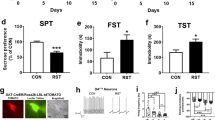
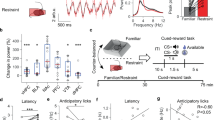
Abstract
Stress is thought to be an important contributing factor for eating disorders; however, neural substrates underlying the complex relationship between stress and appetite are not fully understood. Using in vivo recordings from awake behaving mice, we show that various acute stressors activate catecholaminergic nucleus tractus solitarius (NTSTH) projections in the paraventricular hypothalamus (PVH). Remarkably, the resulting adrenergic tone inhibits MC4R-expressing neurons (PVHMC4R), which are known for their role in feeding suppression. We found that PVHMC4R silencing encodes negative valence in sated mice and is required for avoidance induced by visceral malaise. Collectively, these findings establish PVHMC4R neurons as an effector of stress-activated brainstem adrenergic input in addition to the well-established hypothalamic-pituitary-adrenal axis. Convergent modulation of stress and feeding by PVHMC4R neurons implicates NTSTH → PVHMC4R input in stress-associated appetite disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Goddard AW, Ball SG, Martinez J, Robinson MJ, Yang CR, Russell JM, et al. Current perspectives of the roles of the central norepinephrine system in anxiety and depression. Depress Anxiety. 2010;27:339–50.
Pruccoli J, Parmeggiani A, Cordelli DM, Lanari M. The Role of the Noradrenergic System in Eating Disorders: A Systematic Review. Int J Mol Sci. 2021;22:11086.
Razzoli M, Bartolomucci A. The Dichotomous Effect of Chronic Stress on Obesity. Trends Endocrinol Metab. 2016;27:504–15.
Dallman MF. Stress-induced obesity and the emotional nervous system. Trends Endocrinol Metab. 2010;21:159–65.
Dayas CV, Buller KM, Day TA. Hypothalamic paraventricular nucleus neurons regulate medullary catecholamine cell responses to restraint stress. J Comp Neurol. 2004;478:22–34.
Pezzone MA, Lee WS, Hoffman GE, Pezzone KM, Rabin BS. Activation of brainstem catecholaminergic neurons by conditioned and unconditioned aversive stimuli as revealed by c-Fos immunoreactivity. Brain Res. 1993;608:310–8.
Yamamoto T, Shimura T, Sako N, Azuma S, Bai WZ, Wakisaka S. C-fos expression in the rat brain after intraperitoneal injection of lithium chloride. NeuroReport. 1992;3:1049–52.
Swank MW, Schafe GE, Bernsterin IL. c-Fos induction in response to taste stimuli previously paired with amphetamine or LiCl during taste aversion learning. Brain Res. 1995;673:251–61.
Pacak K, Palkovits M, Kopin IJ, Goldstein DS. Stress-Induced Norepinephrine Release in the Hypothalamic Paraventricular Nucleus and Pituitary-Adrenocortical and Sympathoadrenal Activity: In Vivo Microdialysis Studies. Front Neuroendocrinol. 1995;16:89–150.
Nakata T, Berard W, Kogosov E, Alexander N. Cardiovascular change and hypothalamic norepinephrine release in response to enviornmental stress. Am J Phys. 1993;26:784–89.
Pacak K, Palkovits M, Kvetnansky R, Yadid G, Kopin IJ, Goldstein DS. Effects of Various Stressors on In Vivo Norepinephrine Release in the Hypothalamic Paraventricular Nucleus and on the Pituitary-Adrenocortical Axis. Ann N Y Acad Sci. 1995;771:115–30.
Kvetnansky R, Sabban EL, Palkovits M. Catecholaminergic systems in stress: structural and molecular genetic approaches. Physiol Rev. 2009;89:535–606.
Myers B, Scheimann JR, Franco-Villanueva A, Herman JP. Ascending mechanisms of stress integration: Implications for brainstem regulation of neuroendocrine and behavioral stress responses. Neurosci Biobehav Rev. 2017;74:366–75.
Fuzesi T, Daviu N, Wamsteeker Cusulin JI, Bonin RP, Bains JS. Hypothalamic CRH neurons orchestrate complex behaviours after stress. Nat Commun. 2016;7:11937.
Bains JS, Wamsteeker Cusulin JI, Inoue W. Stress-related synaptic plasticity in the hypothalamus. Nat Rev Neurosci. 2015;16:377–88.
Ritter S, Watts AG, Dinh TT, Sanchez-Watts G, Pedrow C. Immunotoxin lesion of hypothalamically projecting norepinephrine and epinephrine neurons differentially affects circadian and stressor-stimulated corticosterone secretion. Endocrinology. 2003;144:1357–67.
Balcita-Pedicino JJ, Rinaman L. Noradrenergic axon terminals contact gastric preautonomic neurons in the paraventricular nucleus of the hypothalamus in rats. J Comp Neurol. 2007;501:608–18.
Raby WN, Renaud LP. Nucleus tractus solitarius innervation of supraoptic nucleus: anatomical and electrophysiological studies in the rat suggest differential innervation of oxytocin and vasopressin neurons. Prog Brain Res. 1989;81:319–27.
Fuzesi T, Wittmann G, Lechan RM, Liposits Z, Fekete C. Noradrenergic innervation of hypophysiotropic thyrotropin-releasing hormone-synthesizing neurons in rats. Brain Res. 2009;1294:38–44.
Wittmann G. Regulation of hypophysiotrophic corticotrophin-releasing hormone- and thyrotrophin-releasing hormone-synthesising neurones by brainstem catecholaminergic neurones. J Neuroendocrinol. 2008;20:952–60.
Sayar-Atasoy N, Laule C, Aklan I, Kim H, Yavuz Y, Ates T, et al. Adrenergic modulation of melanocortin pathway by hunger signals. Nat Commun. 2023;14:6602.
Savitt JM, Jang SS, Mu W, Dawson VL, Dawson TM. Bcl-x is required for proper development of the mouse substantia nigra. J Neurosci. 2005;25:6721–8.
Garfield AS, Li C, Madara JC, Shah BP, Webber E, Steger JS, et al. A neural basis for melanocortin-4 receptor-regulated appetite. Nat Neurosci. 2015;18:863–71.
Robertson SD, Plummer NW, de Marchena J, Jensen P. Developmental origins of central norepinephrine neuron diversity. Nat Neurosci. 2013;16:1016–23.
Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–40.
Aklan I, Sayar Atasoy N, Yavuz Y, Ates T, Coban I, Koksalar F, et al. NTS Catecholamine Neurons Mediate Hypoglycemic Hunger via Medial Hypothalamic Feeding Pathways. Cell Metab. 2020;31:313–26 e5.
Aklan I, Sayar-Atasoy N, Deng F, Kim H, Yavuz Y, Rysted J, et al. Dorsal raphe serotonergic neurons suppress feeding through redundant forebrain circuits. Mol Metab. 2023;69:101676.
Betley JN, Xu S, Cao ZFH, Gong R, Magnus CJ, Yu Y, et al. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature. 2015;521:180–85.
Nam MH, Han KS, Lee J, Won W, Koh W, Bae JY, et al. Activation of Astrocytic mu-Opioid Receptor Causes Conditioned Place Preference. Cell Rep. 2019;28:1154–66 e5.
McCann MJ, Verbalis JG, Stricker EM. LiCl and CCK inhibit gastric emptying and feeding and stimulate OT secretion in rats. Am J Physiol Regul Integr Comp Physiol. 1989;256:R463–8.
Feng J, Dong H, Lischinsky J, Zhou J, Deng F, Zhuang C, et al. Monitoring norepinephrine release in vivo using next-generation GRAB-NE sensors. BioRxiv. 2023.
Rinaman L. Hindbrain noradrenergic A2 neurons: diverse roles in autonomic, endocrine, cognitive, and behavioral functions. Am J Physiol Regul Integr Comp Physiol. 2011;300:R222–35.
Holt MK, Rinaman L. The role of nucleus of the solitary tract glucagon-like peptide-1 and prolactin-releasing peptide neurons in stress: anatomy, physiology and cellular interactions. Br J Pharmacol. 2022;179:642–58.
Maniscalco JW, Rinaman L. Interoceptive modulation of neuroendocrine, emotional, and hypophagic responses to stress. Physiol Behav. 2017;176:195–206.
Gasparini S, Howland JM, Thatcher AJ, Geerling JC. Central afferents to the nucleus of the solitary tract in rats and mice. J Comp Neurol. 2020;528:2708–28.
Paez X, Stanley BG, Leibowitz SF. Microdialysis analysis of norepinephrine levels in the paraventricular nucleus in association with food intake at dark onset. Brain Res. 1993;606:167–70.
Leibowitz SF, Hammer NJ, Chang K. Feeding Behavior Induced by Central Norepinephrine Injection is Attenuated by Discrete Lesions in the Hypothalamic Paraventricular Nucleus. Pharmacol Biochem Behav. 1983;19:945–50.
Cole RL, Sawchenko PE. Neurotransmitter Regulation of Cellular Activation and Neuropeptide Gene Expression in the Paraventricular Nucleus of the Hypothalamus. J Neurosci. 2002;22:959–69.
Itoi K, Suda T, Tozawa F, Dobashi I, Ohmori N, Sakai Y, et al. Microinjection of Norepinephrine into the Paraventricular Nucleus of the Hypothalamus Stimulates Corticotropin Releasing Factor Gene Expression in Conscious Rats. Endocrinology. 1994;135:2177–82.
Leibowitz SF. Paraventricular Nucleus: A Primary Site Mediating Adrenergic Stimulation of Feeding and Drinking. Pharmacol Biochem Behav. 1978;2:163–75.
Chen J, Cheng M, Wang L, Zhang L, Xu D, Cao P, et al. A Vagal-NTS Neural Pathway that Stimulates Feeding. Curr Biol. 2020;30:3986–98 e5.
Roman CW, Derkach VA, Palmiter RD. Genetically and functionally defined NTS to PBN brain circuits mediating anorexia. Nat Commun. 2016;7:11905.
Murphy S, Collis Glynn M, Dixon TN, Grill HJ, McNally GP, Ong ZY Nucleus of the solitary tract A2 neurons control feeding behaviors via projections to the paraventricular hypothalamus. Neuropsychopharmacology. 2022;48:351–61.
Rinaman L. Hindbrain noradrenergic lesions attenuate anorexia and alter central cFos expression in rats after gastric viscerosensory stimulation. J Neurosci. 2003;23:10084–92.
Wellman PJ, Davies BT, Morien A, McMahon L. Modulation of feeding by hypothalamic paraventricular nucleus a1- and a2-adrenergic receptors. Life Sciences. 1993;53:669–79.
Pace SA, Myers B. Hindbrain adrenergic/noradrenergic control of integrated endocrine and autonomic stress responses. Endocrinology. 2023;165:bqad178.
Li C, Navarrete J, Liang-Guallpa J, Lu C, Funderburk SC, Chang RB, et al. Defined Paraventricular Hypothalamic Populations Exhibit Differential Responses to Food Contingent on Caloric State. Cell Metab. 2019;29:681–94 e5.
Xu S, Yang H, Menon V, Lemire AL, Wang L, Henry FE, et al. Behavioral state coding by molecularly defined paraventricular hypothalamic cell type ensembles. Science. 2020;370:eabb2494.
Kovacs KJ. CRH: the link between hormonal-, metabolic- and behavioral responses to stress. J Chem Neuroanat. 2013;54:25–33.
Sweeney P, Chen C, Rajapakse I, Cone RD. Network dynamics of hypothalamic feeding neurons. Proc Natl Acad Sci USA. 2021;118:e2011140118.
Li MM, Madara JC, Steger JS, Krashes MJ, Balthasar N, Campbell JN, et al. The Paraventricular Hypothalamus Regulates Satiety and Prevents Obesity via Two Genetically Distinct Circuits. Neuron. 2019;102:653–67 e6.
Contreras M, Ceric F, Torrealba F. Inactivation of the Interoceptive Insula Disrupts Drug Craving and Malaise Induced by Lithium. Science. 2007;318:4.
Meachum C, Bernstein I. Behavioral Conditioned Responses to Contextual and Odor Stimuli Paired with LiCl Administration. Physiol Behav. 1992;52:5.
Fang X, Jiang S, Wang J, Bai Y, Kim CS, Blake D, et al. Chronic unpredictable stress induces depression-related behaviors by suppressing AgRP neuron activity. Mol Psychiatry. 2021;26:2299–315.
Fang X, Chen Y, Wang J, Zhang Z, Bai Y, Denney K, et al. Increased intrinsic and synaptic excitability of hypothalamic POMC neurons underlies chronic stress-induced behavioral deficits. Mol Psychiatry. 2023;28:1365–82.
Yam KY, Ruigrok SR, Ziko I, De Luca SN, Lucassen PJ, Spencer SJ, et al. Ghrelin and hypothalamic NPY/AgRP expression in mice are affected by chronic early-life stress exposure in a sex-specific manner. Psychoneuroendocrinology. 2017;86:73–77.
Liu J, Garza JC, Truong HV, Henschel J, Zhang W, Lu XY. The melanocortinergic pathway is rapidly recruited by emotional stress and contributes to stress-induced anorexia and anxiety-like behavior. Endocrinology. 2007;148:5531–40.
Di Bonaventura EM, Botticelli L, Del Bello F, Giorgioni G, Piergentili A, Quaglia W, et al. Investigating the role of the central melanocortin system in stress and stress-related disorders. Pharmacol Res. 2022;185:106521.
Lee EJ, Hanchate NK, Kondoh K, Tong APS, Kuang D, Spray A, et al. A psychological stressor conveyed by appetite-linked neurons. Sci Adv. 2020;6:eaay5366.
Lim BK, Huang KW, Grueter BA, Rothwell PE, Malenka RC. Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature. 2012;487:183–9.
Liu J, Garza JC, Li W, Lu XY. Melanocortin-4 receptor in the medial amygdala regulates emotional stress-induced anxiety-like behaviour, anorexia and corticosterone secretion. Int J Neuropsychopharmacol. 2013;16:105–20.
Cho D, O’Berry K, Possa-Paranhos IC, Butts J, Palanikumar N, Sweeney P. Paraventricular thalamic MC3R circuits link energy homeostasis with anxiety-related behavior. J Neurosci. 2023;43:6280–96.
Jeong JY, Lee DH, Kang SS. Effects of chronic restraint stress on body weight, food intake, and hypothalamic gene expressions in mice. Endocrinol Metab. 2013;28:288–96.
Silva MSD, Zimmerman PM, Meguid MM, Nandi J, Ohinata K, Xu Y, et al. Anorexia in Space and Possible Etiologies: An Overview. Nutrition. 2002;18:805–13.
Weatherford SC, Chiruzzo FY, Laughton WB. Satiety induced by endogenous and exogenous cholecystokinin is mediated by CCK-A receptors in mice. Am J Phys Regul Integr Comp Physiol. 1992;262:R574–8.
Silver AJ, Flood JF, Song AM, Morley JE. Evidence for a physiological role for CCK in the regulation of food intake in mice. Am J Phys Regul Integr Comp Physiol. 1989;256:R646–52.
Moran TH, McHugh PR. Cholecystokinin suppresses food intake by inhibiting gastric emptying. Am J Phys Regul Integr Comp Physiol. 1982;242:R491–7.
Possa-Paranhos IC, Catalbas K, Butts J, O’Berry K, Sweeney P. Establishment of Restraint Stress-induced Anorexia and Social Isolation-induced Anorexia Mouse Models. Bio Protocol. 2023;13:e4597.
Singh U, Jiang J, Saito K, Toth BA, Dickey JE, Rodeghiero SR, et al. Neuroanatomical organization and functional roles of PVN MC4R pathways in physiological and behavioral regulations. Mol Metab. 2022;55:101401.
Acknowledgements
We thank Dr. Kamal Rahmouni and Dr. Deng-Fu Guo for providing Mc4r-cre x ai14 mice. We thank Dr. Huxing Cui and Dr. Uday Singh for providing Mc4r-cre mice.
Funding
This work is supported by NIH to D.A. R01DK126740 and NIH to C.L. F31HL168820.
Author information
Authors and Affiliations
Contributions
CL performed behavioral experiments; CL, NSA performed in vivo imaging experiments; CL, IA performed surgeries; CL performed electrophysiology experiments; CL, HK, TA performed post hoc analysis; NSA performed genotyping; DD managed mouse colony; DA, CL, NSA conceived experiments; DA, CL, NSA analyzed data; DA and CL wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Laule, C., Sayar-Atasoy, N., Aklan, I. et al. Stress integration by an ascending adrenergic-melanocortin circuit. Neuropsychopharmacol. (2024). https://doi.org/10.1038/s41386-024-01810-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41386-024-01810-9