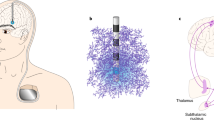
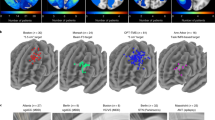
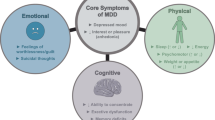
Abstract
Deep brain stimulation (DBS) is an invasive approach to precise modulation of psychiatrically relevant circuits. Although it has impressive results in open-label psychiatric trials, DBS has also struggled to scale to and pass through multi-center randomized trials. This contrasts with Parkinson disease, where DBS is an established therapy treating thousands of patients annually. The core difference between these clinical applications is the difficulty of proving target engagement, and of leveraging the wide range of possible settings (parameters) that can be programmed in a given patient’s DBS. In Parkinson’s, patients’ symptoms change rapidly and visibly when the stimulator is tuned to the correct parameters. In psychiatry, those same changes take days to weeks, limiting a clinician’s ability to explore parameter space and identify patient-specific optimal settings. I review new approaches to psychiatric target engagement, with an emphasis on major depressive disorder (MDD). Specifically, I argue that better engagement may come by focusing on the root causes of psychiatric illness: dysfunction in specific, measurable cognitive functions and in the connectivity and synchrony of distributed brain circuits. I overview recent progress in both those domains, and how it may relate to other technologies discussed in companion articles in this issue.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron 2005;45:651–60.
Greenberg B, Gabriels L, Malone D, Rezai A, Friehs G, Okun M, et al. Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: worldwide experience. Mol Psychiatry. 2010;15:64–79.
Malone DA, Dougherty DD, Rezai AR, Carpenter LL, Friehs GM, Eskandar EN, et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry. 2009;65:267–75.
Holtzheimer PE, Husain MM, Lisanby SH, Taylor SF, Whitworth LA, McClintock S, et al. Subcallosal cingulate deep brain stimulation for treatment-resistant depression: a multisite, randomised, sham-controlled trial. Lancet Psychiatry. 2017;4:839–49.
Dougherty DD, Rezai AR, Carpenter LL, Howland RH, Bhati MT, O’Reardon JP, et al. A randomized sham-controlled trial of deep brain stimulation of the ventral capsule/ventral striatum for chronic treatment-resistant depression. Biol Psychiatry. 2015;78:240–8.
Menchón JM, Real E, Alonso P, Aparicio MA, Segalas C, Plans G, et al. A prospective international multi-center study on safety and efficacy of deep brain stimulation for resistant obsessive-compulsive disorder. Mol Psychiatry. 2021;26:1234–47.
Denys D, Graat I, Mocking R, de Koning P, Vulink N, Figee M, et al. Efficacy of deep brain stimulation of the ventral anterior limb of the internal capsule for refractory obsessive-compulsive disorder: a clinical cohort of 70 patients. AJP. 2020;177:265–71. appi.ajp.2019.19060656
Dougherty DD, Brennan B, Stewart SE, Wilhelm S, Widge AS, Rauch SL. Neuroscientifically informed formulation and treatment planning for patients with obsessive-compulsive disorder: a review. JAMA Psychiatry. 2018;75:1081–7.
Widge AS. Closed-Loop Deep Brain Stimulation for Psychiatric Disorders. Harv Rev Psychiatry. 2023;31:162.
Holtzheimer PE, Kelley ME, Gross RE, Filkowski MM, Garlow SJ, Barrocas A, et al. Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Arch Gen Psychiatry. 2012;69:150–8.
Bergfeld IO, Mantione M, Hoogendorn ML, Ruhé HG, Notten P, van Laarhoven J, et al. Deep brain stimulation of the ventral anterior limb of the internal capsule for treatment-resistant depression: A randomized clinical trial. JAMA Psychiatry. 2016;73:456–64.
Malekmohammadi M, Mustakos R, Sheth S, Pouratian N, McIntyre CC, Bijanki KR, et al. Automated optimization of deep brain stimulation parameters for modulating neuroimaging-based targets. J Neural Eng. 2022;19:046014.
Peña E, Zhang S, Patriat R, Aman JE, Vitek JL, Harel N, et al. Multi-objective particle swarm optimization for postoperative deep brain stimulation targeting of subthalamic nucleus pathways. J Neural Eng. 2018;15:066020.
Slopsema JP, Peña E, Patriat R, Lehto LJ, Gröhn O, Mangia S, et al. Clinical deep brain stimulation strategies for orientation-selective pathway activation. J Neural Eng. 2018;15:056029.
Widge AS, Dougherty DD. Managing patients with psychiatric disorders with deep brain stimulation. In: Marks Jr. WJ, Ostrem JL, editors. Deep Brain Stimulation Management, 3rd ed. Cambridge: New York: Cambridge University Press; 2022. p. 198–214.
van Westen M, Rietveld E, Bergfeld IO, Koning Pde, Vullink N, Ooms P, et al. Optimizing deep brain stimulation parameters in obsessive–compulsive disorder. Neuromodulation: Technology at the Neural. Interface. 2021;24:307–15.
Okun MS, Mann G, Foote KD, Shapira NA, Bowers D, Springer U, et al. Deep brain stimulation in the internal capsule and nucleus accumbens region: responses observed during active and sham programming. J Neurol Neurosurg Psychiatry. 2007;78:310–4.
Choi K, Riva-Posse P, Gross RE, Mayberg HS. Mapping the “depression switch” during intraoperative testing of subcallosal cingulate deep brain stimulation. JAMA Neurol. 2015;72:1252–60.
Krystal JH, Abdallah CG, Sanacora G, Charney DS, Duman RS. Ketamine: A Paradigm Shift for Depression Research and Treatment. Neuron 2019;101:774–8.
Zaki N, Chen L (Nancy), Lane R, Doherty T, Drevets WC, et al. Long-term safety and maintenance of response with esketamine nasal spray in participants with treatment-resistant depression: interim results of the SUSTAIN-3 study. Neuropsychopharmacology. 2023;48:1225–33.
van Westen M, Rietveld E, Denys D. Effective deep brain stimulation for obsessive-compulsive disorder requires clinical expertise. Front Psychol. 2019;10:2294.
Riva-Posse P, Choi KS, Holtzheimer PE, Crowell AL, Garlow SJ, Rajendra JK, et al. A connectomic approach for subcallosal cingulate deep brain stimulation surgery: prospective targeting in treatment-resistant depression. Mol Psychiatry. 2018;23:843–9.
Riva-Posse P, Choi KS, Holtzheimer PE, McIntyre CC, Gross RE, Chaturvedi A, et al. Defining critical white matter pathways mediating successful subcallosal cingulate deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2014;76:963–9.
Sullivan CRP, Olsen S, Widge AS. Deep brain stimulation for psychiatric disorders: From focal brain targets to cognitive networks. NeuroImage 2021;225:117515.
Widge AS, Deckersbach T, Eskandar EN, Dougherty DD. Deep brain stimulation for treatment-resistant psychiatric illnesses: what has gone wrong and what should we do next? Biol Psychiatry. 2016;79:e9–e10.
Widge AS, Malone DAJ, Dougherty DD. Closing the loop on deep brain stimulation for treatment-resistant depression. Front Neurosci. 2018;12:175.
Cuthbert BN. Research Domain Criteria (RDoC): Progress and Potential. Curr Dir Psychol Sci. 2022;31:107–14.
Kapur S, Phillips AG, Insel TR. Why has it taken so long for biological psychiatry to develop clinical tests and what to do about it? Mol Psychiatry. 2012;17:1174–9.
Widge AS, Miller EK. Next-generation clinical brain stimulation: targeting cognition and networks through neural oscillations. JAMA Psychiatry. 2019;76:671–2.
Bergey GK, Morrell MJ, Mizrahi EM, Goldman A, King-Stephens D, Nair D, et al. Long-term treatment with responsive brain stimulation in adults with refractory partial seizures. Neurology 2015;84:810–7.
Waters AC, Veerakumar A, Choi KS, Howell B, Tiruvadi V, Bijanki KR, et al. Test–retest reliability of a stimulation-locked evoked response to deep brain stimulation in subcallosal cingulate for treatment resistant depression. Hum Brain Mapp. 2018;39:4844–56.
Veerakumar A, Tiruvadi V, Howell B, Waters AC, Crowell AL, Voytek B, et al. Field potential 1/f activity in the subcallosal cingulate region as a candidate signal for monitoring deep brain stimulation for treatment-resistant depression. J Neurophysiol. 2019;122:1023–35.
Olsen S, Basu I, Bilge MT, Kanabar A, Boggess MJ, Rockhill AP, et al. Case report of dual-site neurostimulation and chronic recording of cortico-striatal circuitry in a patient with treatment refractory obsessive compulsive disorder. Front Hum Neurosci. 2020;14:569973.
Scangos KW, Makhoul GS, Sugrue LP, Chang EF, Krystal AD. State-dependent responses to intracranial brain stimulation in a patient with depression. Nat Med. 2021;27:229–31.
Scangos KW, Khambhati AN, Daly PM, Makhoul GS, Sugrue LP, Zamanian H, et al. Closed-loop neuromodulation in an individual with treatment-resistant depression. Nat Med. 2021;27:1696–1700.
Baldermann JC, Schüller T, Kohl S, Voon V, Li N, Hollunder B, et al. Connectomic deep brain stimulation for obsessive-compulsive disorder. Biol Psychiatry. 2021. 19 July 2021. https://doi.org/10.1016/j.biopsych.2021.07.010.
Gadot R, Li N, Shofty B, Avendano-Ortega M, McKay S, Bijanki KR, et al. Tractography-Based Modeling Explains Treatment Outcomes in Patients Undergoing Deep Brain Stimulation for Obsessive Compulsive Disorder. Biol Psychiatry. 2023. 31 January 2023. https://doi.org/10.1016/j.biopsych.2023.01.017.
Sheth SA, Bijanki KR, Metzger B, Allawala A, Pirtle V, Adkinson JA, et al. Deep brain stimulation for depression informed by intracranial recordings. Biol Psychiatry. 2021. 22 November 2021. https://doi.org/10.1016/j.biopsych.2021.11.007.
Allawala A, Bijanki KR, Goodman W, Cohn JF, Viswanathan A, Yoshor D, et al. A Novel Framework for Network-Targeted Neuropsychiatric Deep Brain Stimulation. Neurosurgery. 2021. 29 April 2021. https://doi.org/10.1093/neuros/nyab112.
Ramasubbu R, Clark DL, Golding S, Dobson KS, Mackie A, Haffenden A, et al. Long versus short pulse width subcallosal cingulate stimulation for treatment-resistant depression: a randomised, double-blind, crossover trial. Lancet Psychiatry. 2020;7:29–40.
Widge AS, Zhang F, Gosai A, Papadimitrou G, Wilson-Braun P, Tsintou M, et al. Patient-specific connectomic models correlate with, but do not reliably predict, outcomes in deep brain stimulation for obsessive-compulsive disorder. Neuropsychopharmacology 2022;47:965–72.
Graat I, Mocking RJT, Liebrand LC, van den Munckhof P, Bot M, Schuurman PR, et al. Tractography-based versus anatomical landmark-based targeting in vALIC deep brain stimulation for refractory obsessive-compulsive disorder. Mol Psychiatry. 2022:1–7.
Marek S, Tervo-Clemmens B, Calabro FJ, Montez DF, Kay BP, Hatoum AS, et al. Reproducible brain-wide association studies require thousands of individuals. Nature 2022;603:654–60.
Eklund A, Nichols TE, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. PNAS 2016;113:7900–5.
Bullock DN, Hayday EA, Grier MD, Tang W, Pestilli F, Heilbronner SR. A taxonomy of the brain’s white matter: twenty-one major tracts for the 21st century. Cereb Cortex. 2022;32:4524–48.
Grier MD, Zimmermann J, Heilbronner SR. Estimating brain connectivity with diffusion-weighted MRI: Promise and peril. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;5:846–54.
Haber SN, Tang W, Choi EY, Yendiki A, Liu H, Jbabdi S, et al. Circuits, networks, and neuropsychiatric disease: transitioning from anatomy to imaging. Biol Psychiatry. 2020;87:318–27.
Smith EH, Horga G, Yates MJ, Mikell CB, Banks GP, Pathak YJ, et al. Widespread temporal coding of cognitive control in the human prefrontal cortex. Nat Neurosci. 2019;22:1883–91.
Basu I, Yousefi A, Crocker B, Zelmann R, Paulk AC, Peled N, et al. Closed-loop enhancement and neural decoding of cognitive control in humans. Nat Biomed Eng. 2021:s41551-021-00804-y.
Helfrich RF, Knight RT. Oscillatory dynamics of prefrontal cognitive control. Trends Cogn Sci. 2016;20:916–30.
Ezzyat Y, Wanda PA, Levy DF, Kadel A, Aka A, Pedisich I, et al. Closed-loop stimulation of temporal cortex rescues functional networks and improves memory. Nat Commun. 2018;9:365.
Sani OG, Yang Y, Lee MB, Dawes HE, Chang EF, Shanechi MM. Mood variations decoded from multi-site intracranial human brain activity. Nat Biotechnol. 2018;36:954–61.
Regier DA, Narrow WE, Clarke DE, Kraemer HC, Kuramoto SJ, Kuhl EA, et al. DSM-5 field trials in the United States and Canada, part II: test-retest reliability of selected categorical diagnoses. AJP. 2013;170:59–70.
Widge AS, Bilge MT, Montana R, Chang W, Rodriguez CI, Deckersbach T, et al. Electroencephalographic biomarkers for treatment response prediction in major depressive illness: a meta-analysis. Am J Psychiatry. 2019;176:44–56.
Zeier Z, Carpenter LL, Kalin NH, Rodriguez CI, McDonald WM, Widge AS, et al. Clinical implementation of pharmacogenetic decision support tools for antidepressant drug prescribing. Am J Psychiatry. 2018;175:873–86.
Testo AA, Garnaat SL, Corse AK, McLaughlin N, Greenberg BD, Deckersbach T, et al. A case of non-affective psychosis followed by extended response to non-stimulation in deep brain stimulation for obsessive-compulsive disorder. Brain Stimulation: Basic, Transl, Clin Res Neuromodulation. 2020;13:1317–9.
Luyten L, Hendrickx S, Raymaekers S, Gabriëls L, Nuttin B. Electrical stimulation in the bed nucleus of the stria terminalis alleviates severe obsessive-compulsive disorder. Mol Psychiatry. 2016;21:1272–80.
Widge AS, Zorowitz S, Basu I, Paulk AC, Cash SS, Eskandar EN, et al. Deep brain stimulation of the internal capsule enhances human cognitive control and prefrontal cortex function. Nat Commun. 2019;10:1536.
McCracken CB, Grace AA. High-frequency deep brain stimulation of the nucleus accumbens region suppresses neuronal activity and selectively modulates afferent drive in rat orbitofrontal cortex in vivo. J Neurosci. 2007;27:12601–10.
Dunner DL, Aaronson ST, Sackeim HA, Janicak PG, Carpenter LL, Boyadjis T, et al. A multisite, naturalistic, observational study of transcranial magnetic stimulation for patients with pharmacoresistant major depressive disorder: durability of benefit over a 1-year follow-up period. J Clin Psychiatry. 2014;75:1394–401.
Cole EJ, Stimpson KH, Bentzley BS, Gulser M, Cherian K, Tischler C, et al. Stanford Accelerated Intelligent Neuromodulation Therapy for Treatment-Resistant Depression. Am J Psychiatry. 2020;177:716–26.
Redish AD, Kepecs A, Anderson LM, Calvin OL, Grissom NM, Haynos AF, et al. Computational validity: using computation to translate behaviours across species. Philos Trans R Soc. B 2022;377:20200525.
Paulus MP, Huys QJM, Maia TV. A roadmap for the development of applied computational psychiatry. Biol Psychiatry: Cogn Neurosci Neuroimaging. 2016;1:386–92.
Huys QJM. Advancing clinical improvements for patients using the theory-driven and data-driven branches of computational psychiatry. JAMA Psychiatry. 2018;75:225–6.
Sanislow CA, Ferrante M, Pacheco J, Rudorfer MV, Morris SE. Advancing translational research using NIMH research domain criteria and computational methods. Neuron 2019;101:779–82.
Redish A, Gordon J, editors. Computational Psychiatry. The MIT Press; 2016.
Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 2013;11:126.
Durstewitz D, Huys QJM, Koppe G. Psychiatric illnesses as disorders of network dynamics. Biol Psychiatry: Cogn Neurosci Neuroimaging. 2021;6:865–76.
Gordon JA. On being a circuit psychiatrist. Nat Neurosci. 2016;19:1385–6.
Insel TR. Faulty Circuits. Sci Am. 2010;302:44–51.
Haber SN, Yendiki A, Jbabdi S. Four deep brain stimulation targets for obsessive-compulsive disorder: Are they different? Biol Psychiatry. 2021;90:667–77.
Dembek TA, Reker P, Visser‐Vandewalle V, Wirths J, Treuer H, Klehr M, et al. Directional DBS increases side-effect thresholds—A prospective, double-blind trial. Mov Disord. 2017;32:1380–8.
Cagnan H, Denison T, McIntyre C, Brown P. Emerging technologies for improved deep brain stimulation. Nat Biotechnol. 2019;37:1024–33.
Grzenda A, Kraguljac NV, McDonald WM, Nemeroff CB, Torous J, Alpert JE, et al. Evaluating the machine learning literature: a primer and user’s guide for psychiatrists. Am J Psychiatry. 2021;178:715–29.
Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23:28–38.
Dinga R, Schmaal L, Penninx BWJH, van Tol MJ, Veltman DJ, van Velzen L, et al. Evaluating the evidence for biotypes of depression: Methodological replication and extension of Drysdale et al. (2017). NeuroImage: Clin 2019;22:101796.
Birn RM, Molloy EK, Patriat R, Parker T, Meier TB, Kirk GR, et al. The effect of scan length on the reliability of resting-state fMRI connectivity estimates. Neuroimage 2013;83:550–8.
Ball TM, Goldstein-Piekarski AN, Gatt JM, Williams LM. Quantifying person-level brain network functioning to facilitate clinical translation. Transl Psychiatry. 2017;7:e1248.
Gordon EM, Laumann TO, Gilmore AW, Newbold DJ, Greene DJ, Berg JJ, et al. Precision Functional Mapping of Individual Human Brains. Neuron 2017;95:791–807.e7.
Goldstein-Piekarski AN, Ball TM, Samara Z, Staveland BR, Keller AS, Fleming SL, et al. Mapping neural circuit biotypes to symptoms and behavioral dimensions of depression and anxiety. Biol Psychiatry. 2022;91:561–71.
Voon V, Reiter A, Sebold M, Groman S. Model-based control in dimensional psychiatry. Biol Psychiatry. 2017;82:391–400.
Rutledge RB, Skandali N, Dayan P, Dolan RJ. A computational and neural model of momentary subjective well-being. PNAS 2014;111:12252–7.
Cooper JA, Arulpragasam AR, Treadway MT. Anhedonia in depression: biological mechanisms and computational models. Curr Opin Behav Sci. 2018;22:128–35.
Grisanzio KA, Goldstein-Piekarski AN, Wang MY, Ahmed APR, Samara Z, Williams LM. Transdiagnostic symptom clusters and associations with brain, behavior, and daily function in mood, anxiety, and trauma disorders. JAMA Psychiatry. 2017;75:201–9.
Barnett I, Torous J, Staples P, Sandoval L, Keshavan M, Onnela J-P. Relapse prediction in schizophrenia through digital phenotyping: a pilot study. Neuropsychopharmacology 2018;43:1660–6.
Harvey PD, Depp CA, Rizzo AA, Strauss GP, Spelber D, Carpenter LL, et al. Technology and mental health: state of the art for assessment and treatment. AJP. 2022;179:897–914.
Xia CH, Barnett I, Tapera TM, Adebimpe A, Baker JT, Bassett DS, et al. Mobile footprinting: linking individual distinctiveness in mobility patterns to mood, sleep, and brain functional connectivity. Neuropsychopharmacol. 2022;47:1662–71.
Gardner J. A history of deep brain stimulation: Technological innovation and the role of clinical assessment tools. Soc Stud Sci. 2013;43:707–28.
Coffey RJ, Lozano AM. Neurostimulation for chronic noncancer pain: an evaluation of the clinical evidence and recommendations for future trial designs. J Neurosurg. 2006;105:175–89.
Vitek JL, Johnson LA. Understanding Parkinson’s disease and deep brain stimulation: Role of monkey models. Proc Natl Acad Sci USA. 2019;116:26259–65.
Wang J, Nebeck S, Muralidharan A, Johnson MD, Vitek JL, Baker KB. Coordinated reset deep brain stimulation of subthalamic nucleus produces long-lasting, dose-dependent motor improvements in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine non-human primate model of Parkinsonism. Brain Stimulation. 2016;9:609–17.
Spix TA, Nanivadekar S, Toong N, Kaplow IM, Isett BR, Goksen Y, et al. Population-specific neuromodulation prolongs therapeutic benefits of deep brain stimulation. Science 2021;374:201–6.
Monteggia LM, Heimer H, Nestler EJ. Meeting Report: Can We Make Animal Models of Human Mental Illness? Biol Psychiatry. 2018;84:542–5.
Heilbronner SR, Rodriguez-Romaguera J, Quirk GJ, Groenewegen HJ, Haber SN. Circuit based cortico-striatal homologies between rat and primate. Biol Psychiatry. 2016;80:509–21.
Widge AS, Heilbronner SR, Hayden BY. Prefrontal cortex and cognitive control: new insights from human electrophysiology. F1000Res. 2019;8:1696.
Creed M. Current and emerging neuromodulation therapies for addiction: insight from pre-clinical studies. Curr Opin Neurobiol. 2018;49:168–74.
Kravitz AV, Tomasi D, LeBlanc KH, Baler R, Volkow ND, Bonci A, et al. Cortico-striatal circuits: Novel therapeutic targets for substance use disorders. Brain Res. 2015;1628:186–98.
Fenster RJ, Lebois LAM, Ressler KJ, Suh J. Brain circuit dysfunction in post-traumatic stress disorder: from mouse to man. Nat Rev Neurosci. 2018;19:535.
Langevin J-P, Koek RJ, Schwartz HN, Chen JWY, Sultzer DL, Mandelkern MA, et al. Deep brain stimulation of the basolateral amygdala for treatment-refractory posttraumatic stress disorder. Biol Psychiatry. 2016;79:e82–e84.
Luigjes J, Brink Wvanden, Feenstra M, Munckhof Pvanden, Schuurman PR, Schippers R, et al. Deep brain stimulation in addiction: a review of potential brain targets. Mol Psychiatry. 2012;17:572–83.
Ball TM, Gunaydin LA. Measuring maladaptive avoidance: from animal models to clinical anxiety. Neuropsychopharmacol. 2022;47:978–86.
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
Diehl MM, Bravo-Rivera C, Quirk GJ. The study of active avoidance: A platform for discussion. Neurosci Biobehav Rev. 2019;107:229–37.
Zilverstand A, Huang AS, Alia-Klein N, Goldstein RZ. Neuroimaging impaired response inhibition and salience attribution in human drug addiction: a systematic review. Neuron 2018;98:886–903.
McTeague LM, Huemer J, Carreon DM, Jiang Y, Eickhoff SB, Etkin A. Identification of common neural circuit disruptions in cognitive control across psychiatric disorders. AJP. 2017;174:676–85.
Gruner P, Pittenger C. Cognitive inflexibility in Obsessive-Compulsive Disorder. Neuroscience 2017;345:243–55.
Yang Z, Oathes DJ, Linn KA, Bruce SE, Satterthwaite TD, Cook PA, et al. Cognitive behavioral therapy is associated with enhanced cognitive control network activity in major depression and posttraumatic stress disorder. Biol Psychiatry: Cogn Neurosci Neuroimaging. 2018;3:311–9.
Cavanagh JF, Shackman AJ. Frontal midline theta reflects anxiety and cognitive control: Meta-analytic evidence. J Physiol-Paris. 2015;109:3–15.
Robbins TW, Vaghi MM, Banca P. Obsessive-compulsive disorder: puzzles and prospects. Neuron 2019;102:27–47.
Rodman AM, Jenness JL, Weissman DG, Pine DS, McLaughlin KA. Neurobiological markers of resilience to depression and anxiety following childhood maltreatment: The role of neural circuits supporting the cognitive control of emotion. Biol Psychiatry. 2019;86:464–73.
Farchione TJ, Fairholme CP, Ellard KK, Boisseau CL, Thompson-Hollands J, Carl JR, et al. Unified protocol for transdiagnostic treatment of emotional disorders: a randomized controlled trial. Behav Ther. 2012;43:666–78.
Ellard KK, Fairholme CP, Boisseau CL, Farchione TJ, Barlow DH. Unified Protocol for the Transdiagnostic Treatment of Emotional Disorders: Protocol development and initial outcome data. Cogn Behav Pract. 2010;17:88–101.
Foa EB, McLean CP. The efficacy of exposure therapy for anxiety-related disorders and its underlying mechanisms: the case of OCD and PTSD. Annu Rev Clin Psychol. 2016;12:1–28.
Foa EB, McLean CP, Zang Y, Rosenfield D, Yadin E, Yarvis JS, et al. Effect of Prolonged Exposure Therapy Delivered Over 2 Weeks vs 8 Weeks vs Present-Centered Therapy on PTSD Symptom Severity in Military Personnel: A Randomized Clinical Trial. JAMA 2018;319:354–64.
Abramowitz JS, Deacon BJ, Whiteside SPH. Exposure Therapy for Anxiety: Principles and Practice. Second edition. New York: The Guilford Press; 2019.
de Haan S, Rietveld E, Stokhof M, Denys D. Effects of deep brain stimulation on the lived experience of obsessive-compulsive disorder patients: in-depth interviews with 18 patients. PLoS ONE. 2015;10:e0135524.
Graat I, Franken S, Rooijen G van, Koning P de, Vulink N, Kroo M de, et al. Cognitive behavioral therapy in patients with deep brain stimulation for obsessive-compulsive disorder: a matched controlled study. Psychol Med. 2022:1–7.
Mantione M, Nieman DH, Figee M, Denys D. Cognitive–behavioural therapy augments the effects of deep brain stimulation in obsessive–compulsive disorder. Psychol Med. 2014;44:3515–22.
Sharpe MJ, Stalnaker T, Schuck NW, Killcross S, Schoenbaum G, Niv Y. An integrated model of action selection: distinct modes of cortical control of striatal decision making. Annu Rev Psychol. 2018;70:1–24.
Bari A, Robbins TW. Inhibition and impulsivity: Behavioral and neural basis of response control. Prog Neurobiol. 2013;108:44–79.
McLaughlin NCR, Dougherty DD, Eskandar E, Ward H, Foote KD, Malone DA, et al. Double blind randomized controlled trial of deep brain stimulation for obsessive-compulsive disorder: Clinical trial design. Contemp Clin Trials Commun. 2021;22:100785.
Cavanagh JF, Frank MJ. Frontal theta as a mechanism for cognitive control. Trends Cogn Sci. 2014;18:414–21.
Cooper PS, Karayanidis F, McKewen M, McLellan-Hall S, Wong ASW, Skippen P, et al. Frontal theta predicts specific cognitive control-induced behavioural changes beyond general reaction time slowing. NeuroImage 2019;189:130–40.
Cohen MX, Donner TH. Midfrontal conflict-related theta-band power reflects neural oscillations that predict behavior. J Neurophysiol. 2013;110:2752–63.
Vaghi MM, Vértes PE, Kitzbichler MG, Apergis-Schoute AM, van der Flier FE, Fineberg NA, et al. Specific frontostriatal circuits for impaired cognitive flexibility and goal-directed planning in obsessive-compulsive disorder: evidence from resting-state functional connectivity. Biol Psychiatry. 2017;81:708–17.
Allawala A, Vartany S, Mathura R, Ritz H, Adkinson JA, Oswalt D, et al. Characterization and modulation of neural substrates underlying cognitive control in treatment-resistant depression. San Diego, CA: 2022 Society for Neuroscience Annual Meeting; 2022.
Yousefi A, Paulk AC, Basu I, Dougherty DD, Eskandar EN, Eden UT, et al. COMPASS: an open-source, general-purpose software toolkit for computational psychiatry. Front Neurosci. 2019;12:957.
Haber SN. Corticostriatal circuitry. Dialogues Clin Neurosci. 2016;18:7–21.
Nagrale SS, Yousefi A, Netoff TI, Widge AS. In silico development and validation of Bayesian methods for optimizing deep brain stimulation to enhance cognitive control. J Neural Eng. 2023;20:036015.
Braunstein LM, Gross JJ, Ochsner KN. Explicit and implicit emotion regulation: a multi-level framework. Soc Cogn Affect Neurosci. 2017;12:1545–57.
Gross JJ. Emotion Regulation: Current Status and Future Prospects. Psychol Inq. 2015;26:1–26.
Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013;14:609–25.
Cox J, Witten IB. Striatal circuits for reward learning and decision-making. Nat Rev Neurosci. 2019;20:482.
Enkavi AZ, Eisenberg IW, Bissett PG, Mazza GL, MacKinnon DP, Marsch LA, et al. Large-scale analysis of test–retest reliabilities of self-regulation measures. Proc Natl Acad Sci. 2019;116:5472–7.
Enkavi AZ, Poldrack RA. Implications of the Lacking Relationship Between Cognitive Task and Self-report Measures for Psychiatry. Biol Psychiatry: Cogn Neurosci Neuroimaging. 2021;6:670–2.
Gillan CM, Kosinski M, Whelan R, Phelps EA, Daw ND. Characterizing a psychiatric symptom dimension related to deficits in goal-directed control. ELife 2016;5:e11305.
Adamchic I, Hauptmann C, Barnikol UB, Pawelczyk N, Popovych O, Barnikol TT, et al. Coordinated reset neuromodulation for Parkinson’s disease: Proof-of-concept study. Mov Disord. 2014;29:1679–84.
Popovych OV, Tass PA. Desynchronizing electrical and sensory coordinated reset neuromodulation. Front Hum Neurosci. 2012;6:58.
Mobbs D, Headley DB, Ding W, Dayan P. Space, time, and fear: survival computations along defensive circuits. Trends Cogn Sci. 2020;24:228–41.
Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3:760–73.
Hebb DO The organization of behavior; a neuropsychological theory. Oxford, England: Wiley; 1949.
Fetz EE Chapter 12 - Restoring motor function with bidirectional neural interfaces. In: Dancause N, Nadeau S, Rossignol S, editors. Progress in Brain Research, vol. 218, Elsevier; 2015. p. 241–52.
Nishimura Y, Perlmutter SI, Eaton RW, Fetz EE. Spike-timing-dependent plasticity in primate corticospinal connections induced during free behavior. Neuron 2013;80:1301–9.
Jackson A, Mavoori J, Fetz EE. Long-term motor cortex plasticity induced by an electronic neural implant. Nature 2006;44:56–60.
Moritz CT. Now is the critical time for engineered neuroplasticity. Neurotherapeutics 2018;15:628–34.
Moorjani S, Walvekar S, Fetz EE, Perlmutter SI. Movement-dependent electrical stimulation for volitional strengthening of cortical connections in behaving monkeys. Proc Natl Acad Sci USA. 2022;119:e2116321119.
Bi G, Poo M. Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. J Neurosci. 1998;18:10464–72.
Widge AS, Moritz CT. Pre-frontal control of closed-loop limbic neurostimulation by rodents using a brain-computer interface. J Neural Eng. 2014;11:024001.
Seeman SC, Mogen BJ, Fetz EE, Perlmutter SI. Paired stimulation for spike-timing-dependent plasticity in primate sensorimotor cortex. J Neurosci. 2017;37:1935–49.
Udupa K, Bahl N, Ni Z, Gunraj C, Mazzella F, Moro E, et al. Cortical plasticity induction by pairing subthalamic nucleus deep-brain stimulation and primary motor cortical transcranial magnetic stimulation in Parkinson’s disease. J Neurosci. 2016;36:396–404.
Veniero D, Ponzo V, Koch G. Paired associative stimulation enforces the communication between interconnected areas. J Neurosci. 2013;33:13773–83.
Lo M, Younk R, Widge AS. Paired electrical pulse trains for controlling connectivity in emotion-related brain circuitry. IEEE Trans Neural Syst Rehabil Eng. 2020;20:2721–30.
Barra B, Conti S, Perich MG, Zhuang K, Schiavone G, Fallegger F, et al. Epidural electrical stimulation of the cervical dorsal roots restores voluntary upper limb control in paralyzed monkeys. Nat Neurosci. 2022;25:924–34.
Borton D, Bonizzato M, Beauparlant J, DiGiovanna J, Moraud EM, Wenger N, et al. Corticospinal neuroprostheses to restore locomotion after spinal cord injury. Neurosci Res. 2014;78:21–29.
Peña Pino I, Hoover C, Venkatesh S, Ahmadi A, Sturtevant D, Patrick N, et al. Long-term spinal cord stimulation after chronic complete spinal cord injury enables volitional movement in the absence of stimulation. Front Syst Neurosci. 2020;14:35.
Webler RD, Oathes DJ, van Rooij SJH, Gewirtz JC, Nahas Z, Lissek SM, et al. Causally mapping human threat extinction relevant circuits with depolarizing brain stimulation methods. Neurosci Biobehav Rev. 2023;144:105005.
Bick SK, Patel SR, Katnani HA, Peled N, Widge A, Cash SS, et al. Caudate stimulation enhances learning. Brain 2019;142:2930–7.
Milad MR, Vidal-Gonzalez I, Quirk GJ. Electrical stimulation of medial prefrontal cortex reduces conditioned fear in a temporally specific manner. Behav Neurosci. 2004;118:389–94.
Herman AB, Widge AS. Dynamic network targeting for closed loop deep brain stimulation. Neuropsychopharmacology 2018;44:219–20.
Vinogradov S, Herman A. Psychiatric Illnesses as Oscillatory Connectomopathies. Neuropsychopharmacology 2016;41:387–8.
Mathalon DH, Sohal VS. Neural oscillations and synchrony in brain dysfunction and neuropsychiatric disorders: It’s about time. JAMA Psychiatry. 2015;72:840–4.
Başar E, Schmiedt-Fehr C, Mathes B, Femir B, Emek-Savaş DD, Tülay E, et al. What does the broken brain say to the neuroscientist? Oscillations and connectivity in schizophrenia, Alzheimer’s disease, and bipolar disorder. Int J Psychophysiol. 2016;103:135–48.
Fries P. Rhythms for cognition: communication through coherence. Neuron 2015;88:220–35.
Hahn G, Ponce-Alvarez A, Deco G, Aertsen A, Kumar A. Portraits of communication in neuronal networks. Nat Rev Neurosci. 2019;20:117.
Hultman R, Ulrich K, Sachs BD, Blount C, Carlson DE, Ndubuizu N, et al. Brain-wide electrical spatiotemporal dynamics encode depression vulnerability. Cell 2018;173:166–180.e14.
Provenza NR, Paulk AC, Peled N, Restrepo MI, Cash SS, Dougherty DD, et al. Decoding task engagement from distributed network electrophysiology in humans. J Neural Eng. 2019;16:056015.
de Hemptinne C, Swann NC, Ostrem JL, Ryapolova-Webb ES, San Luciano M, Galifianakis NB, et al. Therapeutic deep brain stimulation reduces cortical phase-amplitude coupling in Parkinson’s disease. Nat Neurosci. 2015;18:779–86.
Wang DD, de Hemptinne C, Miocinovic S, Ostrem JL, Galifianakis NB, Luciano MS, et al. Pallidal deep-brain stimulation disrupts pallidal beta oscillations and coherence with primary motor cortex in Parkinson’s disease. J Neurosci. 2018;38:4556–68.
Wendt K, Denison T, Foster G, Krinke L, Thomson A, Wilson S, et al. Physiologically informed neuromodulation. J Neurological Sci. 2022;434:120121.
Grover S, Nguyen JA, Reinhart RMG. Synchronizing Brain Rhythms to Improve Cognition. Annu Rev Med. 2021;72:29–43.
Gordon PC, Belardinelli P, Stenroos M, Ziemann U, Zrenner C. Prefrontal theta phase-dependent rTMS-induced plasticity of cortical and behavioral responses in human cortex. Brain Stimulation. 2022;15:391–402.
Mansouri F, Shanbour A, Mazza F, Fettes P, Zariffa J, Downar J. Effect of theta transcranial alternating current stimulation and phase-locked transcranial pulsed current stimulation on learning and cognitive control. Front Neurosci. 2019;13:1181.
Cagnan H, Pedrosa D, Little S, Pogosyan A, Cheeran B, Aziz T, et al. Stimulating at the right time: phase-specific deep brain stimulation. Brain. 2017;140:132–45.
Holt AB, Kormann E, Gulberti A, Pötter-Nerger M, McNamara CG, Cagnan H, et al. Phase-dependent suppression of beta oscillations in Parkinson’s disease patients. J Neurosci. 2019;39:1119–34.
Hosseinian T, Yavari F, Biagi MC, Kuo M-F, Ruffini G, Nitsche MA, et al. External induction and stabilization of brain oscillations in the human. Brain Stimulation. 2021;14:579–87.
Hosseinian T, Yavari F, Kuo M-F, Nitsche MA, Jamil A. Phase synchronized 6 Hz transcranial electric and magnetic stimulation boosts frontal theta activity and enhances working memory. NeuroImage 2021;245:118772.
Widge AS, Boggess M, Rockhill AP, Mullen A, Sheopory S, Loonis R, et al. Altering alpha-frequency brain oscillations with rapid analog feedback-driven neurostimulation. PLOS ONE. 2018;13:e0207781.
McNamara CG, Rothwell M, Sharott A. Stable, interactive modulation of neuronal oscillations produced through brain-machine equilibrium. Cell Rep. 2022;41:111616.
Escobar Sanabria D, Johnson LA, Yu Y, Busby Z, Nebeck S, Zhang J, et al. Real-time suppression and amplification of frequency-specific neural activity using stimulation evoked oscillations. Brain Stimulation. 2020;13:1732–42.
Zanos S, Rembado I, Chen D, Fetz EE. Phase-locked stimulation during cortical beta oscillations produces bidirectional synaptic plasticity in awake monkeys. Curr Biol. 2018;28:2515–2526.e4.
Carlson D, David LK, Gallagher NM, Vu M-AT, Shirley M, Hultman R, et al. Dynamically timed stimulation of corticolimbic circuitry activates a stress-compensatory pathway. Biol Psychiatry. 2017;82:904–13.
Bastos AM, Vezoli J, Bosman CA, Schoffelen J-M, Oostenveld R, Dowdall JR, et al. Visual areas exert feedforward and feedback influences through distinct frequency channels. Neuron 2015;85:390–401.
Lüscher C. The emergence of a circuit model for addiction. Annu Rev Neurosci. 2016;39:257–76.
Hearing MC, Jedynak J, Ebner SR, Ingebretson A, Asp AJ, Fischer RA, et al. Reversal of morphine-induced cell-type–specific synaptic plasticity in the nucleus accumbens shell blocks reinstatement. PNAS 2016;113:757–62.
Tan LL, Kuner R. Neocortical circuits in pain and pain relief. Nat Rev Neurosci. 2021;22:458–71.
Peirs C, Seal RP. Neural circuits for pain: Recent advances and current views. Science 2016;354:578–84.
Boccard SGJ, Prangnell SJ, Pycroft L, Cheeran B, Moir L, Pereira EAC, et al. Long-term results of deep brain stimulation of the anterior cingulate cortex for neuropathic pain. World Neurosurg. 2017;106:625–37.
Huang Y, Cheeran B, Green AL, Denison TJ, Aziz TZ. Applying a sensing-enabled system for ensuring safe anterior cingulate deep brain stimulation for pain. Brain Sci. 2019;9:150.
Williams LM. Defining biotypes for depression and anxiety based on large-scale circuit dysfunction: a theoretical review of the evidence and future directions for clinical translation. Depression Anxiety. 2016;34:9–24.
Yang Y, Shanechi MM A framework for identification of brain network dynamics using a novel binary noise modulated electrical stimulation pattern. 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), 2015. p. 2087–90.
Grado LL, Johnson MD, Netoff TI. Bayesian adaptive dual control of deep brain stimulation in a computational model of Parkinson’s disease. PLOS Comput Biol. 2018;14:e1006606.
Funding
Preparation of this article was supported by R01MH125429, R01MH124687, R01MH123634, UH3NS100548, R01NS120851, R01MH119384, R21DA052568, and the MnDRIVE Brain Conditions Initiative. The views are entirely those of the author(s), not any funding body.
Author information
Authors and Affiliations
Contributions
ASW wrote the article.
Corresponding author
Ethics declarations
Competing interests
ASW receives DBS device donations from Medtronic and consulting fees related to DBS for psychiatric illness from Abbott. He holds multiple granted and pending patents in the area of closed-loop deep brain stimulation.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Widge, A.S. Closing the loop in psychiatric deep brain stimulation: physiology, psychometrics, and plasticity. Neuropsychopharmacol. 49, 138–149 (2024). https://doi.org/10.1038/s41386-023-01643-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-023-01643-y