Abstract
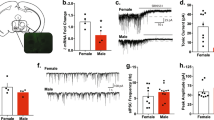
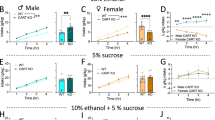
The central nucleus of the amygdala is known to play key roles in alcohol use and affect. Neurotensin neurons in the central nucleus of the amygdala have been shown to regulate alcohol drinking in male mice. However, little is known about which neurotransmitters released by these cells drive alcohol consumption or whether these cells drive alcohol consumption in female mice. Here we show that knockdown of GABA release from central amygdala neurotensin neurons using a Nts-cre-dependent vGAT-shRNA-based AAV strategy reduces alcohol drinking in male, but not female, mice. This manipulation did not impact avoidance behavior, except in a fasted novelty-suppressed feeding test, in which vGAT shRNA mice demonstrated increased latency to feed on a familiar high-value food reward, an effect driven by male mice. In contrast, vGAT shRNA female mice showed heightened sensitivity to thermal stimulation. These data show a role for GABA release from central amygdala neurotensin neurons in modulating consumption of rewarding substances in different motivational states.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Gilpin NW, Herman MA, Roberto M. The Central Amygdala as an Integrative Hub for Anxiety and Alcohol Use Disorders. Biol Psychiatry. 2015;77:859–69.
Kim J, Zhang X, Muralidhar S, LeBlanc SA, Tonegawa S. Basolateral to Central Amygdala Neural Circuits for Appetitive Behaviors. Neuron. 2017;93:1464–79.e5.
Koob GF. Brain stress systems in the amygdala and addiction. Brain Res. 2009;1293:61–75.
Ventura-Silva AP, Melo A, Ferreira AC, Carvalho MM, Campos FL, Sousa N, et al. Excitotoxic lesions in the central nucleus of the amygdala attenuate stress-induced anxiety behavior. Front Behav Neurosci. 2013;7:32.
Gereau GB, Garrison SD, McElligott ZA. Neurotensin and energy balance. J Neurochem. 2023;166:189–200.
Torruella-Suárez ML, McElligott ZA. Neurotensin in reward processes. Neuropharmacology. 2020;167:108005.
Torruella-Suárez ML, Vandenberg JR, Cogan ES, Tipton GJ, Teklezghi A, Dange K, et al. Manipulations of central amygdala neurotensin neurons alter the consumption of ethanol and sweet fluids in mice. J Neurosci. 2020;40:632–47.
Roussy G, Dansereau MA, Baudisson S, Ezzoubaa F, Belleville K, Beaudet N, et al. Evidence for a role of NTS2 receptors in the modulation of tonic pain sensitivity. Mol Pain. 2009;5:1–15.
Dobner PR. Neurotensin and pain modulation. Peptides. 2006;27:2405–14.
Mazella J, Béraud-Dufour S, Devader C, Massa F, Coppola T. Neurotensin and its receptors in the control of glucose homeostasis. Front Endocrinol. 2012;3:143.
Ramirez-Virella J, Leinninger GM. The Role of Central Neurotensin in Regulating Feeding and Body Weight. Endocrinology. 2021;162:1–14.
Brethvad AO, Zakariassen HL, Holt J, Lundgren JR, Jakobsen A, Hartmann B, et al. Increased meal-induced neurotensin response predicts successful maintenance of weight loss – Data from a randomized controlled trial. Metabolism. 2023;143:155534.
Ehlers CL, Somes C, Li T-K, Lumeng L, Kinkead B, Owensk MJ, et al. Neurotensin Studies In Alcohol Naive, Preferring And Non-Preferring Rats*. Neuroscience. 1999;93:227–36.
Lee MR, Hinton DJ, Unal SS, Richelson E, Choi DS. Increased Ethanol Consumption and Preference in Mice Lacking Neurotensin Receptor Type 2. Alcohol Clin Exp Res. 2011;35:99–107.
Lee MR, Hinton DJ, Song JY, Lee KW, Choo C, Johng H, et al. Neurotensin receptor type 1 regulates ethanol intoxication and consumption in mice. Pharmacol Biochem Behav. 2010;95:235–41.
Ma H, Huang Y, Zhang B, Wang Y, Zhao H, Du H, et al. Association between neurotensin receptor 1 gene polymorphisms and alcohol dependence in a male Han Chinese population. J Mol Neurosci. 2013;51:408–15.
Pomrenze MB, Giovanetti SM, Maiya R, Gordon AG, Kreeger LJ, Messing RO. Dissecting the Roles of GABA and Neuropeptides from Rat Central Amygdala CRF Neurons in Anxiety and Fear Learning. Cell Rep. 2019;29:13–21.e4.
Torres-Rodriguez JM, Wilson TD, Singh S, Torruella-Suárez ML, Chaudhry S, Adke AP, et al. The parabrachial to central amygdala pathway is critical to injury-induced pain sensitization in mice. Neuropsychopharmacology. 2023. https://doi.org/10.1038/s41386-023-01673-6.
Wilson TD, Valdivia S, Khan A, Ahn H-S, Adke AP, Martinez Gonzalez S, et al. Dual and Opposing Functions of the Central Amygdala in the Modulation of Pain. Cell Rep. 2019;29:332–46.e5.
Leinninger GM, Opland DM, Jo YH, Faouzi M, Christensen L, Cappellucci LA, et al. Leptin action via neurotensin neurons controls orexin, the mesolimbic dopamine system and energy balance. Cell Metab. 2011;14:313–23.
Leger M, Quiedeville A, Bouet V, Haelewyn B, Boulouard M, Schumann-Bard P, et al. Object recognition test in mice. Nat Protoc. 2013;8:2531–7.
Brenner DS, Golden JP, Gereau RW IV. A novel behavioral assay for measuring cold sensation in mice. PLoS ONE. 2012;7:e39765.
Yu W, Pati D, Pina MM, Schmidt KT, Boyt KM, Hunker AC, et al. Periaqueductal gray/dorsal raphe dopamine neurons contribute to sex differences in pain-related behaviors. Neuron. 2021;109:1365–80.e5.
Bonin RP, Bories C, De Koninck Y. A Simplified Up-Down Method (SUDO) for Measuring Mechanical Nociception in Rodents Using von Frey Filaments. Mol Pain. 2014;10:1744–8069.
Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88.
Cunningham CL, Gremel CM, Groblewski PA. Drug-induced conditioned place preference and aversion in mice. Nat Protoc. 2006;1:1662–70.
Winnicka MM, Braszko JJ. 6-OHDA lesions to the central amygdala abolish angiotensins facilitation of object recognition in rats. Gen Pharmacol Vasc Syst. 1997;29:239–43.
Gallagher M, Graham P, Holland P. The amygdala central nucleus and appetitive Pavlovian conditioning: lesions impair one class of conditioned behavior. J Neurosci. 1990;10:1906–11.
Koob GF, Sanna PP, Bloom FE. Neuroscience of Addiction. Neuron. 1998;21:467–76.
László K, Tóth K, Kertes E, Péczely L, Lénárd L. The role of neurotensin in positive reinforcement in the rat central nucleus of amygdala. Behav Brain Res. 2010;208:430–5.
Agoglia AE, Tella J, Herman MA. Sex differences in corticotropin releasing factor peptide regulation of inhibitory control and excitability in central amygdala corticotropin releasing factor receptor 1-neurons. Neuropharmacology. 2020;180:108296.
Kirson D, Khom S, Rodriguez L, Wolfe SA, Varodayan FP, Gandhi PJ, et al. Sex Differences in Acute Alcohol Sensitivity of Naïve and Alcohol Dependent Central Amygdala GABA Synapses. Alcohol Alcohol. 2021;56:581–8.
Logrip ML, Oleata C, Roberto M. Sex differences in responses of the basolateral-central amygdala circuit to alcohol, corticosterone and their interaction. Neuropharmacology. 2017;114:123–34.
Borgonetti V, Cruz B, Vozella V, Khom S, Steinman MQ, Bullard R, et al. IL-18 Signaling in the Rat Central Amygdala Is Disrupted in a Comorbid Model of Post-Traumatic Stress and Alcohol Use Disorder. Cells. 2023;12:1943.
Lagrange AH, Botzolakis EJ, Macdonald RL. Enhanced macroscopic desensitization shapes the response of α4 subtype-containing GABA A receptors to synaptic and extrasynaptic GABA: Enhanced fast desensitization in α4 subunit-containing GABA A receptors. J Physiol. 2007;578:655–76.
Gingrich KJ. Dependence of the GABAA receptor gating kinetics on the ac-subunit isoform: implications for structure-function relations and synaptic transmission. J Physiol. 1995;489:529–43.
Sallard E, Letourneur D, Legendre P. Electrophysiology of ionotropic GABA receptors. Cell Mol Life Sci. 2021;78:5341–70.
Watters JJ, Dorsa DM. Transcriptional Effects of Estrogen on Neuronal Neurotensin Gene Expression Involve cAMP/Protein Kinase A-Dependent Signaling Mechanisms. J Neurosci. 1998;18:6672–80.
Boissoneault J, Lewis B, Nixon SJ. Characterizing chronic pain and alcohol use trajectory among treatment-seeking alcoholics. Alcohol. 2019;75:47–54.
Ferguson E, Zale E, Ditre J, Wesolowicz D, Stennett B, Robinson M, et al. CANUE: A Theoretical Model of Pain as an Antecedent for Substance Use. Ann Behav Med. 2021;55:489–502.
Warlow SM, Naffziger EE, Berridge KC. The central amygdala recruits mesocorticolimbic circuitry for pursuit of reward or pain. Nat Commun. 2020;11:2716.
Sharfman NM, Kelley LK, Secci ME, Gilpin NW. Melanocortin-4 receptor signaling in the central amygdala mediates chronic inflammatory pain effects on nociception. Neuropharmacology. 2022;210:109032.
Avegno EM, Lobell TD, Itoga CA, Baynes BB, Whitaker AM, Weera MM, et al. Central Amygdala Circuits Mediate Hyperalgesia in Alcohol-Dependent Rats. J Neurosci. 2018;38:7761–73.
Acknowledgements
The authors thank Drs. Robert Messing, for the AAV8-hSyn-FLEX-GFP-shvGAT virus.
Funding
Funded by NIAAA R01 AA026363 (ZAM), NIAAA U01 AA020911 (ZAM and TLK), and NINDS R01 NS122230 (TLK).
Author information
Authors and Affiliations
Contributions
GBG, MLTS, ZAM, and TLK designed the research. MLTS did surgeries and drinking studies. GBG did surgeries and behavioral assays. MX, KMB, and GBG did sensory studies. SES did electrophysiology. GBG, DZ, LAW, AAP, ATT perfused mice and used microscopy to validate viral targeting and function. DZ scored novel object recognition test. GBG and ZAM wrote the paper with contributions from MLTS, SES, and MX.
Corresponding author
Ethics declarations
Competing interests
ZAM is subcontracted by Epicypher on a project unrelated to this work DA057749. The other authors have nothing to disclose.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gereau, G.B., Torruella-Suárez, M.L., Sizer, S.E. et al. GABA release from central amygdala neurotensin neurons differentially modulates ethanol consumption in male and female mice. Neuropsychopharmacol. (2024). https://doi.org/10.1038/s41386-024-01830-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41386-024-01830-5