein levels. Indeed, HER2 protein abundance is markedly reduced in all three cell lines treated with Melatonin (Fig. 2A). To examine whether Melatonin decreases HER2 through downregulation of its transcription, we conducted quantitative reverse transcription PCR (qRT-PCR) analysis. Melatonin treatment did not downregulate HER2 mRNA levels (Supplementary Fig. 2), suggesting that Melatonin-induced reduction at HER2 protein levels cannot be explained at the mRNA level of HER2.
A Western blot analysis of HER2 protein levels in the HER2+ breast cancer cells treated with Melatonin at the indicated concentrations for 24 h. Western blot analysis of HER2 proteins levels in the cells treated with Vehicle control or Melatonin for 24 h followed by the addition of cycloheximide (CHX) treatment for the indicated time. Melatonin, 2 mM. CHX, HCC1954 (B), 20 μg/ml; MDA-MB-361(C), 40 μg/ml; MCF7/HER2 (D), 150 μg/ml. Vinculin was used as a loading control. The quantification of HER2 protein abundance and half-lives are shown. T1/2, half-lives. Data are representative of three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001 (Student’s t test).
Next, we asked whether Melatonin may affect HER2 protein stability. For this, we examined the effect of Melatonin on existing HER2 protein pools in HER2+ breast cancer cells using the protein synthesis inhibitor cycloheximide (CHX). As expected, the HER2 protein level gradually decreased in the vehicle-treated cells after CHX treatment in a time-dependent manner. In contrast, more striking degradation of HER2 was observed in the Melatonin-treated cells (Fig. 2B–D). Together, these results suggest that Melatonin treatment decreases HER2 protein stability.
Melatonin treatment destructs HER2 through promoting its endocytosis and lysosomal degradation
Receptor stability is known to be regulated by ubiquitin-proteasomal and/or lysosomal mechanisms [25,26,27,28]. We next sought to understand how Melatonin mediates HER2 downregulation. Interestingly, we found that proteasome inhibition by either MG132 or Velcade further reduced HER2 protein abundance upon treatment with Melatonin (Fig. 3A, B). In contrast, the lysosome inhibitor Bafilomycin A1 (BAF) restored the reduced HER2 levels (Fig. 3C), suggesting that Melatonin may induce HER2 downregulation primarily through the endocytic lysosomal degradation.
HCC1954 cells were treated with proteasome inhibitor MG132 (A) or Velcade (B) for 0.5 h or DMSO as control followed by the addition of Melatonin (0, 12, and 24 h). Western blot analysis and quantification of HER2 protein levels were shown. Melatonin, 2 mM; MG-132, 10 μM; Velcade, 500 nM. Vinculin was used as a loading control. C HCC1954 cells were treated with lysosome inhibitor BAF for 0.5 h or DMSO as control followed by the addition of Melatonin (0, 12, and 24 h). Western blot analysis and quantification of HER2 protein levels were shown. Melatonin, 2 mM; Bafilomycin A1 (BAF), 20 nM. D Representative images of immunofluorescent staining of HER2 in the HCC1954 cells treated with Melatonin at indicated concentrations for 24 h. Scale bar, 25 μm. E Flow cytometric analysis of HER2 protein levels on the surface of cells as in D. Quantification of HER2 abundance is shown as Mean ± S.D. Data are representative of three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001 (Student’s t test).
Receptors can be internalized from plasma membrane and subjected to degradation by the endosomal-lysosomal pathway [26, 28,29,30]. To examine the potential role of Melatonin in HER2 endocytosis, we first examined the effect of Melatonin on the cellular localization of HER2 protein in HER2+ breast cancer cells. While HER2 protein was expressed strongly on the membrane and diffused in the cytoplasm of the untreated HCC1954 cells, HER2 appeared as intracellular puncta in the Melatonin-treated cells (Fig. 3D), suggesting that Melatonin may induce HER2 subcellular trafficking. Consistently, flow cytometric analysis revealed that Melatonin treatment markedly reduced the amount of HER2 present on the cell surface in a dose-dependent manner (Fig. 3E and Supplementary Fig. 3). These data implicate a potential role for Melatonin in promoting HER2 endocytosis.
The molecular chaperone system involving HSP90 and HSP70 isoforms (HSP70 and HSC70) has been shown to protect their client protein such as HER2 from ubiquitin-mediated endocytosis [31, 32]. Melatonin treatment resulted in decreased expression of HSP90 and HSC70 and a compensatory increase in HSP70 expression (Fig. 4A), similar to the effect of HSP90 inhibitor 17-AAG [33]. Meanwhile, the amounts of HSP90 co-immunoprecipitated with HER2 were also markedly reduced in the Melatonin-treated cells, suggesting that Melatonin may induce dissociation of HSP90 from HER2 (Fig. 4B). Meanwhile, Melatonin treatment led to increased levels of ubiquitylated HER2 (Fig. 4B). In addition, immunofluorescence staining analysis revealed that Melatonin treatment yielded evident localization of internalized HER2 as puncta structures to endosomal compartment stained with EEA1 (an early endosome marker) (Fig. 4C). Moreover, HER2 was found to colocalize with LAMP1 (a late endosome/lysosomal marker) in the Melatonin-treated HCC1954 cells (Fig. 4D). Together with our finding that the lysosome inhibitor BAF could rescue downregulation of HER2 induced by Melatonin (Fig. 3C), these data support the notion that Melatonin destructs HER2 through promoting its endocytosis and lysosomal degradation.
A Western blot analysis of proteins as indicated in the HCC1954 cells treated with or without Melatonin. Drug treatment conditions were shown as indicated. Vinculin was used as a loading control. The quantification of protein abundance is shown. B Co-Immunoprecipitation (Co-IP) of HER2 and HSP90 from the lysates of the Melatonin-treated HCC1954 cells or control cells. Melatonin, 2 mM, 48 h. Co-immunoprecipitated HSP90 and ubiquitylated HER2 band intensities were quantified and adjusted to the corresponding normalized HER2 signal and shown in the bar charts. Data from three independent experiments are shown as Mean ± S.D. *p < 0.05, **p < 0.01, ***p < 0.001 (Student’s t test). C Representative images of immunofluorescent staining of HER2 and EEA1 proteins in the HCC1954 cells treated with 2 mM Melatonin for 24 h. D Representative images of immunofluorescent staining of HER2 and LAMP1 proteins in the HCC1954 cells treated as in C. Scale bar, 25 μm.
Melatonin enhances the cytotoxic effect of Neratinib in HER2+ breast cancer cells
Built upon our finding that Melatonin decreases HER2 protein stability in this study, we next investigated whether Melatonin could potentiate the therapeutic effect of the pan-HER kinase inhibitor Neratinib in the treatment of HER2+ breast cancer cells. To test this hypothesis, we assessed the response of HER2+ breast cancer cell lines to these drugs as single-agents or in combination. In all four breast cancer cell lines tested, we observed synergistic treatment response as evaluated by the CalcuSyn model (Supplementary Fig. 4A–C). We further examined the synergistic growth inhibitory effects of the drug combination by clonogenic survival assays. Combined use of Melatonin and Neratinib induced a significantly stronger growth inhibitory effect than either single-agent treatment (Fig. 5A, B). To examine the effect of drug combination on the migration potential of HER2+ breast cancer cells, we conducted the wound-healing assays. We found that the gaps between the scratched area were larger in the Melatonin + Neratinib group than those in the single-agent group 48 h post-wounding (Fig. 5C). While single-agent Neratinib [27], and Melatonin to a lesser extent, attenuated HER2 protein abundance as well as phospho-HER2 signal, the combination treatment yielded a more potent inhibitory effect (Fig. 5D). Consistently, while Melatonin or Neratinib alone yielded a moderate cytotoxic effect, the combination treatment resulted in substantially increased cell death (Fig. 5E). Together, these data indicate that the combination of Melatonin and Neratinib exerts synergistic therapeutic activity against HER2+ breast cancer cells.
Long-term cell viability of HER2+ breast cancer cells treated with Melatonin and Neratinib, either alone or in combination, was examined by crystal violet assay. HCC1954, 2 mM Melatonin, 50 nM Neratinib; MDA-MB-453, 2 mM Melatonin, 100 nM Neratinib; MDA-MB-361, 2 mM Melatonin, 100 nM Neratinib; MCF7/HER2, 1 mM Melatonin, 50 nM Neratinib. A, 96-well format; B, 24-well format. Representative images of plates and the quantification of cell viability are shown. C Cell migration potential of the HER2+ breast cancers cells treated as in A was determined using a wound-healing assay. The images of the wound areas are shown at 0 and 48 h. Scale bar, 200 μm. D Western blot analysis of proteins as indicated in cells treated as in A for 24 h. Vinculin was used as a loading control. The quantification of protein abundance is shown. E Cell death in the HER2+ breast cancer cells treated as in A for 48 h was examined by PI staining and FACS analysis. Data from three independent experiments are shown as Mean ± S.D. *p < 0.05, **p < 0.01, ***p < 0.001 (Student’s t test).
Combined use of Melatonin and Neratinib effectively blocks the growth of HCC1954 tumor xenografts
To validate our in vitro findings, we next evaluated the combinatorial effect of Melatonin and Neratinib in the HCC1954 xenograft tumor model. Compared to the control group, Melatonin and Neratinib monotherapies significantly reduced the tumor growth of the HCC1954 xenografts (Fig. 6A). Strikingly, combined use of Melatonin and Neratinib led to greater inhibition of tumor growth (in terms of average tumor volumes and endpoint tumor weights) than with either agent alone (Fig. 6A–D). Notably, only the combination treatment achieved tumor regression in a fraction of tumors (5 out of 8) (Fig. 6A, B). In keeping with our findings that Melatonin destructed HER2 protein in vitro, the tumors from mice treated with Melatonin alone revealed substantially reduced abundance of HER2 protein (Fig. 6E). Of note, consistent with the recent report [27], we also found that the pan-HER TKI Neratinib decreased HER2 protein abundance as well as HER2 signaling (Fig. 6E and Fig. 5D). In addition, combination treatment did not yield overt toxic effects and mouse body weights were not significantly affected throughout the course of the treatment (Supplementary Fig. 5). Together, these results suggest that combined use of Melatonin and Neratinib may have the potential to improve the treatment response against HER2+ breast cancer.
A HCC1954 xenograft tumor-bearing mice were treated with Melatonin (50 mg/kg/day, intraperitoneal administration) and Neratinib (5 mg/kg/day, oral gavage), either alone or in combination. Vehicle, n = 7; Melatonin, n = 8; Neratinib, n = 8; Melatonin + Neratinib, n = 8. Tumor volume (Mean ± S.E.M) measured at the indicated time points was shown. **p < 0 .01, ***p < 0.001 (Two-way ANOVA with Tukey’s multiple comparison tests). B The waterfall plot indicates fold changes in tumor volume of the mice treated as indicated. Fold changes were calculated by (endpoint tumor volume-baseline tumor volume)/baseline tumor volume, multiplied by 100%. Baseline, tumor volume at treatment Day 0. Representative images of tumors (C) and tumor weight (D) at the endpoint (Day 26) were shown. The data are shown as the mean tumor weight ± S.E.M. E The HCC1954 xenograft tumor-bearing mice were treated as in A for 3 days and were sacrificed 4 h after the last treatment. Western blot analysis of total- and phospho-HER2 in tumor lysates from the mice treated as indicated. Vinculin was used as a loading control. The quantification of protein abundance is shown. The data are shown as Mean ± S.E.M. (n = 3). *p < 0.01; **p < 0.01; ***p < 0.001 (Student’s t test).
Discussion
The oncogenic driver HER2 remains a major therapeutic target in breast cancer harboring HER2 amplification/overexpression. Despite the success of HER2-targeted therapy, disease relapse will eventually occur. Intense clinical efforts have been focused on combining HER2-targeted agents of different mechanisms of action or extending the duration of HER2-targeted inhibition. Our study uncovers an unexpected action of Melatonin on destruction of HER2 through inducing HER2 endocytosis and lysosomal degradation. Importantly, our work reveals that Melatonin potentiates the cytotoxic effects of the pan-HER kinase inhibitor Neratinib in HER2+ breast cancer both in vitro and in vivo.
The prevention and treatment effects of Melatonin on cancer have been widely studied [14,15,16,17,18,19,20]. Previous reports have implicated anticancer properties of Melatonin in HER2+ breast cancer [34,35,36,37,38]. Melatonin inhibited cell invasion through suppressing the MAPK/ERK pathway in the studies using HER2+ breast cancer cell line modin vitroels [34, 35]. In our study, we showed that Melatonin monotherapy significantly attenuated the growth of HCC1954 xenografts, an effect consistent with previous reports using the MMTV-Neu mouse model of breast cancer [36]. In addition, when co-administered with estradiol-progesterone therapy or Metformin, Melatonin reduced tumor incidence and metastatic burden in the animal model of HER2+ breast cancer [36,37,38]. However, while these and our findings have reported the potential therapeutic effects of Melatonin in the setting of HER2+ breast cancer [34,35,36,37,38,39], the impact of Melatonin on HER2 expression per se has not been previously reported.
In the current study, Melatonin treatment led to significant downregulation of multiple HER2-mediated signal transduction in HER2+ breast cancer cells, recapitulating many effects exerted by HER2-targeted agents. These data prompted us to investigate whether Melatonin may inhibit the expression and/or activity of HER2 protein itself. Our work identified an unexpected role of Melatonin in dysregulating HER2 protein stability and potentiating the cytotoxic effect of Neratinib in HER2 + breast cancer. Mechanistically, Melatonin treatment triggered dissociation of HER2 from the HSP90 chaperone complex, and consequently ubiquitin-mediated endocytic trafficking and lysosomal degradation of HER2. Noteworthily, the lysosome inhibitor restored the HER2 protein abundance reduced by Melatonin treatment, whereas the proteasome inhibitor led to accelerated HER2 downregulation (Fig. 3A–C). It has been shown that HER2 ubiquitination is involved in both proteasomal and endocytic degradation [40]. Given that proteasome inhibition results in accumulation of ubiquitylated HER2 [41,42,43] and that Melatonin induces efficient HER2 ubiquitylation and endocytic lysosomal degradation (Fig. 4B), we reason that the resulting overall increase in HER2 ubiquitylation may accelerate its turnover through the endo-lysosomal degradation pathway [41, 44].
Melatonin is a naturally produced hormone with an impressive long-term safety profile [45,46,47]. In addition, to enhance therapeutic effects, Melatonin also showed the ability to reduce the adverse effects or general toxicity arising from chemotherapies or radiation in animal models and clinical studies [48,49,50]. In our study, combined use of Melatonin with Neratinib led to potent inhibitory anti-tumor activities with no overt mouse body weight changes, supporting the potential of Melatonin as a combination adjuvant in the treatment of HER2+ breast cancer. Given the prevalence of HER2 positivity in other solid tumors, such as gastric cancer and bladder cancer [51], our finding may expand the potential utility of Melatonin in HER2-targeted therapies beyond breast cancer.
Activated HER2 protein is exclusively present on the plasma membrane in breast cancer cells with HER2 amplification. The HSP90 and its associated molecular chaperone system protects HER2 from degradation, accounting for at least in part the stabilization of HER2 at the plasma membrane [32, 52, 53]. Our data show that Melatonin treatment resulted in decreased levels of HSP90 and HSC70 but a compensatory induction in HSP70 levels. The impairment in the chaperone system arising from Melatonin treatment is indeed consistent with previous reports that HSP70 expression is often concurrently induced in cells following treatment with HSP90 inhibitors or silencing of HSC70 [33, 54]. More importantly, we found that Melatonin treatment also led to attenuated association of HER2 from HSP90. Since HSP90 also chaperones a number of other therapeutic targets, such as CDK4, AKT, and steroid hormone receptors [55,56,57,58], it is conceivable that Melatonin may also confer the ability to selectively destruct other oncoproteins chaperoned by HSP90. As such, our study may be broadened to other cancer types with specific actionable vulnerabilities and shed new light on the utility of Melatonin as a promising agent in molecularly targeted cancer therapies.
Neratinib is an irreversible pan-HER receptor TKI. In addition to blocking the activities of pan-HER kinases, Neratinib has also been reported as a HER2 degradation-inducing factor through HER2 endocytosis [27]. In our study, the potential of understanding the activity of Melatonin on HER2 destruction is exemplified by the combinatorial therapeutic effect of Melatonin and Neratinib in HER2+ breast cancer cells. Our results from in vitro and in vivo experiments showed that Melatonin significantly enhances the cytotoxicity of Neratinib in a panel of HER2+ breast cancer cells harboring PIK3CA mutations known to be less responsive to HER2-targeted agents. Future studies will be needed to examine whether such drug combinations would also be effective in breast cancers resistant to HER2-targeted agents. Collectively, our finding offers a novel dual HER2 blockade (Melatonin plus Neratinib) strategy with effective duration on HER2 destruction.
Materials and methods
Cell culture and reagents
All cell lines were obtained from the American Type Culture Collection (ATCC, VA, USA) and maintained in culture medium (HCC1954 and MCF7/HER2 cells in RPMI-1640, MDA-MB-361 and MDA-MB-453 cells in Dulbecco’s Modified Eagle Medium) supplemented with 10% fetal bovine serum (FBS, Biological Industries, Kibbutz, Israel) and 1% penicillin/streptomycin (Gibco, CA, USA) in a humidified incubator at 5% CO2 and 37 °C. The cell identity was confirmed by short tandem repeat profiling at the beginning of this study. All cells were negative for mycoplasma during this investigation. Cycloheximide, MG132, Bafilomycin A1, Velcade, Melatonin, and Neratinib were purchased from Med Chem Express (MCE, Shanghai, China).
Clonogenic survival assay
Cells were seeded on 96-well and 24-well plates, and cultured with or without drugs for days as indicated. Fresh media containing drugs were replaced every other day. At the endpoint, cells were fixed and stained with 0.5% crystal violet solution. Images of stained plates were captured using Epson Scan (Nagano, Japan). Bound crystal violet was resolved by 50% acetic acid solution. The optical absorbance (OD) of bound crystal violet was measured at 570 nm using the Multi-functional microplate reader xMARKTM (Bio-Rad, CA, USA).
Determination of drug synergy
Cells were plated on 96-well plates and treated with or without drugs for three days, cell viability was assayed using the MTT solution (Aladdin, Shanghai, China). Synergistic effects were determined by the Chou-Talalay method to calculate the combination index [59].
Western blot analysis
Cell lysates were prepared using ice-cold lysis buffer (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 1 mM C3H7Na3O6P, 1 mM NaF, 1 mM Na4O7P2, 1 mM Na3VO4, and 5 mM PMSF) supplemented with protease/phosphatase inhibitors (Roche, Basel, Switzerland). Western blot experiments were conducted as described previously [60]. The blots were probed with the following primary antibodies: Anti-HER2 (#2165), anti-HSP90 (#4877), anti-phospho-HER2 (Tyr1221/1222, #2243), anti-phospho-AKT (S473, #4060), anti-phospho-ERK1/2 (Thr202/Tyr204, #4370), anti-phospho-NF-κB (Ser536, #3033), and anti-phospho-SRC family (Tyr416, #2101) were obtained from Cell Signaling Technology (CST, MA, USA). Anti-HER2(sc-08) and anti-N-cadherin (sc-271386) were obtained from Santa Cruz Biotechnology (TX, USA). Anti-ubiquitin (10201-2-AP), anti-HSP70 (10995-1-AP), and anti-HSC70 (10654-1-AP) were obtained from Proteintech (Wuhan, China). Anti-E-Cadherin (610181) was obtained from Becton, Dickinson and Company (BD Biosciences, NJ, USA). Anti-Vinculin (V9131) was obtained from Sigma Aldrich (MO, USA). Fluorescent-labeled secondary antibodies against mouse IgG and rabbit IgG were used (Li-COR, Nebraska, USA). Western blots were imaged and quantified by Odyssey Infrared Imaging System (Li-COR, Nebraska, USA).
Immunoprecipitation
One milligram of whole-cell lysates isolated from the HCC1954 cells treated with or without Melatonin was incubated with anti-HER2 antibody for 4 h at 4 °C followed by the addition of Protein A/G PLUS-Agarose (Santa Cruz Biotechnology) at 4 °C overnight. Beads were washed three times with ice-cold PBS supplemented with protease/phosphatase inhibitors (Roche, Switzerland). The immune complexes were eluted with 2×SDS PAGE sample buffer and analyzed by Western blot.
Flow cytometric analysis
Cell death assays were performed by using the Propidium Iodide (PI) solution (Dojindo Molecular Technologies, Kumamoto, Japan) according to the manufacturer’s protocol. Briefly, following drug treatment, cells were harvested and stained with PI solution in dark for 15 min. For flow cytometric analysis of HER2 protein levels present on the cell surface, cells were stained with APC anti-HER2 antibody (324407, Biolegend, CA, USA) or APC mouse IgG1 Isotype control antibody (400119, Biolegend). Stained cells were analyzed on NovoCyte Fluidics Station II (Agilent, CA, USA).
Wound-healing assay
Cells were seeded in 12-well plates and grown until a confluent state. The cells were scratched to generate an artificial wound by using sterile pipette tips. The cell monolayer was then rinsed with PBS three times to remove cell debris. Fresh culture medium with or without drug was added. Images of wound were captured by phase-contrast microscope (Leica, Wetzlar, Germany). The mean width of each scratch was measured using ImageJ software.
Immunofluorescence staining analysis
Immunofluorescence staining was performed as previously described [61]. Briefly, cells were fixed with 4% formaldehyde, permeabilized with 0.1% Triton X-100, and then blocked in 5% BSA. Then cells were incubated with anti-HER2 (ab134182, Abcam), anti-LAMP1 (#15665, CST) or anti-EEA1 (sc-137130, Santa Cruz) antibodies at 4 °C overnight followed by staining with fluorescence-conjugated secondary antibodies and DAPI solution (Sigma-Aldrich, Missouri, USA). Cells were photographed with a fluorescence microscope (Leica, Germany).
Quantitative reverse transcription PCR (qRT-PCR)
Total RNA was isolated using NucleoZOL Reagent (Macherey-Nagel, Düren Germany) according to the manufacturer’s instructions. For gene expression analysis, reverse transcription reaction was performed using total RNA by the cDNA synthesis kit (TaKaRa, Dalian, China), and gene expression levels were analyzed by qRT-PCR using SYBR Select Master Mix (Monad, Shanghai, China) in the QuantStudio™ 5 Real-Time PCR system (Thermo Fisher, MA, USA). The relative expression levels of target genes were normalized to ACTB and assessed by the delta-delta-Ct (ΔΔCT) method (expressed as 2−ΔΔCT). The following primers were used:
ACTB
5′-CATGTACGTTGCTATCCAGGC-3′ (Forward)
5’-CTCCTTAATGTCACGCACGAT-3′ (Reverse)
HER2
5′-CCGAGGGCCGGTATACATTC-3′ (Forward)
5′-TGCTGTCACCTCTTGGTTGT-3′ (Reverse)
Analysis of RNA sequencing data
We recently deposited the RNA-seq dataset to the Gene Expression Omnibus (GEO) with accession number GSE175906 [39]. Heatmap showing the expression of leading-edge subsets of gene sets using Omicshare (https://www.omicshare.com/tools/Home/Soft/heatmap). Parametric t test p values and false discovery rate (FDR) values were reported for each gene. GSEA was performed by the JAVA program (http://software.broadinstitute.org/gsea/index.jsp) using Molecular Signatures Database. 1000 random sample permutations were carried out, and the significance threshold was set at p < 0.05 and nominal FDR < 0.05.
In vivo mouse xenograft study
All the animal experiments were carried out in accordance with the approval of the Animal Research Committee of Dalian Medical University. Eight-week-old female NOD/SCID mice (Vital River Laboratory Animal Technology Co. Ltd, Beijing, China) were maintained in a pathogen-free environment. 5 × 106 HCC1954 cells mixed with Matrigel (BD Biosciences, NJ, USA) were inoculated into NOD/SCID mouse mammary fat pad. Tumor-bearing mice were randomized to four treatment groups (Vehicle, Melatonin, Neratinib, and Melatonin + Neratinib) so that the average starting tumor volumes (about 120 mm3) among different groups were similar before drug treatment. Neratinib was dissolved in 0.5% methylcellulose with 0.4% Tween-80 and administered via oral gavage at 5 mg/kg/day. Melatonin was dissolved in ethanol (13%) and administered via intraperitoneal administration at 50 mg/kg/day. Mouse body weights were measured daily during the course of treatment. Tumor volumes were measured every other day with a digital caliper and calculated according to the following formula: tumor volume = (length × width2)/2.
Statistical analysis
The in vitro data are expressed as Mean ± S.D. from three independent experiments. The in vivo data are expressed as Mean ± S.E.M. The unpaired student’s t tests and the two-way ANOVA with Tukey’s multiple-comparisons tests were performed using GraphPad Prism software for analysis of the data as specified in the corresponding figure legends. p < 0.05 was considered as statistical significance.
References
Gingras I, Gebhart G, de Azambuja E, Piccart-Gebhart M. HER2-positive breast cancer is lost in translation: time for patient-centered research. Nat Rev Clin Oncol. 2017;14:669–81.
Arteaga CL, Sliwkowski MX, Osborne CK, Perez EA, Puglisi F, Gianni L. Treatment of HER2-positive breast cancer: current status and future perspectives. Nature reviews. Clin Oncol. 2011;9:16–32.
Wang J, Xu B. Targeted therapeutic options and future perspectives for HER2-positive breast cancer. Signal Transduct Target Ther. 2019;4:34.
Goutsouliak K, Veeraraghavan J, Sethunath V, De Angelis C, Osborne CK, Rimawi MF, et al. Towards personalized treatment for early-stage HER2-positive breast cancer. Nature reviews. Clin Oncol. 2020;17:233–50.
Wissner A, Overbeek E, Reich MF, Floyd MB, Johnson BD, Mamuya N, et al. Synthesis and structure-activity relationships of 6,7-disubstituted 4-anilinoquinoline-3-carbonitriles. The design of an orally active, irreversible inhibitor of the tyrosine kinase activity of the epidermal growth factor receptor (EGFR) and the human epidermal growth factor receptor-2 (HER-2). J Med Chem. 2003;46:49–63.
Rabindran SK, Discafani CM, Rosfjord EC, Baxter M, Floyd MB, Golas J, et al. Antitumor activity of HKI-272, an orally active, irreversible inhibitor of the HER-2 tyrosine kinase. Cancer Res. 2004;64:3958–65.
Wissner A, Mansour TS. The development of HKI-272 and related compounds for the treatment of cancer. Arch Pharm. 2008;341:465–77.
Deeks ED. Neratinib: first global approval. Drugs. 2017;77:1695–704.
Burstein HJ, Sun Y, Dirix LY, Jiang Z, Paridaens R, Tan AR, et al. Neratinib, an irreversible ErbB receptor tyrosine kinase inhibitor, in patients with advanced ErbB2-positive breast cancer. J Clin Oncol. 2010;28:1301–7.
Xia W, Petricoin EF, Zhao S, Liu L, Osada T, Cheng Q, et al. An heregulin-EGFR-HER3 autocrine signaling axis can mediate acquired lapatinib resistance in HER2+ breast cancer models. Breast Cancer Res. 2013;15:R85.
Breslin S, Lowry MC, O’Driscoll L. Neratinib resistance and cross-resistance to other HER2-targeted drugs due to increased activity of metabolism enzyme cytochrome P4503A4. Br J Cancer. 2017;116:620–5.
Karakas B, Ozmay Y, Basaga H, Gul O, Kutuk O. Distinct apoptotic blocks mediate resistance to panHER inhibitors in HER2+ breast cancer cells. Biochim Biophys Acta Mol Cell Res. 2018;1865:1073–87.
Hyman DM, Piha-Paul SA, Won H, Rodon J, Saura C, Shapiro GI, et al. HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature. 2018;554:189–94.
Pariente R, Pariente JA, Rodríguez AB, Espino J. Melatonin sensitizes human cervical cancer HeLa cells to cisplatin-induced cytotoxicity and apoptosis: effects on oxidative stress and DNA fragmentation. J Pineal Res. 2016;60:55–64.
Chuffa LGA, Reiter RJ, Lupi LA. Melatonin as a promising agent to treat ovarian cancer: molecular mechanisms. Carcinogenesis. 2017;38:945–52.
Li Y, Li S, Zhou Y, Meng X, Zhang JJ, Xu DP, et al. Melatonin for the prevention and treatment of cancer. Oncotarget. 2017;8:39896–921.
Galano A, Tan DX, Reiter RJ. Melatonin: a versatile protector against oxidative DNA damage. Molecules. 2018;23:530–65.
Talib WH. Melatonin and cancer hallmarks. Molecules. 2018;23:518–34.
Wang M, Xue Y, Shen L, Qin P, Sang X, Tao Z, et al. Inhibition of SGK1 confers vulnerability to redox dysregulation in cervical cancer. Redox Biol. 2019;24:101225.
Kong X, Gao R, Wang Z, Wang X, Fang Y, Gao J, et al. Melatonin: a potential therapeutic option for breast cancer. Trends Endocrinol Metab. 2020;31:859–71.
Bhat-Nakshatri P, Sweeney CJ, Nakshatri H. Identification of signal transduction pathways involved in constitutive NF-kappaB activation in breast cancer cells. Oncogene. 2002;21:2066–78.
Moasser MM. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene. 2007;26:6469–87.
Zhang S, Huang WC, Li P, Guo H, Poh SB, Brady SW, et al. Combating trastuzumab resistance by targeting SRC, a common node downstream of multiple resistance pathways. Nat Med. 2011;17:461–9.
Chung SS, Giehl N, Wu Y, Vadgama JV. STAT3 activation in HER2-overexpressing breast cancer promotes epithelial-mesenchymal transition and cancer stem cell traits. Int J Oncol. 2014;44:403–11.
Clague MJ, Urbe S. Ubiquitin: same molecule, different degradation pathways. Cell. 2010;143:682–5.
Li BT, Michelini F, Misale S, Cocco E, Baldino L, Cai Y, et al. HER2-mediated internalization of cytotoxic agents in ERBB2 amplified or mutant lung cancers. Cancer Discov. 2020;10:674–87.
Zhang Y, Zhang J, Liu C, Du S, Feng L, Luan X, et al. Neratinib induces ErbB2 ubiquitylation and endocytic degradation via HSP90 dissociation in breast cancer cells. Cancer Lett. 2016;382:176–85.
Zhang J, Liu S, Li Q, Shi Y, Wu Y, Liu F, et al. The deubiquitylase USP2 maintains ErbB2 abundance via counteracting endocytic degradation and represents a therapeutic target in ErbB2-positive breast cancer. Cell Death Differ. 2020;27:2710–25.
Harari D, Yarden Y. Molecular mechanisms underlying ErbB2/HER2 action in breast cancer. Oncogene. 2000;19:6102–14.
Hendriks BS, Opresko LK, Wiley HS, Lauffenburger D. Coregulation of epidermal growth factor receptor/human epidermal growth factor receptor 2 (HER2) levels and locations: quantitative analysis of HER2 overexpression effects. Cancer Res. 2003;63:1130–7.
Goetz MP, Toft DO, Ames MM, Erlichman C. The Hsp90 chaperone complex as a novel target for cancer therapy. Ann Oncol. 2003;14:1169–76.
Pedersen NM, Madshus IH, Haslekås C, Stang E. Geldanamycin-induced down-regulation of ErbB2 from the plasma membrane is clathrin-dependent but proteasomal activity independent. Mol Cancer Res. 2008;6:491–500.
Powers MV, Clarke PA, Workman P. Dual targeting of HSC70 and HSP72 inhibits HSP90 function and induces tumor-specific apoptosis. Cancer Cell. 2008;14:250–62.
Mao L, Yuan L, Slakey LM, Jones FE, Burow ME, Hill SM. Inhibition of breast cancer cell invasion by melatonin is mediated through regulation of the p38 mitogen-activated protein kinase signaling pathway. Breast Cancer Res. 2010;12:R107.
Mao L, Summers W, Xiang S, Yuan L, Dauchy RT, Reynolds A, et al. Melatonin represses metastasis in Her2-positive human breast cancer cells by suppressing RSK2 expression. Mol Cancer Res. 2016;14:1159–69.
Anisimov VN, Egormin PA, Piskunova TS, Popovich IG, Tyndyk ML, Yurova MN, et al. Metformin extends life span of HER-2/neu transgenic mice and in combination with melatonin inhibits growth of transplantable tumors in vivo. Cell Cycle. 2010;9:188–97.
Dodda BR, Bondi CD, Hasan M, Clafshenkel WP, Gallagher KM, Kotlarczyk MP, et al. Co-administering melatonin with an estradiol-progesterone menopausal hormone therapy represses mammary cancer development in a mouse model of HER2-positive breast cancer. Front Oncol. 2019;9:525.
Anisimov VN, Alimova IN, Baturin DA, Popovich IG, Zabezhinski MA, Manton KG, et al. The effect of melatonin treatment regimen on mammary adenocarcinoma development in HER-2/neu transgenic mice. Int J Cancer. 2003;103:300–5.
Sang X, Li L, Rui C, Liu Y, Liu Z, Tao Z, et al. Induction of EnR stress by Melatonin enhances the cytotoxic effect of Lapatinib in HER2-positive breast cancer. Cancer Lett. 2021;518:82–93.
Clague MJ, Liu H, Urbe S. Governance of endocytic trafficking and signaling by reversible ubiquitylation. Dev Cell. 2012;23:457–67.
Huynh TK, Ho CY, Tsai CH, Wang CK, Chen YJ, Bau DT, et al. Proteasome inhibitors suppress ErbB family expression through HSP90-mediated lysosomal degradation. Int J Mol Sci. 2019;20:4812–26.
Ren Y, Chen D, Zhai Z, Chen J, Li A, Liang Y, et al. JAC1 suppresses proliferation of breast cancer through the JWA/p38/SMURF1/HER2 signaling. Cell Death Discov. 2021;7:85.
Marx C, Held JM, Gibson BW, Benz CC. ErbB2 trafficking and degradation associated with K48 and K63 polyubiquitination. Cancer Res. 2010;70:3709–17.
Strous GJ, Govers R. The ubiquitin-proteasome system and endocytosis. J Cell Sci. 1999;112:1417–23.
Andersen LP, Gögenur I, Rosenberg J, Reiter RJ. The safety of melatonin in humans. Clin drug Investig. 2016;36:169–75.
Sletten TL, Magee M, Murray JM, Gordon CJ, Lovato N, Kennaway DJ, et al. Efficacy of melatonin with behavioural sleep-wake scheduling for delayed sleep-wake phase disorder: a double-blind, randomised clinical trial. PLoS Med. 2018;15:e1002587.
Malow BA, Findling RL, Schroder CM, Maras A, Breddy J, Nir T, et al. Sleep, growth, and puberty after 2 years of prolonged-release melatonin in children with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2021;60:252–61.
Lissoni P, Barni S, Mandalà M, Ardizzoia A, Paolorossi F, Vaghi M, et al. Decreased toxicity and increased efficacy of cancer chemotherapy using the pineal hormone melatonin in metastatic solid tumour patients with poor clinical status. Eur J Cancer. 1999;35:1688–92.
Sookprasert A, Johns NP, Phunmanee A, Pongthai P, Cheawchanwattana A, Johns J, et al. Melatonin in patients with cancer receiving chemotherapy: a randomized, double-blind, placebo-controlled trial. Anticancer Res. 2014;34:7327–37.
Zhang J, Xie T, Zhong X, Jiang HL, Li R, Wang BY, et al. Melatonin reverses nasopharyngeal carcinoma cisplatin chemoresistance by inhibiting the Wnt/β-catenin signaling pathway. Aging. 2020;12:5423–38.
Oh DY, Bang YJ. HER2-targeted therapies - a role beyond breast cancer. Nat Rev Clin Oncol. 2020;17:33–48.
Tikhomirov O, Carpenter G. Identification of ErbB-2 kinase domain motifs required for geldanamycin-induced degradation. Cancer Res. 2003;63:39–43.
Xu W, Yuan X, Xiang Z, Mimnaugh E, Marcu M, Neckers L. Surface charge and hydrophobicity determine ErbB2 binding to the Hsp90 chaperone complex. Nat Struct Mol Biol. 2005;12:120–6.
Shevtsov M, Multhoff G, Mikhaylova E, Shibata A, Guzhova I, Margulis B. Combination of anti-cancer drugs with molecular chaperone inhibitors. Int J Mol Sci. 2019;20.
Basso AD, Solit DB, Chiosis G, Giri B, Tsichlis P, Rosen N. Akt forms an intracellular complex with heat shock protein 90 (Hsp90) and Cdc37 and is destabilized by inhibitors of Hsp90 function. J Biol Chem. 2002;277:39858–66.
Workman P. Combinatorial attack on multistep oncogenesis by inhibiting the Hsp90 molecular chaperone. Cancer Lett. 2004;206:149–57.
Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5:761–72.
Hallett ST, Pastok MW, Morgan RML, Wittner A, Blundell K, Felletar I, et al. Differential regulation of G1 CDK complexes by the Hsp90-Cdc37 chaperone system. Cell Rep. 2017;21:1386–98.
Ashton JC. Drug combination studies and their synergy quantification using the Chou-Talalay method—letter. Cancer Res. 2015;75:2400.
Ding J, Wang X, Zhang Y, Sang X, Yi J, Liu C, et al. Inhibition of BTF3 sensitizes luminal breast cancer cells to PI3Kα inhibition through the transcriptional regulation of ERα. Cancer Lett. 2019;440-441:54–63.
Yi J, Liu C, Tao Z, Wang M, Jia Y, Sang X, et al. MYC status as a determinant of synergistic response to Olaparib and Palbociclib in ovarian cancer. EBioMedicine. 2019;43:225–37.
Acknowledgements
We acknowledge the support of the Dalian Key Laboratory of Molecular Targeted Cancer Therapy and the Molecular Biology Laboratory of National Administration of Traditional Chinese Medicine (Class III). This work was supported by the National Natural Science Foundation of China (Nos. 81672575 and 81874111 to HC; No. 81972482 to PL), the Liaoning Provincial Key Research and Development Program (No. 2020JH2/10300049 to PL), Liaoning Revitalization Talents Program (XLYC2002043 to PL), Dalian Leading Talents Fund (203598 to PL), the Liaoning Provincial Climbing Scholars Supporting Program of China (HC and PL), the Scientific Research Fund of Liaoning Provincial Department of Education (LZ2020006 to PL; LZ2020069 to XS), and Science and Technology Innovation Fund of Dalian Department of Science and Technology (2020JJ27SN079 to HC, 2021JJ12SN39 to PL.).
Author information
Authors and Affiliations
Contributions
HC, PL, and XS made substantial contributions to conception, design, and interpretation of data. HC, PL, XS, and ZL drafted and revised the manuscript critically. ZL performed majority of the experiments. XW helped with western blot experiments. XS performed FACS analysis and bioinformatics analysis. ZL and MW performed the co-immunoprecipitation analysis. YL, JL, and ZL performed animal experiments.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, Z., Sang, X., Wang, M. et al. Melatonin potentiates the cytotoxic effect of Neratinib in HER2+ breast cancer through promoting endocytosis and lysosomal degradation of HER2. Oncogene 40, 6273–6283 (2021). https://doi.org/10.1038/s41388-021-02015-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-021-02015-w
This article is cited by
-
Elucidating molecular mechanisms and therapeutic synergy: irreversible HER2-TKI plus T-Dxd for enhanced anti-HER2 treatment of gastric cancer
Gastric Cancer (2024)
-
Neratinib for HER2-positive breast cancer with an overlooked option
Molecular Medicine (2023)
-
Melatonin inhibits bladder tumorigenesis by suppressing PPARγ/ENO1-mediated glycolysis
Cell Death & Disease (2023)
-
Melatonin inhibits ESCC tumor growth by mitigating the HDAC7/β-catenin/c-Myc positive feedback loop and suppressing the USP10-maintained HDAC7 protein stability
Military Medical Research (2022)
-
Synchronous intracellular delivery of EGFR-targeted antibody–drug conjugates by p38-mediated non-canonical endocytosis
Scientific Reports (2022)