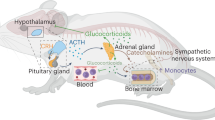
Abstract
There is an urgent need to develop more effective treatments for stress-related illnesses, which include depression, post-traumatic stress disorder, and anxiety. We view animal models as playing an essential role in this effort, but to date, such approaches have generally not succeeded in developing therapeutics with new mechanisms of action. This is partly due to the complexity of the brain and its disorders, but also to inherent difficulties in modeling human disorders in rodents and to the incorrect use of animal models: namely, trying to recapitulate a human syndrome in a rodent which is likely not possible as opposed to using animals to understand underlying mechanisms and evaluating potential therapeutic paths. Recent transcriptomic research has established the ability of several different chronic stress procedures in rodents to recapitulate large portions of the molecular pathology seen in postmortem brain tissue of individuals with depression. These findings provide crucial validation for the clear relevance of rodent stress models to better understand the pathophysiology of human stress disorders and help guide therapeutic discovery. In this review, we first discuss the current limitations of preclinical chronic stress models as well as traditional behavioral phenotyping approaches. We then explore opportunities to dramatically enhance the translational use of rodent stress models through the application of new experimental technologies. The goal of this review is to promote the synthesis of these novel approaches in rodents with human cell-based approaches and ultimately with early-phase proof-of-concept studies in humans to develop more effective treatments for human stress disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–9.
Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the national comorbidity survey. Arch Gen Psychiatry. 1995;52:1048–60.
Kessler RC. The effects of stressful life events on depression. Annu Rev Psychol. 1997;48:191–214.
Russo SJ, Murrough JW, Han M-H, Charney DS, Nestler EJ. Neurobiology of resilience. Nat Neurosci. 2012;15:1475–84.
Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902.
De Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:6.
Levey DF, Stein MB, Wendt FR, Pathak GA, Zhou H, Aslan M, et al. Bi-ancestral depression GWAS in the Million Veteran Program and meta-analysis in >1.2 million individuals highlight new therapeutic directions. Nat Neurosci. 2021. 2021. https://doi.org/10.1038/s41593-021-00860-2.
Howard DM, Adams MJ, Clarke T-K, Hafferty JD, Gibson J, Shirali M, et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat Neurosci. 2019;22:343–52.
Scarpa JR, Fatma M, Loh YHE, Traore SR, Stefan T, Chen TH, et al. Shared transcriptional signatures in major depressive disorder and mouse chronic stress models. Biol Psychiatry. 2020;88:159–68.
Friedrich MJ. Depression is the leading cause of disability around the world. J Am Med Assoc. 2017;317:1517.
Flory JD, Yehuda R. Comorbidity between post-traumatic stress disorder and major depressive disorder: alternative explanations and treatment considerations. Dialog Clin Neurosci. 2015;17:141–50.
van Praag HM. Can stress cause depression? Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:891–907.
Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 1999;156:837–41.
Nestler EJ, Gould E, Manji H, Bucan M, Duman RS, Gershenfeld HK, et al. Preclinical models: status of basic research in depression. Biol Psychiatry. 2002;52:503–28.
Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharm. 1978;47:379–91.
Can A, Dao DT, Terrillion CE, Piantadosi SC, Bhat S, Gould TD. The tail suspension test. J Vis Exp. 2012;59:e3769.
Pryce CR, Azzinnari D, Sigrist H, Gschwind T, Lesch KP, Seifritz E. Establishing a learned-helplessness effect paradigm in C57BL/6 mice: Behavioural evidence for emotional, motivational and cognitive effects of aversive uncontrollability per se. Neuropharmacology. 2012;62:358–72.
Yao L, Li Y, Qian Z, Wu M, Yang H, Chen N, et al. Loss of control over mild aversive events produces significant helplessness in mice. Behav Brain Res. 2019;376:112173.
Hodes GE, Pfau ML, Purushothaman I, Francisca Ahn H, Golden SA, Christoffel DJ, et al. Sex differences in nucleus accumbens transcriptome profiles associated with susceptibility versus resilience to subchronic variable stress. J Neurosci. 2015;35:16362–76.
Borrow AP, Bales NJ, Stover SA, Handa RJ. Chronic variable stress induces sex-specific alterations in social behavior and neuropeptide expression in the mouse. Endocrinology. 2018;159:2803–14.
Labonté B, Engmann O, Purushothaman I, Menard C, Wang J, Tan C, et al. Sex-specific transcriptional signatures in human depression. Nat Med. 2017;23:1102–11.
Coutellier L, Gilbert V, Shepard R. Npas4 deficiency increases vulnerability to juvenile stress in mice. Behav Brain Res. 2015;295:17–25.
Lopez MF, Doremus-Fitzwater TL, Becker HC. Chronic social isolation and chronic variable stress during early development induce later elevated ethanol intake in adult C57BL/6J mice. Alcohol. 2011;45:355–64.
Hillis S, Mercy J, Amobi A, Kress H. Global prevalence of past-year violence against children: a systematic review and minimum estimates. Pediatrics. 2016;137:e20154079.
Krishnan V, Han M-H, Graham DL, Berton O, Renthal W, Russo SJ, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404.
Iñiguez SD, Riggs LM, Nieto SJ, Dayrit G, Zamora NN, Shawhan KL, et al. Social defeat stress induces a depression-like phenotype in adolescent male c57BL/6 mice. Stress. 2014;17:247–55.
Berton O, Mcclung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–8.
Charney DS. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiatry. 2004;161:195–216.
Bagot RC, Cates HM, Purushothaman I, Lorsch ZS, Walker DM, Wang J, et al. Circuit-wide transcriptional profiling reveals brain region-specific gene networks regulating depression susceptibility. Neuron. 2016;90:969–83.
Vialou V, Robison AJ, Laplant QC, Covington HE, Dietz DM, Ohnishi YN, et al. ΔfosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat Neurosci. 2010;13:745–52.
Pizarro JM, Lumley LA, Medina W, Robison CL, Chang WE, Alagappan A, et al. Acute social defeat reduces neurotrophin expression in brain cortical and subcortical areas in mice. Brain Res. 2004;1025:10–20.
Keeney A, Jessop DS, Harbuz MS, Marsden CA, Hogg S, Blackburn-Munro RE. Differential effects of acute and chronic social defeat stress on hypothalamic-pituitary-adrenal axis function and hippocampal serotonin release in mice. J Neuroendocrinol. 2006;18:330–8.
Warren BL, Vialou VF, Iñiguez SD, Alcantara LF, Wright KN, Feng J, et al. Neurobiological sequelae of witnessing stressful events in adult mice. Biol Psychiatry. 2013;73:7–14.
Qi G, Zhang P, Li T, Li M, Zhang Q, He F, et al. NAc-VTA circuit underlies emotional stress-induced anxiety-like behavior in the three-chamber vicarious social defeat stress mouse model. Nat Commun. 2022;13:1.
Sial OK, Warren BL, Alcantara LF, Parise EM, Bolaños-Guzmán CA. Vicarious social defeat stress: bridging the gap between physical and emotional stress. J Neurosci Methods. 2016;258:94–103.
Iñiguez SD, Flores-Ramirez FJ, Riggs LM, Alipio JB, Garcia-Carachure I, Hernandez MA, et al. Vicarious social defeat stress induces depression-related outcomes in female mice. Biol Psychiatry. 2018;83:9–17.
Garcia-Carachure I, Flores-Ramirez FJ, Castillo SA, Themann A, Arenivar MA, Preciado-Piña J, et al. Enduring effects of adolescent ketamine exposure on cocaine- and sucrose-induced reward in male and female C57BL/6 mice. Neuropsychopharmacology. 2020;45:1536–44.
Harris AZ, Atsak P, Bretton ZH, Holt ES, Alam R, Morton MP, et al. A novel method for chronic social defeat stress in female mice. Neuropsychopharmacology. 2018;43:1276–83.
Takahashi A, Chung JR, Zhang S, Zhang H, Grossman Y, Aleyasin H, et al. Establishment of a repeated social defeat stress model in female mice. Sci Rep. 2017;7:12838.
Yohn CN, Dieterich A, Bazer AS, Maita I, Giedraitis M, Samuels BA. Chronic non-discriminatory social defeat is an effective chronic stress paradigm for both male and female mice. Neuropsychopharmacology. 2019;44:2220–9.
Newman EL, Covington HE, Suh J, Bicakci MB, Ressler KJ, DeBold JF, et al. Fighting females: neural and behavioral consequences of social defeat stress in female mice. Biol Psychiatry. 2019;86:657–68.
Heim C, Binder EB. Current research trends in early life stress and depression: review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Exp Neurol. 2012;233:102–11.
Heim C, Shugart M, Craighead WE, Nemeroff CB. Neurobiological and psychiatric consequences of child abuse and neglect. Dev Psychobiol. 2010;52:671–90.
Nelson EC, Heath AC, Madden PAF, Cooper; M Lynne, Dinwiddie SH, et al. Association between self-reported childhood sexual abuse and adverse psychosocial outcomes results from a twin study. Arch Gen Psychiatry. 2002;59:139–45.
Kendler KS, Bulik CM, Silberg J, Hettema JM, Myers J, Prescott CA. Childhood sexual abuse and adult psychiatric and substance use disorders in women an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57:953–9.
Gallo M, Shleifer DG, Godoy LD, Ofray D, Olaniyan A, Campbell T, et al. Limited bedding and nesting induces maternal behavior resembling both hypervigilance and abuse. Front Behav Neurosci. 2019;13:167.
Peña CJ, Smith M, Ramakrishnan A, Cates HM, Bagot RC, Kronman HG, et al. Early life stress alters transcriptomic patterning across reward circuitry in male and female mice. Nat Commun. 2019;10:5098.
Stuart SA, Hinchcliffe JK, Robinson ESJ. Evidence that neuropsychological deficits following early life adversity may underlie vulnerability to depression. Neuropsychopharmacology. 2019;44:1623–30.
Goodwill HL, Manzano-Nieves G, Gallo M, Lee HI, Oyerinde E, Serre T, et al. Early life stress leads to sex differences in development of depressive-like outcomes in a mouse model. Neuropsychopharmacology. 2019;44:711–20.
Block CL, Eroglu O, Mague SD, Smith CJ, Ceasrine AM, Sriworarat C, et al. Prenatal environmental stressors impair postnatal microglia function and adult behavior in males. Cell Rep. 2022;40:111161.
Van Den Hove DLA, Blanco CE, Aendekerk B, Desbonnet L, Bruschettini M, Steinbusch HP, et al. Prenatal restraint stress and long-term affective consequences. Dev Neurosci. 2005;27:313–20.
Begni V, Zampar S, Longo L, Riva MA. Sex differences in the enduring effects of social deprivation during adolescence in rats: implications for psychiatric disorders. Neuroscience. 2020;437:11–22.
Walker DM, Zhou X, Cunningham AM, Ramakrishnan A, Cates HM, Lardner CK, et al. Crystallin Mu in medial amygdala mediates the effect of social experience on cocaine seeking in males but not in females. Biol Psychiatry. 2022;92:895–906.
Fosnocht AQ, Lucerne KE, Ellis AS, Olimpo NA, Briand LA. Adolescent social isolation increases cocaine seeking in male and female mice. Behav Brain Res. 2019;359:589–96.
Walker DM, Zhou X, Cunningham AM, Lipschultz AP, Ramakrishnan A, Cates HM, et al. Sex-specific transcriptional changes in response to adolescent social stress in the brain’s reward circuitry. Biol Psychiatry. 2022;91:118–28.
Daskalakis NP, Xu C, Bader HN, Chatzinakos C, Weber P, Makotkine I, et al. Intergenerational trauma is associated with expression alterations in glucocorticoid- and immune-related genes. Neuropsychopharmacology. 2021;46:763–73.
Gourley SL, Taylor JR. Recapitulation and reversal of a persistent depression-like syndrome in rodents. Curr Protoc Neurosci. 2009;Chapter 9:Unit 9.32.
Johnson SA, Fournier NM, Kalynchuk LE. Effect of different doses of corticosterone on depression-like behavior and HPA axis responses to a novel stressor. Behav Brain Res. 2006;168:280–8.
David DJ, Samuels BA, Rainer Q, Wang J-W, Marsteller D, Mendez I, et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479–93.
Liu YN, Peng YL, Lei-Liu, Wu TY, Zhang Y, Lian YJ, et al. TNFα mediates stress-induced depression by upregulating indoleamine 2,3-dioxygenase in a mouse model of unpredictable chronic mild stress. Eur Cytokine Netw. 2015;26:15–25.
Renner V, Schellong J, Bornstein S, Petrowski K. Stress-induced pro- and anti-inflammatory cytokine concentrations in female PTSD and depressive patients. Transl Psychiatry. 2022;12:158.
O’Connor JC, Lawson MA, André C, Moreau M, Lestage J, Castanon N, et al. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. 2009;14:511–22.
Walker AK, Budac DP, Bisulco S, Lee AW, Smith RA, Beenders B, et al. NMDA receptor blockade by ketamine abrogates lipopolysaccharide-induced depressive-like behavior in C57BL/6 J mice. Neuropsychopharmacology. 2013;38:1609–16.
Feder Y, Nesher E, Ogran A, Kreinin A, Malatynska E, Yadid G, et al. Selective breeding for dominant and submissive behavior in Sabra mice. J Affect Disord. 2010;126:214–22.
Nesher E, Gross M, Lisson S, Tikhonov T, Yadid G, Pinhasov A. Differential responses to distinct psychotropic agents of selectively bred dominant and submissive animals. Behav Brain Res. 2013;236:225–35.
Flagel SB, Waselus M, Clinton SM, Watson SJ, Akil H. Antecedents and consequences of drug abuse in rats selectively bred for high and low response to novelty. Neuropharmacology. 2014;76:425–36.
Davis BA, Clinton SM, Akil H, Becker JB. The effects of novelty-seeking phenotypes and sex differences on acquisition of cocaine self-administration in selectively bred High-Responder and Low-Responder rats. Pharm Biochem Behav. 2008;90:331–8.
Calvo N, Cecchi M, Kabbaj M, Watson SJ, Akil H. Differential effects of social defeat in rats with high and low locomotor response to novelty. Neuroscience. 2011;183:81–89.
Peña CJ, Kronman HG, Walker DM, Cates HM, Bagot RC, Purushothaman I, et al. Early life stress confers lifelong stress susceptibility in mice via ventral tegmental area OTX2. Science. 2017;356:1185–8.
Kronman H, Torres-Berrío A, Sidoli S, Issler O, Godino A, Ramakrishnan A, et al. Long-term behavioral and cell-type-specific molecular effects of early life stress are mediated by H3K79me2 dynamics in medium spiny neurons. Nat Neurosci. 2021;24:667–76.
Hollis F, Wang H, Dietz D, Gunjan A, Kabbaj M. The effects of repeated social defeat on long-term depressive-like behavior and short-term histone modifications in the hippocampus in male Sprague–Dawley rats. Psychopharmacol (Berl). 2010;211:69–77.
Venzala E, García-García AL, Elizalde N, Tordera RM. Social vs. environmental stress models of depression from a behavioural and neurochemical approach. Eur Neuropsychopharmacol. 2013;23:697–708.
Covington HE, Vialou VF, LaPlant Q, Ohnishi YN, Nestler EJ. Hippocampal-dependent antidepressant-like activity of histone deacetylase inhibition. Neurosci Lett. 2011;493:122–6.
Slattery DA, Uschold N, Magoni M, Bär J, Popoli M, Neumann ID, et al. Behavioural consequences of two chronic psychosocial stress paradigms: anxiety without depression. Psychoneuroendocrinology. 2012;37:702–14.
ver Hoeve ES, Kelly G, Luz S, Ghanshani S, Bhatnagar S. Short-term and long-term effects of repeated social defeat during adolescence or adulthood in female rats. Neuroscience. 2013;249:63–73.
Bächli H, Steiner MA, Habersetzer U, Wotjak CT. Increased water temperature renders single-housed C57BL/6J mice susceptible to antidepressant treatment in the forced swim test. Behav Brain Res. 2008;187:67–71.
Ayash S, Schmitt U, Müller MB. Chronic social defeat-induced social avoidance as a proxy of stress resilience in mice involves conditioned learning. J Psychiatr Res. 2020;120:64–71.
Cunningham CL, Gremel CM, Groblewski PA. Drug-induced conditioned place preference and aversion in mice. Nat Protoc. 2006;1:1662–70.
Childs E, de Wit H. Amphetamine-induced place preference in humans. Biol Psychiatry. 2009;65:900–4.
Tombaugh TN, Grandmaison LJ, Zito KA. Establishment of secondary reinforcement in sign tracking and place preference tests following pimozide treatment. Pharm Biochem Behav. 1982;17:665–70.
Tenk CM, Wilson H, Zhang Q, Pitchers KK, Coolen LM. Sexual reward in male rats: effects of sexual experience on conditioned place preferences associated with ejaculation and intromissions. Horm Behav. 2009;55:93–7.
Peartree NA, Hood LE, Thiel KJ, Sanabria F, Pentkowski NS, Chandler KN, et al. Limited physical contact through a mesh barrier is sufficient for social reward-conditioned place preference in adolescent male rats. Physiol Behav. 2012;105:749–56.
Li L, Durand-de Cuttoli R, Aubry AV, Burnett CJ, Cathomas F, Parise LF, et al. Social trauma engages lateral septum circuitry to occlude social reward. Nature. 2023;613:696–703.
Baron D, Holland CM, Carlson K, Wolfrum E, Thompson BL. Adapting social conditioned place preference for use in young children. Neurobiol Learn Mem. 2020;172:107235.
Kiser DP, Gromer D, Pauli P, Hilger K. A virtual reality social conditioned place preference paradigm for humans: Does trait social anxiety affect approach and avoidance of virtual agents? Front Virtual Real. 2022;3:916575.
Guo B, Chen J, Chen Q, Ren K, Feng D, Mao H, et al. Anterior cingulate cortex dysfunction underlies social deficits in Shank3 mutant mice. Nat Neurosci. 2019;22:1223–34.
Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, et al. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302.
Park MJ, Seo BA, Lee B, Shin HS, Kang MG. Stress-induced changes in social dominance are scaled by AMPA-type glutamate receptor phosphorylation in the medial prefrontal cortex. Sci Rep. 2018;8:15008.
Franco D, Wulff AB, Lobo MK, Fox ME. Chronic physical and vicarious psychosocial stress alter fentanyl consumption and nucleus accumbens Rho GTPases in male and female C57BL/6 mice. Front Behav Neurosci. 2022;16:821080.
Morais-Silva G, Campbell RR, Nam H, Basu M, Pagliusi M, Fox ME, et al. Molecular, circuit, and stress response characterization of ventral pallidum Npas1-neurons. J Neurosci. 2023;43:405–18.
Matsumoto M, Yoshida M, Jayathilake BW, Inutsuka A, Nishimori K, Takayanagi Y, et al. Indispensable role of the oxytocin receptor for allogrooming toward socially distressed cage mates in female mice. J Neuroendocrinol. 2021;33:e12980.
Peñagarikano O, Lázaro MT, Lu X-H, Gordon A, Dong H, Lam HA, et al. Exogenous and evoked oxytocin restores social behavior in the Cntnap2 mouse model of autism. Sci Transl Med. 2015;7:271ra8.
Kim J-W, Park K, Kang RJ, Gonzales ELT, Kim DG, Oh HA, et al. Pharmacological modulation of AMPA receptor rescues social impairments in animal models of autism. Neuropsychopharmacology. 2019;44:314–23.
Du Preez A, Law T, Onorato D, Lim YM, Eiben P, Musaelyan K, et al. The type of stress matters: repeated injection and permanent social isolation stress in male mice have a differential effect on anxiety- and depressive-like behaviours, and associated biological alterations. Transl Psychiatry. 2020;10:325.
Samuels BA, Hen R. Novelty-Suppressed Feeding in the Mouse. In: Gould T, editor. Mood and Anxiety Related Phenotypes in Mice. Neuromethods, vol 63. 2011. p. 107–21.
Dieterich A, Srivastava P, Sharif A, Stech K, Floeder J, Yohn SE, et al. Chronic corticosterone administration induces negative valence and impairs positive valence behaviors in mice. Transl Psychiatry. 2019;9:337.
Stedenfeld KA, Clinton SM, Kerman IA, Akil H, Watson SJ, Sved AF. Novelty-seeking behavior predicts vulnerability in a rodent model of depression. Physiol Behav. 2011;103:210–6.
Surget A, Saxe M, Leman S, Ibarguen-Vargas Y, Chalon S, Griebel G, et al. Drug-dependent requirement of hippocampal neurogenesis in a model of depression and of antidepressant reversal. Biol Psychiatry. 2008;64:293–301.
DiFazio LE, Fanselow M, Sharpe MJ. The effect of stress and reward on encoding future fear memories. Behav Brain Res. 2022;417:113587.
Ressler KJ, Berretta S, Bolshakov VY, Rosso IM, Meloni EG, Rauch SL, et al. Post-traumatic stress disorder: clinical and translational neuroscience from cells to circuits. Nat Rev Neurol. 2022;18:273–88.
Gorwood P. Neurobiological mechanisms of anhedonia. Dialog Clin Neurosci 2008;10:291–9.
Stanton CH, Holmes AJ, Chang SWC, Joormann J. From stress to anhedonia: molecular processes through functional circuits. Trends Neurosci. 2019;42:23–42.
Eagle AL, Gajewski PA, Yan M, Kechner ME, Al Masraf BS, Kennedy PJ. Experience-dependent induction of hippocampal DeltaFosB controls learning. J Neurosci. 2015;35:13773–83.
Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacol (Berl). 1987;93:358–64.
Liu MY, Yin CY, Zhu LJ, Zhu XH, Xu C, Luo CX, et al. Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat Protoc. 2018;13:1686–98.
Peciña S, Cagniard B, Berridge KC, Aldridge JW, Zhuang X. Behavioral/systems/cognitive hyperdopaminergic mutant mice have higher ‘wanting’ but not ‘liking’ for sweet rewards. J Neurosci. 2003;23:9395–402.
Ghiglieri O, Gambarana C, Scheggi S, Tagliamonte A, Willner P, De Montis MG. Palatable food induces an appetitive behaviour in satiated rats which can be inhibited by chronic stress. Behav Pharmacol. 1997;8:619–28.
Gavrilov VV, Onufriev MV, Moiseeva YV, Alexandrov YI, Gulyaeva NV. Chronic social isolation stress and crowding in rats have different effects on learning an operant behavior and the state of the hypothalamo-hypophyseal-adrenocortical system. Neurosci Behav Physiol. 2022;52:698–704.
Thai CA, Zhang Y, Howland JG. Effects of acute restraint stress on set-shifting and reversal learning in male rats. Cogn Affect Behav Neurosci. 2013;13:164–73.
Scott K, Phan TT, Boukelmoune N, Heijnen CJ, Dantzer R. Chronic restraint stress impairs voluntary wheel running but has no effect on food-motivated behavior in mice. Brain Behav Immun. 2023;107:319–29.
Freidin E, Mustaca AE. Frustration and sexual behavior in male rats. Anim Learn Behav. 2004;32:311–20.
Gorzalka BB, Hanson LA, Brotto LA. Chronic stress effects on sexual behavior in male and female rats. Pharm Biochem Behav. 1998;61:405–12.
Hu RK, Zuo Y, Ly T, Wang J, Meera P, Wu YE, et al. An amygdala-to-hypothalamus circuit for social reward. Nat Neurosci. 2021;24:831–42.
Martin L, Sample H, Gregg M, Wood C. Validation of operant social motivation paradigms using BTBR T+tf/J and C57BL/6J inbred mouse strains. Brain Behav. 2014;4:754–64.
Carlezon WA, Chartoff EH. Intracranial self-stimulation (Icss) in rodents to study the neurobiology of motivation. Nat Protoc. 2007;2:2987–95.
Donahue RJ, Muschamp JW, Russo SJ, Nestler EJ, Carlezon WA. Effects of striatal ΔFosB over expression and ketamine on social defeat stress-induced anhedonia in mice. Biol Psychiatry. 2014;76:550–8.
Der-Avakian A, Mazei-Robison MS, Kesby JP, Nestler EJ, Markou A. Enduring deficits in brain reward function after chronic social defeat in rats: Susceptibility, resilience, and antidepressant response. Biol Psychiatry. 2014;76:542–9.
Engeln M, Fox ME, Lobo MK. Housing conditions during self-administration determine motivation for cocaine in mice following chronic social defeat stress. Psychopharmacol (Berl). 2021;238:41–54.
Dunn TW, Marshall JD, Severson KS, Aldarondo DE, Hildebrand DGC, Chettih SN, et al. Geometric deep learning enables 3D kinematic profiling across species and environments. Nat Methods. 2021;18:564–73.
Hobson L, Bains RS, Greenaway S, Wells S, Nolan PM. Phenotyping in mice using continuous home cage monitoring and ultrasonic vocalization recordings. Curr Protoc Mouse Biol. 2020;10:e80.
Sweis BM, Nestler EJ. Pushing the boundaries of behavioral analysis could aid psychiatric drug discovery. PLoS Biol. 2022;20:e3001904.
Mathis A, Mamidanna P, Cury KM, Abe T, Murthy VN, Mathis MW, et al. DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat Neurosci. 2018;21:1281–9.
Pereira TD, Tabris N, Matsliah A, Turner DM, Li J, Ravindranath S, et al. SLEAP: a deep learning system for multi-animal pose tracking. Nat Methods. 2022;19:486–95.
Willmore L, Cameron C, Yang J, Witten IB, Falkner AL. Behavioural and dopaminergic signatures of resilience. Nature. 2022;611:124–32.
Grieco F, Bernstein BJ, Biemans B, Bikovski L, Burnett CJ, Cushman JD, et al. Measuring behavior in the home cage: study design, applications, challenges, and perspectives. Front Behav Neurosci. 2021;15:219.
Alexandrov V, Brunner D, Hanania T, Leahy E. High-throughput analysis of behavior for drug discovery. Eur J Pharm. 2015;750:82–89.
Prevot TD, Misquitta KA, Fee C, Newton DF, Chatterjee D, Nikolova YS, et al. Residual avoidance: a new, consistent and repeatable readout of chronic stress-induced conflict anxiety reversible by antidepressant treatment. Neuropharmacology 2019;153:98–110.
Kiryk A, Janusz A, Zglinicki B, Turkes E, Knapska E, Konopka W, et al. IntelliCage as a tool for measuring mouse behavior – 20 years perspective. Behav Brain Res. 2020;388:112620.
Heinla I, Åhlgren J, Vasar E, Voikar V. Behavioural characterization of C57BL/6N and BALB/c female mice in social home cage – effect of mixed housing in complex environment. Physiol Behav. 2018;188:32–41.
Robinson L, Spruijt B, Riedel G. Between and within laboratory reliability of mouse behaviour recorded in home-cage and open-field. J Neurosci Methods. 2018;300:10–19.
de Visser L, van den Bos R, Kuurman WW, Kas MJH, Spruijt BM. Novel approach to the behavioural characterization of inbred mice: automated home cage observations. Genes Brain Behav. 2006;5:458–66.
Jhuang H, Garrote E, Yu X, Khilnani V, Poggio T, Steele AD, et al. Automated home-cage behavioural phenotyping of mice. Nat Commun. 2010;1:68.
Bass JS, Tuo AH, Ton LT, Jankovic MJ, Kapadia PK, Schirmer C, et al. On the digital psychopharmacology of valproic acid in mice. Front Neurosci. 2020;14:594612.
Singh S, Bermudez-Contreras E, Nazari M, Sutherland RJ, Mohajerani MH. Low-cost solution for rodent home-cage behaviour monitoring. PLoS One. 2019;14:e0220751.
Leahy E. Validated phenotypic approach to neuropsychiatric drug discovery. Drug Develop Delivery. 2019:46–49.
Roberds SL, Filippov I, Alexandrov V, Hanania T, Brunner D. Rapid, computer vision-enabled murine screening system identifies neuropharmacological potential of two new mechanisms. Front Neurosci. 2011;5:103
Lorsch ZS, Ambesi-Impiombato A, Zenowich R, Morganstern I, Leahy E, Bansal M, et al. Computational analysis of multidimensional behavioral alterations after chronic social defeat stress. Biol Psychiatry. 2021;89:920–8.
Koblan KS, Kent J, Hopkins SC, Krystal JH, Cheng H, Goldman R, et al. A non–D2-receptor-binding drug for the treatment of schizophrenia. N. Engl J Med. 2020;382:1497–506.
Durand-de Cuttoli R, Martínez-Rivera FJ, Li L, Minier-Toribio A, Holt LM, Cathomas F, et al. Distinct forms of regret linked to resilience versus susceptibility to stress are regulated by region-specific CREB function in mice. Sci Adv. 2022;8:eadd5579.
Havenith MN, Zijderveld PM, van Heukelum S, Abghari S, Glennon JC, Tiesinga P. The Virtual-Environment-Foraging Task enables rapid training and single-trial metrics of attention in head-fixed mice. Sci Rep. 2018;8:17371.
Gire DH, Kapoor V, Arrighi-Allisan A, Seminara A, Murthy VN. Mice develop efficient strategies for foraging and navigation using complex natural stimuli. Curr Biol. 2016;26:1261–73.
Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA. Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated camp response element-binding protein expression in nucleus accumbens. J Neurosci. 2001;21:7397–403.
Krystal AD, Pizzagalli DA, Smoski M, Mathew SJ, Nurnberger J, Lisanby SH, et al. A randomized proof-of-mechanism trial applying the ‘fast-fail’ approach to evaluating κ-opioid antagonism as a treatment for anhedonia. Nat Med. 2020;26:760–8.
Costi S, Morris LS, Kirkwood KA, Hoch M, Corniquel M, Vo-Le B, et al. Impact of the KCNQ2/3 channel opener ezogabine on reward circuit activity and clinical symptoms in depression: results from a randomized controlled trial. Am J Psychiatry. 2021;178:437–46.
Acknowledgements
We would like to thank Jill Gregory for graphic design and illustrations.
Funding
National Institutes of Health grants R01MH051399 and R01MH129306 (EJN); Hope for Depression Research Foundation (EJN), and the Brain & Behavior Research Foundation (EMP).
Author information
Authors and Affiliations
Contributions
TMG wrote this article. EMP and EJN reviewed and edited this article.
Corresponding author
Ethics declarations
Competing interests
EJN is a co-founder and Chair of the Scientific Advisory Board of PsychoGenics. TMG and EMP declare that they have no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gyles, T.M., Nestler, E.J. & Parise, E.M. Advancing preclinical chronic stress models to promote therapeutic discovery for human stress disorders. Neuropsychopharmacol. 49, 215–226 (2024). https://doi.org/10.1038/s41386-023-01625-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-023-01625-0
This article is cited by
-
An Integrated General Theory of Psychopathology and Suicide
Evolutionary Psychological Science (2023)