Abstract
Oncogene HER2 is amplified in 20%–25% of human breast cancers and 6.1%–23.0% of gastric cancers, and HER2-directed therapy significantly improves the outcome for patients with HER2-positive cancers. However, drug resistance is still a clinical challenge due to primary or acquired mutations and drug-induced negative regulatory feedback. In this study, we discovered a potent irreversible HER2 kinase inhibitor, CHMFL-26, which covalently targeted cysteine 805 of HER2 and effectively overcame the drug resistance caused by HER2 V777L, HER2 L755S, HER2 exon 20 insertions, and p95-HER2 truncation mutations. CHMFL-26 displayed potent antiproliferation efficacy against HER2-amplified and mutant cells through constant HER2-mediated signaling pathway inhibition and apoptosis induction. In addition, CHMFL-26 suppressed tumor growth in a dose-dependent manner in xenograft mouse models. Together, these results suggest that CHMFL-26 may be a potential novel anti-HER2 agent for overcoming drug resistance in HER2-positive cancer therapy.
Similar content being viewed by others
Introduction
HER2 is a receptor tyrosine kinase that belongs to the epidermal growth factor receptor kinase family [1]. No soluble ligand has been identified for this protein thus far, and it is activated by heterodimerization with other members of EGFR family, such as EGFR or HER3 [2]. The HER2-mediated signaling pathway plays an important role in cell proliferation and survival [3]. HER2 gene amplification and somatic mutations are closely related to cancer cell transformation and oncogenesis [4]. Therefore, HER2 is as an oncogene expressed in several cancers, and amplification of HER2 has been found in 20%–25% of human breast cancers [5] and 6.1%–23.0% of gastric cancers [6] and is thus considered a therapeutic target for disease treatment and prognosis.
With the rapid drug discovery realized in the past few decades, a variety of drugs with different action mechanisms targeting HER2 have been clinically approved, such as the monoclonal antibodies trastuzumab [7, 8] and pertuzumab [9], the antibody-drug conjugate T-DM1 [10, 11], and the small-molecule inhibitors lapatinib [12, 13] and neratinib [14, 15]. Although HER2-targeted therapies have significantly improved the prognosis for HER2-positive cancers, the use of anti-HER2 therapies is still limited, and one reason is primary or acquired drug-resistant mutations. For example, both the kinase domain mutation V777L and a p95 truncation mutation confer resistance to trastuzumab [16, 17], and the L755S mutation leads to resistance to lapatinib [18, 19]. The compensatory upregulation of HER3 and downstream PI3K/AKT signaling may also contribute to lapatinib and trastuzumab resistance [20]. In addition, the side effects of HER2 small-molecule inhibitors have raised concerns about their clinical use. As a pan-HER inhibitor, neratinib shows poor selectivity between EGFR and HER2 kinases. As a result, neratinib inhibits EGFR in normal tissues, causing side effects that include vomiting and diarrhea [21]. Thus, new HER2 inhibitors with the ability to overcome drug resistance and that have a safer therapeutic window are still in great demand.
We previously described CHMFL-26, a selective mutant-specific EGFR inhibitor [22]. CHMFL-26 showed the ability to distinguish between mutant and wild-type EGFR within a selectivity window and rarely affected the proliferation of EGFR wt-expressing cells. Here, we report that CHMFL-26 is a highly potent irreversible HER2 inhibitor that can be used to overcome drug-resistant mutations and that displays high antitumor efficacy both in vitro and in vivo.
Materials and methods
Chemical reagents
CHMFL-26 and CHMFL-26R were synthesized in the laboratory as previously described [22]; lapatinib (CAS #231277-92-2) was purchased from Shanghai Haoyuan Chemexpress Co., Ltd. (Shanghai, China).
Cell lines
Transgenic BaF3 cell lines expressing HER2 mutations were constructed in our laboratory as previously reported [23]. The BT-474 human breast cancer cell line, OE19 esophageal carcinoma cell line, and NCI-N87 gastric cancer cell line were purchased from Cobioer Biosciences Co., Ltd. (Nanjing, China). The BT-474, OE19, NCI-N87, and transgenic BaF3 cell lines were cultured in RPMI-1640 medium (Corning, Midland, NY, USA) with 10% FBS (v/v) (ExcellBio, Shanghai, China) supplemented with 1% penicillin/streptomycin (v/v).
Antibodies
The anti-phospho-HER2/ErbB2(Tyr1248) rabbit mAb (#2247), anti-phospho-HER2/ErbB2 (Tyr1221/1222) (6B12) rabbit mAb (#2243), anti-HER2/ErbB2 rabbit mAb (#4290), anti-EGF receptor (D38B1) XP rabbit mAb (#4267), anti-phospho-EGF receptor (Tyr1068) (D7A5) XP rabbit mAb (#3777), anti-Akt (pan) (C67E7) rabbit mAb (#4691), anti-phospho-Akt (Ser473) (D9E) XP rabbit mAb (#4060), anti-phospho-Akt (Thr308) (D25E6) XP rabbit mAb (#13038), anti-p44/42 MAPK (Erk1/2) (137F5) rabbit mAb (#4695), anti-phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) (D13.14.4E) XP rabbit mAb (#4370), anti-PARP rabbit mAb (#9542), anti-caspase-3 rabbit mAb (#9662) and anti-GAPDH XP rabbit mAb (#D16H11) were obtained from Cell Signaling Technology (Danvers, MA, USA). All antibodies were used at a 1:1000 dilution in immunoblotting experiments.
Biochemical assay
The enzymatic inhibition assay of CHMFL-26 was performed using SelectScreen technology according to the manufacturer’s instructions (Thermo Fisher Scientific).
Apoptosis assay
BT-474, OE19, and NCI-N87 cells were treated with DMSO, CHMFL-26 (0.03, 0.1, 0.3, and 1 μM), and lapatinib (1 μM) for 24 to 48 h before they were harvested. The cells were then washed in PBS and lysed in cell lysis buffer for immunoblot analysis using anti-PARP, anti-caspase-3, and anti-GAPDH antibodies.
OE19 xenograft tumor model
Five-week-old female nu/nu mice were purchased from the Nanjing Biomedical Research Institute of Nanjing University (Nanjing, China). All animals were housed in a specific pathogen-free facility and used according to the animal care regulations of Hefei Institutes of Physical Science, Chinese Academy of Sciences (Hefei, China). Five million OE19 cells in PBS were formulated in a 1:1 mixture with Matrigel (BD Biosciences) and injected into the subcutaneous space on the right flank of the nu/nu mice. Animals were then randomly assigned to treatment groups, each with 5 mice, for efficacy studies. Daily oral administration was initiated when tumors reached a size of 200–400 mm3. CHMFL-26 was delivered in an HKI suspension (0.5% methylcellulose/0.4% Tween 80 in ddH2O) by p.o. (BID). A range of doses of CHMFL-26 or a vehicle was administered as described in Fig. 5 legend. Body weight and tumor growth were measured daily after CHMFL-26 treatment. Tumor volumes were calculated as follows: tumor volume (mm3) = [(W2 × L)/2], in which width (W) was defined as the smaller of the two measurements and length (L) was defined as the larger of the two measurements.
Data and statistical analysis
All results are presented as the means ± SEM, and Student’s t test was performed for comparing different groups; IC50 and GI50 values were calculated using Prism 8.0 (GraphPad Software, San Diego, CA, USA) using a normalized inhibitory dose response curve (variable slope).
Results
CHMFL-26 is an irreversible HER2 inhibitor
In a previous study [22], we described a covalent EGFR mutant-specific inhibitor, CHMFL-EGFR-26, which selectively inhibited the cell growth of NSCLC cells harboring EGFR mutations (EGFR T790M, L858R, and del19). Biochemical assays showed that CHMFL-26 also potently inhibited HER2 kinase with an IC50 of 16.36 nM. However, the reversible form CHMFL-26R, which was generated by saturation of acrylamide to propionamide almost completely lost the activity to the HER2 kinase (Fig. 1a). In addition, CHMFL-26 effectively inhibited the proliferation of HER2-WT HER2-P95-BaF3 (GI50: 6.4 nM) but not HER2-mutation at the covalent binding site HER2-P95-C805S-BaF3 (GI50: 3813.7 nM). The reversible form CHMFL-26R almost completely lost the activity to the CHMFL-26 sensitive mutants (GI50: 1007 nM) not to mention the CHMFL-26 insensitive mutants (GI50: >10000 nM) (Fig. 1b). We then used a washout assay to explore the reversibility of CHMFL-26, and the data showed that CHMFL-26 consistently inhibited HER2 autophosphorylation in NCI-N87 cells for up to 48 h, even after CHMFL-26 was removed at hour 4 of the experiment (Fig. 1c). In addition, we constructed two transiently transfected cell lines that expressed HER2-P95 and HER2-P95-C805S mutants. CHMFL-26 inhibited HER2 phosphorylation in HER2-P95-293T cells but not in HER2-P95-C805S-293T cells. Lapatinib, a reversible inhibitor, inhibited HER2 phosphorylation in 293T cells transfected with either HER2-P95 or HER2-P95-C805S plasmid (Fig. 1d). As expected, molecular modeling of CHMFL-26 using the reported crystal structure of HER2 kinase (PDB ID: 3RCD) suggested that the electrophilic acrylamide is poised in a suitable position to allow covalent bond formation with the Cys805 residue (Fig. 1e). Taken together, the acrylamide saturation to propionamide, reactive cysteine residue mutation, washout, and molecular docking experiments all provided clear evidence that CHMFL-26 covalently binds to HER2 and forms a covalent bond with the Cys805 residue.
a IC50 determination of CHMFL-26, CHMFL-26R, and lapatinib with purified HER2 using the ADP-Glo assay (n = 3). b Antiproliferative effects of CHMFL-26, CHMFL-26R, and lapatinib against HER2-p95 wt and HER2-p95-C805S mutant transformed BaF3 cells. c Inhibition of HER2 phosphorylation in NCI-N87 cells cultured with 1 μM CHMFL-26 for 4 h followed by drug wash out for 0, 1, 2, 4, 8, 24 and 48 h. d Inhibition of HER2 phosphorylation in 293T cells transfected with HER2-p95 or HER2-p95-C805S plasmids after treatment with CHMFL-26, CHMFL-26R and lapatinib. e The irreversible binding mode of CHMFL-26 with HER2 (PDB ID: 3RCD).
CHMFL-26 selectively inhibits the proliferation of HER2-positive cells
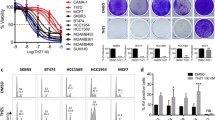
Next, we tested CHMFL-26 against a panel of cancer cell lines and found that it exhibited potent antiproliferation effects against cell lines with HER2 amplifications, such as the BT-474 breast cancer cell line (GI50 = 2.6 nM), NCI-N87 gastric cancer cell line (GI50 = 1 nM), and OE19 esophageal carcinoma cancer cell line (GI50 = 29 nM). Moreover, CHMFL-26 did not affect the growth of ER-positive or triple-negative breast cancer cells, indicating that it selectively inhibited the proliferation of HER2-positive cells. In addition, MDA-MB-231 and MDA-MB-468 triple-negative breast cancer cells were not sensitive to CHMFL-26 (GI50 = 9300 nM and 5734 nM) (Fig. 2a). To confirm CHMFL-26 selectivity between HER2 and EGFR, we examined the phosphorylation of HER2 (EC50 = 7.6 nM) and EGFR (EC50 = 64.4 nM) in NCI-N87 cells, and the results showed that CHMFL-26 achieved greater than 8-fold selectivity between HER2 and EGFR (Fig. 2b). These findings indicate that CHMFL-26 potently and preferentially inhibited the proliferation of HER2-positive cells compared to EGFR-expressing cells.
a Antiproliferative effects of CHMFL-26 on a panel of cell lines. Cell viability was measured by CellTiter–Glo assay (error bars, the mean ± SEM, n = 3). b Inhibition of HER2 and EGFR phosphorylation in NCI-N87 cells treated with CHMFL-26 for 4 h. c Determination of the growth inhibition effects of CHMFL-26 against a panel of BaF3-engineered cell lines (including different HER2 fusions and mutants). Cells were treated with CHMFL-26, CHMFL-26R, or lapatinib (maximum concentration 10 μM) for 72 h. d EC50 determination of CHMFL-26 in different HER2 fusion- and/or mutant BaF3-engineered cell lines. The results are reported as the mean ± SD of three independent experiments; ns not statistically significant; *P < 0.05, **P < 0.01, and ***P < 0.001.
We then investigated the antiproliferation activity of CHMFL-26 against a panel of engineered BaF3 cell lines expressing wild-type or mutant HER2 kinases. In this study, CHMFL-26 showed inhibitory effects against cells carrying HER2 fusions (MDK-HER2-BaF3 GI50 = 13 nM, ZNF207-HER2-BaF3 GI50 < 1.5 nM) and displayed significant selectivity over parental BaF3 cell lines (GI50 > 10000 nM). More importantly, the compound effectively inhibited the growth of cells expressing lapatinib-resistant mutation HER2 L775S (GI50 = 78 nM), trastuzumab-resistant mutation HER2 V777L (GI50 = 15 nM), and truncated p95-HER2 (GI50 = 6.4 nM), and those with de novo resistance to covalent and noncovalent TKIs HER2 exon 20 insertions (GI50 = 21 nM). Consistently, the reversible form CHMFL-26R lost inhibitory activity in all of the aforementioned sensitive cells tested (Fig. 2c, Supplemental Table 1), which confirmed that CHMFL-26 irreversibly binds to HER2 kinase.
To verify the on-target effect of CHMFL-26, we examined its inhibitory effect on HER2 activation in HER2 wt and mutant BaF3 cells. CHMFL-26 effectively blocked the phosphorylation of HER2 with an EC50 of 6.5, 29.3 and 10.8 nM in MDK-HER2-BaF3, ZNF207-HER2-BaF3 and HER2-P95-BaF3 cells, respectively. Similar inhibitory activities were observed for HER2-mutant cells (HER2 L755S EC50 = 97.7 nM, HER2 V777L EC50 = 18.8 nM, and MDK-HER2-ex20 Ins EC50 = 30.7 nM) (Fig. 2d). These results showed that CHMFL-26 effectively inhibited the activation of HER2 kinase in both HER2-amplified and HER2-mutant cells.
CHMFL-26 effectively blocks the oncogenic activities mediated by HER2 kinase in HER2-positive cancer cell lines
Since HER2 inhibitors exert their pharmacological effects mainly through inhibition of HER2 activity and its downstream signaling pathways, we next examined the ability of CHMFL-26 to suppress the HER2-mediated signaling pathway. Our results showed that CHMFL-26 treatment significantly reduced the phosphorylation of HER2 and the downstream targets Akt and ERK1/2 in HER2-amplified BT-474, OE19, and NCI-N87 cancer cell lines (Fig. 3a). After 24 or 48 h of treatment, CHMFL-26 also induced PARP and caspase-3 cleavage in a dose-dependent manner (Fig. 3b). To investigate the mechanism by which CHMFL-26 inhibits the growth of HER2-positive cancer cells, we performed a cell cycle analysis with these cells. A significant increase in the number of HER2-amplified BT-474, OE19, and NCI-N87 cells in the G0/G1 phase was observed after 24 h of treatment with CHMFL-26 (Fig. 3c). In addition, CHMFL-26 dramatically inhibited colony formation in these HER2-amplified cell lines after 7 days or 14 days of treatment (Fig. 3d). These results revealed that CHMFL-26 effectively suppressed HER2-positive cell growth by inducing cell cycle arrest and apoptosis.
CHMFL-26 exhibits persistent inhibition of HER2, HER3, and their downstream signaling pathways in OE19 and NCI-N87 cells
One of the issues with lapatinib, a HER2 inhibitor approved by the FDA for breast cancer treatment, is the reactivation of HER3, AKT, and ERK1/2 after 48 h of treatment, probably due to negative regulatory signaling feedback [24] (Fig. 4a, b). To determine whether CHMFL-26 can prevent this reactivation, we examined the long-term effects of CHMFL-26 on OE19 and NCI-N87 cells and found that it persistently inhibited HER3, AKT, and ERK1/2 for 48 h without feedback effects which had been observed in lapatinib group.
CHMFL-26 inhibits tumor progression in vivo
To assess the in vivo antitumor activity of CHMFL-26, we treated xenograft model mice inoculated with OE19 cells with different dosages of CHMFL-26 and observed them for 28 days. CHMFL-26 displayed dose-dependent tumor growth suppression with a tumor growth inhibition rate of 85.9%, and no mouse body weight loss was observed (200 mg/kg dosage, bid) (Fig. 5a). In addition, an immunohistochemical analysis showed that CHMFL-26 induced cell apoptosis (TUNEL staining) and reduced cell growth (Ki67 staining) in the tumor tissues (Fig. 5b). Furthermore, CHMFL-26 persistently suppressed the phosphorylation of HER2 and the downstream targets AKT and ERK1/2 in tumor tissues in a dose-dependent manner (Fig. 5c). However, negative regulatory feedback of HER3 and its downstream signaling pathway was evident in the tumor tissues of the lapatinib group. Taken together, these results showed that CHMFL-26 exhibited potent in vivo antitumor efficacy in HER2-positive cancer cells.
Female nu/nu mice bearing an established OE19 tumor xenograft model were treated with CHMFL-26 at 50, 100, and 200 mg/kg bid dosages or vehicle. Twice-daily oral administration was initiated when tumors had reached a size of 200–400 mm3. Each group contained five animals. Data are reported as the means ± SEM. a Body weight changes in mice in each twice-daily CHMFL-26 or lapatinib dosing group. Initial body weight was set at 100%. Relative tumor size measurements of OE19 xenograft mice after CHMFL-26 or lapatinib treatment. Representative photographs of tumors in each group after 50, 100, and 200 mg/kg bid CHMFL-26, lapatinib or vehicle treatment. Comparison of the final tumor weight in each group during the 28-day treatment period. b Immunohistochemical analysis of tumors was performed with the indicated antibodies. c Western blot analysis of CHMFL-26 on HER2 signaling pathways in tumor tissues after the 28-day treatment period.
Discussion
HER2-targeted therapy significantly improved the outcome of HER2-positive cancers [25]. However, poor drug response and drug resistance remain clinical challenges. It was reported [26] that the MET, HER3, IGF1R, and INSR pathways are determinants of lapatinib unresponsiveness in HER2-positive gastric cancer. Several mechanisms contribute to drug resistance, such as gain-of-function mutations during the long-term use of drugs and drug-induced negative regulatory feedback [27]. Here, we report that an irreversible HER2 inhibitor, CHMFL-26, with a pyrazolopyrimidine pharmacophore effectively inhibited HER2 kinase activity and the proliferation of HER2-amplified and mutant cells. Saturation of acrylamide to propionamide, reactive cysteine residue mutation, washout experiments, and molecular docking analyses provided clear evidence that CHMFL-26 covalently binds to HER2 at the Cys805 residue in a cellular context. The effect of the reversible inhibitor lapatinib was meaningfully different in 293T-transfected HER2-P95 and HER2-P95-C805S plasmid-carrying cells. These results confirmed that the irreversible binding mode observed with CHMFL-26 in the molecular docking analysis was biologically relevant and indicated a mode of action that differs from that of lapatinib and trastuzumab. The selective effects of CHMFL-26 on HER2 and EGFR suggested that CHMFL-26 may induce less side effects, such as rash, diarrhea, and hepatic disorders [28]. CHMFL-26 intensely inhibited the proliferation of BaF3 isogenic cell lines expressing drug-resistant HER2 kinase wt/mutants, strongly induced cell cycle arrest and cell apoptosis, and inhibited colony formation of HER2-positive cancer cell lines, which indicates the capability of this compound to overcome drug-resistant mutations at the cellular level. In addition, consistent with irreversible inhibitors known to form stable complexes with target enzymes [29] and to dissociate very slowly [30], CHMFL-26 constantly blocked HER2-mediated signaling without reactivating HER3 in OE19 and NCI-N87 cells for 48 h, but the reactivation was particularly notable in the lapatinib group. Additionally, after 28 days of continuous drug treatment in vivo, CHMFL-26 maintained effective inhibition of the phosphorylation levels of HER2, HER3, AKT and ERK in tumor tissues. The expression of HER3 is a potential target in tumorigenic signaling and drug resistance; in particular, the HER3 pathway is a determinant of lapatinib unresponsiveness in HER2-positive gastric cancer [31], which may explain the reason that lapatinib showed a poor effect in the OE19 xenograft tumor model.
Although the short half-life of irreversible inhibitors has been appreciated because of the possible adverse effect that they can induce during circulation in vivo [32], the relatively short life of CHMFL-26 (t1/2 = 0.8 h) leads to relatively low in vivo efficacy [22] and a low tumor penetration rate (Supplementary Table 2, Fig. 3).Therefore, we needed to use a higher dosage (200 mg/kg bid, po) to achieve antitumor effects, which may increase the risk of side effects. Therefore, further chemical optimization is needed to improve the pharmacological properties of CHMFL-26.
In summary, we discovered a HER2 irreversible inhibitor, CHMFL-26, with potent antitumor efficacy both in vitro and in vivo in HER2-positive cancer cells. CHMFL-26 overcame the acquired drug resistance to multiple at the cellular level and consistently inhibited HER3 pathways both in vitro and in vivo, making it a potential drug candidate for HER2-positive cancer therapy.
References
Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341.
Lemmon MA, Schlessinger J, Ferguson KM. The EGFR family: not so prototypical receptor tyrosine kinases. Cold Spring Harb Perspect Biol. 2014;6:a020768.
Hynes NE, MacDonald G. ErbB receptors and signaling pathways in cancer. Curr Opin Cell Biol. 2009;21:177.
Moasser MM. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene. 2007;26:6469.
Arteaga CL, Sliwkowski MX, Osborne CK, Perez EA, Puglisi F, Gianni L. Treatment of HER2-positive breast cancer: current status and future perspectives. Nat Rev Clin Oncol. 2012;9:16.
Wang DS, Liu ZX, Lu YX, Bao H, Wu X, Zeng ZL, et al. Liquid biopsies to track trastuzumab resistance in metastatic HER2-positive gastric cancer. Gut. 2019;68:1152.
Pinto AC, Ades F, de Azambuja E, Piccart-Gebhart M. Trastuzumab for patients with HER2 positive breast cancer: delivery, duration and combination therapies. Breast. 2013;22:S152.
Shak S, Herceptin Multinational Investigator Study Group. Overview of the trastuzumab (Herceptin) anti-HER2 monoclonal antibody clinical program in HER2-overexpressing metastatic breast cancer. Semin Oncol. 1999;26:71.
von Minckwitz G, Procter M, de Azambuja E, Zardavas D, Benyunes M, Viale G, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med. 2017;377:702.
Lambert JM, Chari RVJ. Ado-trastuzumab emtansine (T-DM1): an antibody-drug conjugate (ADC) for HER2-positive breast cancer. J Med Chem. 2014;57:6949.
Montemurro F, Ellis P, Anton A, Wuerstlein R, Delaloge S, Bonneterre J, et al. Safety of trastuzumab emtansine (T-DM1) in patients with HER2-positive advanced breast cancer: primary results from the KAMILLA study cohort 1. Eur J Cancer. 2019;109:92.
Burris HA. Dual kinase inhibition in the treatment of breast cancer: Initial experience with the EGFR/ErbB-2 inhibitor lapatinib. Oncologist. 2004;9:10.
Xia W, Husain I, Liu L, Bacus S, Saini S, Spohn J, et al. Lapatinib antitumor activity is not dependent upon phosphatase and tensin homologue deleted on chromosome 10 in ErbB2-overexpressing breast cancers. Cancer Res. 2007;67:1170.
Awada A, Dirix L, Manso Sanchez L, Xu B, Luu T, Dieras V, et al. Safety and efficacy of neratinib (HKI-272) plus vinorelbine in the treatment of patients with ErbB2-positive metastatic breast cancer pretreated with anti-HER2 therapy. Ann Oncol. 2013;24:109.
Echavarria I, Lopez-Tarruella S, Marquez-Rodas I, Jerez Y, Martin M. Neratinib for the treatment of HER2-positive early stage breast cancer. Expert Rev Anticancer Ther. 2017;17:669.
Eliyatkin NO, Aktas S, Ozgur H, Ercetin P, Kupelioglu A. The role of p95HER2 in trastuzumab resistance in breast cancer. J Buon. 2016;21:382.
Hirotsu Y, Nakagomi H, Amemiya K, Oyama T, Inoue M, Mochizuki H, et al. Intrinsic HER2 V777L mutation mediates resistance to trastuzumab in a breast cancer patient. Med Oncol. 2017;34:3.
Bose R, Kavuri SM, Searleman AC, Shen W, Shen D, Koboldt DC, et al. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov. 2013;3:224.
Kancha RK, von Bubnoff N, Bartosch N, Peschel C, Engh RA, Duyster J. Differential sensitivity of ERBB2 kinase domain mutations towards lapatinib. PLoS ONE. 2011;6:e26760.
Sergina NV, Rausch M, Wang D, Blair J, Hann B, Shokat KM, et al. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature. 2007;445:437.
Cortes J, Dieras V, Ro J, Barriere J, Bachelot T, Hurvitz S, et al. Afatinib alone or afatinib plus vinorelbine versus investigator’s choice of treatment for HER2-positive breast cancer with progressive brain metastases after trastuzumab, lapatinib, or both (LUX-Breast 3): a randomised, open-label, multicentre, phase 2 trial. Lancet Oncol. 2015;16:1700.
Hu C, Wang A, Wu H, Qi Z, Li X, Yan X-E, et al. Discovery and characterization of a novel irreversible EGFR mutants selective and potent kinase inhibitor CHMFL-EGFR-26 with a distinct binding mode. Oncotarget. 2017;8:18359.
Wang AL, Wu H, Chen C, Hu C, Qi ZP, Wang WC, et al. Dual inhibition of AKT/FLT3-ITD by A674563 overcomes FLT3 ligand-induced drug resistance in FLT3-ITD positive AML. Oncotarget. 2016;7:29131.
Leto SM, Sassi F, Catalano I, Torri V, Migliardi G, Zanella ER, et al. Sustained inhibition of HER3 and EGFR is necessary to induce regression of HER2-amplified gastrointestinal carcinomas. Clin Cancer Res. 2015;21:5519.
Jelovac D, Emens LA. HER2-directed therapy for metastatic breast cancer. Oncology. 2013;27:166.
Zhang Z, Wang J, Ji D, Wang C, Liu R, Wu Z, et al. Functional genetic approach identifies MET, HER3, IGF1R, INSR pathways as determinants of lapatinib unresponsiveness in HER2-positive gastric cancer. Clin Cancer Res. 2014;20:4559.
Majewski IJ, Nuciforo P, Mittempergher L, Bosma AJ, Eidtmann H, Holmes E, et al. PIK3CA mutations are associated with decreased benefit to neoadjuvant human epidermal growth factor receptor 2-targeted therapies in breast cancer. J Clin Oncol. 2015;33:1334.
Chatsiproios D. Safety profile and clinical recommendations for the use of lapatinib. Breast Care. 2010;5:16.
Roskoski R. Modulation of enzyme activity. In: Enna SJ, Bylund DB (editors). xPharm: the comprehensive pharmacology reference. Elsevier; 2007. p 1–11.
Berg JM, Tymoczko JL, Stryer L. Enzymes can be inhibited by specific molecules, 5th ed.; W H Freeman, 2002.
Amin DN, Sergina N, Lim L, Goga A, Moasser MM. HER3 signalling is regulated through a multitude of redundant mechanisms in HER2-driven tumour cells. Biochem J. 2012;447:417.
Li X, Zuo Y, Tang G, Wang Y, Zhou Y, Wang X, et al. Discovery of a series of 2,5-diaminopyrimidine covalent irreversible inhibitors of Bruton’s tyrosine kinase with in vivo antitumor activity. J Med Chem. 2014;57:5112.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81903659, 81872748, 81903650), Plan for Major Anhui Provincial Science and Technology Project (Grant No. 202003a07020006), the Natural Science Foundation of Anhui Province (Grant Nos. 1908085MH259, 2108085QH377, 2008085MH274), the China Postdoctoral Science Foundation (Grants No. 2020M671916), the Frontier Science Key Research Program of CAS (Grant No. QYZDB-SSW-SLH037), the Collaborative Innovation Program of Hefei Science Center, CAS (Grant No. 2020HSC-CIP009), and the CASHIPS Director’s Fund (Grant Nos. YZJJZX202011, BJPY2019A03, YZJJ2021QN38). We are also grateful for the support of the Youth Innovation Promotion Association of CAS support (No. 2019437) for HW. A portion of this work was supported by the High Magnetic Field Laboratory of Anhui Province.
Author information
Authors and Affiliations
Contributions
Project design and supervision: QSL, WCW, and JL; methodology development: JYC, SQ, and HW; data acquisition and/or analysis: JYC, SQ, HW, ALW, QWL, XXL, BLW, JG, FMZ, CC, JJW, and CH; writing, review, and/or revision of the manuscript: QSL, WCW, JL, HW, and JYC.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Cao, Jy., Qi, S., Wu, H. et al. CHMFL-26 is a highly potent irreversible HER2 inhibitor for use in the treatment of HER2-positive and HER2-mutant cancers. Acta Pharmacol Sin 43, 2678–2686 (2022). https://doi.org/10.1038/s41401-022-00882-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-022-00882-x