Abstract
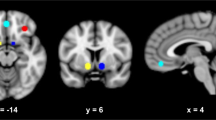
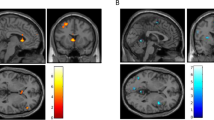
Neuroendocrine tolerance to alcohol, i.e., a blunted cortisol response to alcohol, has been linked to Ventromedial Prefrontal Cortex (VmPFC) alcohol cue reactivity and relapse risk in severe Alcohol Use Disorders (AUDs), but its role in the development of AUDs is not clear. Recent work suggests that blunted cortisol responses to alcohol cues in individuals who engage in binge drinking (BD) may play a role in motivation to consume larger amounts of alcohol, but the link between this dysregulated endocrine response and BD’s neural responses to alcohol cues remains unclear. To examine this, two groups of participants were recruited based on their recent drinking history. Thirty-three BD and 31 non-binging, social drinkers (SD) were exposed to alcohol cues and water cues in two separate 7 T functional magnetic resonance imaging (fMRI) scans. Each scan was followed by the Alcohol Taste Test (ATT) of implicit motivation for alcohol and a post-experiment, one-month prospective measurement of their “real world” drinking behavior. During each scan session, blood plasma was collected repeatedly to examine the separate effects of alcohol cues and alcohol consumption on cortisol levels. Relative to water cues and SD, BD demonstrated blunted cortisol cue reactivity that was negatively associated with VmPFC cue reactivity. In BD, both blunted cortisol and greater VmPFC cue reactivity were related to immediate and future alcohol consumption in the month following the scans. Thus, neuroendocrine tolerance in BD may be associated with increased incentive salience of cues and contribute mechanistically to increased alcohol consumption seen in the development of AUDs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Administration SAaMHS. Key substance use and mental health indicators in the United States: Results from the 2021 National Survey on Drug Use and Health (HHS Publication No. PEP22-07-01-005, NSDUH Series H-57). Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. https://www.samhsa.gov/data/report/2021-nsduh-annual-national-report. 2022.
Blaine SK, Sinha R. Alcohol, stress, and glucocorticoids: from risk to dependence and relapse in alcohol use disorders. Neuropharmacology. 2017;122:136–47.
Richardson HN, Lee SY, O’Dell LE, Koob GF, Rivier CL. Alcohol self-administration acutely stimulates the hypothalamic-pituitary-adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. Eur J Neurosci. 2008;28:1641–53.
Lee S, Rivier C. Alcohol increases the expression of type 1, but not type 2 alpha corticotropin-releasing factor (CRF) receptor messenger ribonucleic acid in the rat hypothalamus. brain Res Mol brain Res. 1997;52:78–89.
Blaine SK, Milivojevic V, Fox H, Sinha R. Alcohol effects on stress pathways: impact on craving and relapse risk. Can J Psychiatry. 2016;61:145–53.
Abernathy K, Chandler LJ, Woodward JJ. Alcohol and the prefrontal cortex. Int Rev Neurobiol. 2010;91:289–320.
Lu Y-L, Richardson H. Alcohol, stress hormones, and the prefrontal cortex: a proposed pathway to the dark side of addiction. Neuroscience 2014;277:139–51.
Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2010;35:217–38.
Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–69.
Seo D, Lacadie CM, Tuit K, Hong KI, Constable RT, Sinha R. Disrupted ventromedial prefrontal function, alcohol craving, and subsequent relapse risk. JAMA Psychiatry. 2013;70:727–39.
Blaine SK, Seo D, Sinha R. Peripheral and prefrontal stress system markers and risk of relapse in alcoholism. Addict Biol. 2017;22:468–78.
Cofresí RU, Bartholow BD, Piasecki TM. Evidence for incentive salience sensitization as a pathway to alcohol use disorder. Neurosci Biobehav Rev. 2019;107:897–926.
Schacht JP, Anton RF, Voronin KE, Randall PK, Li X, Henderson S, et al. Interacting effects of naltrexone and OPRM1 and DAT1 variation on the neural response to alcohol cues. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2013;38:414–22.
Zeng J, Yu S, Cao H, Su Y, Dong Z, Yang X. Neurobiological correlates of cue-reactivity in alcohol-use disorders: a voxel-wise meta-analysis of fMRI studies. Neurosci Biobehav Rev. 2021;128:294–310.
Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive–sensitization view. Addiction. 2000;95:91–117.
Du Hoffmann J, Nicola SM. Dopamine invigorates reward seeking by promoting cue-evoked excitation in the nucleus accumbens. J Neurosci. 2014;34:14349–64.
Heinz A, Beck A, Grüsser SM, Grace AA, Wrase J. Identifying the neural circuitry of alcohol craving and relapse vulnerability. Addict Biol. 2009;14:108–18.
Blaine SK, Wemm S, Fogelman N, Lacadie C, Seo D, Scheinost D, et al. Association of prefrontal-striatal functional pathology with alcohol abstinence days at treatment initiation and heavy drinking after treatment initiation. Am J Psychiatry. 2020;177:1048–59.
Blaine SK, Nautiyal N, Hart R, Guarnaccia J, Sinha R. Craving, cortisol and behavioral alcohol motivation responses to stress and alcohol cue contexts and discrete cues in binge and non‐binge drinkers. Addict Biol. 2019;24:1096–108.
Blaine S, Fogelman N, Lacadie C, Constable T, Sinha R. Blunted neural reward response to alcohol and greater alcohol motivation in binge drinkers in a randomized clinical experiment. Alcohol Clin Exp Res. 2023;47:1067–78.
King A, Munisamy G, de Wit H, Lin S. Attenuated cortisol response to alcohol in heavy social drinkers. Int J Psychophysiol. 2006;59:203–9.
King AC, de Wit H, McNamara PJ, Cao D. Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Arch Gen psychiatry. 2011;68:389–99.
Mick I, Spring K, Uhr M, Zimmermann US. Alcohol administration attenuates hypothalamic–pituitary–adrenal (HPA) activity in healthy men at low genetic risk for alcoholism, but not in high‐risk subjects. Addict Biol. 2013;18:863–71.
Gilman JM, Ramchandani VA, Crouss T, Hommer DW. Subjective and neural responses to intravenous alcohol in young adults with light and heavy drinking patterns. Neuropsychopharmacology. 2012;37:467–77.
Arias AJ, Ma L, Bjork JM, Hammond CJ, Zhou Y, Snyder A, et al. Altered effective connectivity of the reward network during an incentive‐processing task in adults with alcohol use disorder. Alcohol Clin Exp Res. 2021;45:1563–77.
Dager AD, Anderson BM, Rosen R, Khadka S, Sawyer B, Jiantonio‐Kelly RE, et al. Functional magnetic resonance imaging (fMRI) response to alcohol pictures predicts subsequent transition to heavy drinking in college students. Addiction. 2014;109:585–95.
Ray S, Hanson C, Hanson SJ, Bates ME. fMRI BOLD response in high-risk college students (part 1): during exposure to alcohol, marijuana, polydrug and emotional picture cues. Alcohol Alcohol. 2010;45:437–43.
Kambouropoulos N, Staiger PK. ‘Cue reward salience’predicts craving in response to alcohol cues. Personal Individ Differ. 2009;46:78–82.
Abuse NIoA, Alcoholism. NIAAA council approves definition of binge drinking. NIAAA Newsl. 2004;3:3.
Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported ethanol consumption. In: Allen L, Litten R, editors. Measuring alcohol consumption: psychosocial and biological methods. Totowa, NJ: Humana Press; 1992. p. 41–72.
Cahalan D, Cisin IH, Crossley HM. American drinking practices: a national study of drinking behaviors and attitudes. Monogr Rutgers Cent Alcohol Stud. 1969;6:2223–4.
Mann RE, Sobell LC, Sobell MB, Pavan D. Reliability of a family tree questionnaire for assessing family history of alcohol problems. Drug Alcohol Depend. 1985;15:61–7.
Jones A, Button E, Rose AK, Robinson E, Christiansen P, Di Lemma L, et al. The ad-libitum alcohol ‘taste test’: secondary analyses of potential confounds and construct validity. Psychopharmacology. 2016;233:917–24.
Rose R, Kreuz L, Holaday J, Sulak K, Johnson C. Diurnal variation of plasma testosterone and cortisol. J Endocrinol. 1972;54:177–8.
Esteban O, Markiewicz CJ, Blair RW, Moodie CA, Isik AI, Erramuzpe A, et al. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat methods. 2019;16:111–6.
Gorgolewski K, Burns CD, Madison C, Clark D, Halchenko YO, Waskom ML, et al. Nipype: a flexible, lightweight and extensible neuroimaging data processing framework in python. Front Neuroinform. 2011;13.
Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage. 2004;21:1732–47.
JASP Team (2023). JASP (Version 0.17.3)[Mac].
Goldfarb EV. Participant stress in the COVID-19 era and beyond. Nat Rev Neurosci. 2020;21:663–4.
Taylor BK, Fung MH, Frenzel MR, Johnson HJ, Willett MP, Badura-Brack AS, et al. Increases in circulating cortisol during the COVID-19 pandemic are associated with changes in perceived positive and negative affect among adolescents. Res Child Adolesc Psychopathol. 2022;50:1543–55.
Koob GF. Addiction is a reward deficit and stress surfeit disorder. Front psychiatry. 2013;4:72.
NIAAA. Rethinking drinking: alcohol and your health. US Dep Health Hum Serv. 2012.
Funding
Funding for this work was provided by National Institutes of Health grant R00 AA025401 (SKB).
Author information
Authors and Affiliations
Contributions
SKB designed the study, obtained funding, and supervised all aspects of data collection, analysis, and wrote the manuscript. CR, BC, and LC collected the data. RM, JLR, EBA, and EDC provided necessary assistance in setting up data collection and in data analysis, in addition to interpretation of results. EDC performed crucial analyses and edited the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Blaine, S.K., Ridner, C., Campbell, B. et al. People who binge drink show neuroendocrine tolerance to alcohol cues that is associated with immediate and future drinking- results from a randomized clinical experiment. Neuropsychopharmacol. 48, 1968–1974 (2023). https://doi.org/10.1038/s41386-023-01735-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-023-01735-9