Abstract
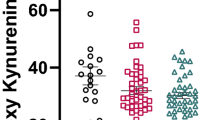
Cognitive impairments predict poor functional outcomes in people with schizophrenia. These impairments may be causally related to increased levels of kynurenic acid (KYNA), a major metabolic product of tryptophan (TRYP). In the brain, KYNA acts as an antagonist of the of α7-nicotinic acetylcholine and NMDA receptors, both of which are involved in cognitive processes. To examine whether KYNA plays a role in the pathophysiology of schizophrenia, we compared the acute effects of a single oral dose of TRYP (6 g) in 32 healthy controls (HC) and 37 people with either schizophrenia (Sz), schizoaffective or schizophreniform disorder, in a placebo-controlled, randomized crossover study. We examined plasma levels of KYNA and its precursor kynurenine; selected cognitive measures from the MATRICS Consensus Cognitive Battery; and resting cerebral blood flow (CBF) using arterial spin labeling imaging. In both cohorts, the TRYP challenge produced significant, time-dependent elevations in plasma kynurenine and KYNA. The resting CBF signal (averaged across all gray matter) was affected differentially, such that TRYP was associated with higher CBF in HC, but not in participants with a Sz-related disorder. While TRYP did not significantly impair cognitive test performance, there was a trend for TRYP to worsen visuospatial memory task performance in HC. Our results demonstrate that oral TRYP challenge substantially increases plasma levels of kynurenine and KYNA in both groups, but exerts differential group effects on CBF. Future studies are required to investigate the mechanisms underlying these CBF findings, and to evaluate the impact of KYNA fluctuations on brain function and behavior. (Clinicaltrials.gov: NCT02067975).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–30.
Singh S, Khanna D, Kalra S. Role of neurochemicals in schizophrenia. Curr Psychopharmacol. 2020;9:144–61.
Sarter M, Bruno JP. Cognitive functions of cortical acetylcholine: toward a unifying hypothesis. Brain Res Brain Res Rev. 1997;23:28–46.
Selvaraj S, Arnone D, Cappai A, Howes O. Alterations in the serotonin system in schizophrenia: a systematic review and meta-analysis of postmortem and molecular imaging studies. Neurosci Biobehav Rev. 2014;45:233–45.
Laruelle M, Kegeles LS, Abi-Dargham A. Glutamate, dopamine, and schizophrenia: from pathophysiology to treatment. Ann N. Y Acad Sci. 2003;1003:138–58.
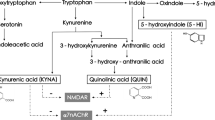
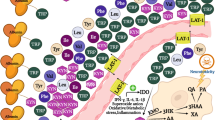
Schwarcz R, Stone TW. The kynurenine pathway and the brain: challenges, controversies and promises. Neuropharmacology. 2017;112:237–47.
Muneer A. Kynurenine pathway of tryptophan metabolism in neuropsychiatric disorders: pathophysiologic and therapeutic considerations. Clin Psychopharmacol Neurosci. 2020;18:507–26.
Badawy AA-B, Dougherty DM. Assessment of the human kynurenine pathway: comparisons and clinical implications of ethnic and gender differences in plasma tryptophan, kynurenine metabolites, and enzyme expressions at baseline and after acute tryptophan loading and depletion. Int J Tryptophan Res. 2016;9:31–49.
Alexander KS, Pocivavsek A, Wu H-Q, Pershing ML, Schwarcz R, Bruno JP. Early developmental elevations of brain kynurenic acid impair cognitive flexibility in adults: reversal with galantamine. Neuroscience. 2013;238:19–28.
Shepard PD, Joy B, Clerkin L, Schwarcz R. Micromolar brain levels of kynurenic acid are associated with a disruption of auditory sensory gating in the rat. Neuropsychopharmacology. 2003;28:1454–62.
Erhardt S, Schwieler L, Emanuelsson C, Geyer M. Endogenous kynurenic acid disrupts prepulse inhibition. Biol Psychiatry. 2004;56:255–60.
Chess AC, Simoni MK, Alling TE, Bucci DJ. Elevations of endogenous kynurenic acid produce spatial working memory deficits. Schizophr Bull. 2007;33:797–804.
Erhardt S, Blennow K, Nordin C, Skogh E, Lindström LH, Engberg G. Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci Lett. 2001;313:96–8.
Schwarcz R, Rassoulpour A, Wu HQ, Medoff D, Tamminga CA, Roberts RC. Increased cortical kynurenate content in schizophrenia. Biol Psychiatry. 2001;50:521–30.
Miller CL, Llenos IC, Dulay JR, Weis S. Upregulation of the initiating step of the kynurenine pathway in postmortem anterior cingulate cortex from individuals with schizophrenia and bipolar disorder. Brain Res. 2006;1073–1074:25–37.
Sathyasaikumar KV, Stachowski EK, Wonodi I, Roberts RC, Rassoulpour A, McMahon RP, et al. Impaired kynurenine pathway metabolism in the prefrontal cortex of individuals with schizophrenia. Schizophr Bull. 2011;37:1147–56.
Linderholm KR, Skogh E, Olsson SK, Dahl M-L, Holtze M, Engberg G, et al. Increased levels of kynurenine and kynurenic acid in the CSF of patients with schizophrenia. Schizophr Bull. 2012;38:426–32.
Schwarcz R, Bruno JP, Muchowski PJ, Wu H-Q. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci. 2012;13:465–477.
Timofeeva OA, Levin ED. Glutamate and nicotinic receptor interactions in working memory: importance for the cognitive impairment of schizophrenia. Neuroscience. 2011;195:21–36.
Yang Y, Paspalas CD, Jin LE, Picciotto MR, Arnsten AFT, Wang M. Nicotinic α7 receptors enhance NMDA cognitive circuits in dorsolateral prefrontal cortex. Proc Natl Acad Sci USA. 2013;110:12078–83.
Phenis D, Vunck SA, Valentini V, Arias H, Schwarcz R, Bruno JP. Activation of alpha7 nicotinic and NMDA receptors is necessary for performance in a working memory task. Psychopharmacol (Berl). 2020;237:1723–35.
Pocivavsek A, Wu H-Q, Potter MC, Elmer GI, Pellicciari R, Schwarcz R. Fluctuations in endogenous kynurenic acid control hippocampal glutamate and memory. Neuropsychopharmacology. 2011;36:2357–67.
Green AR, Aronson JK, Curzon G, Woods HF. Metabolism of an oral tryptophan load. I: effects of dose and pretreatment with tryptophan. Br J Clin Pharm. 1980;10:603–10.
Møller SE. Pharmacokinetics of tryptophan, renal handling of kynurenine and the effect of nicotinamide on its appearance in plasma and urine following L-tryptophan loading of healthy subjects. Eur J Clin Pharm. 1981;21:137–42.
Heuther G, Hajak G, Reimer A, Poeggeler B, Blömer M, Rodenbeck A, et al. The metabolic fate of infused L-tryptophan in men: possible clinical implications of the accumulation of circulating tryptophan and tryptophan metabolites. Psychopharmacol (Berl). 1992;109:422–32.
Yuwiler A, Brammer GL, Morley JE, Raleigh MJ, Flannery JW, Geller E. Short-term and repetitive administration of oral tryptophan in normal men. Effects on blood tryptophan, serotonin, and kynurenine concentrations. Arch Gen Psychiatry. 1981;38:619–26.
Silber BY, Schmitt JAJ. Effects of tryptophan loading on human cognition, mood, and sleep. Neurosci Biobehav Rev. 2010;34:387–407.
Selvaggi P, Hawkins PC, Dipasquale O, Rizzo G, Bertolino A, Dukart J, et al. Increased cerebral blood flow after single dose of antipsychotics in healthy volunteers depends on dopamine D2 receptor density profiles. NeuroImage. 2019;188:774–84.
Dukart J, Holiga Š, Chatham C, Hawkins P, Forsyth A, McMillan R, et al. Cerebral blood flow predicts differential neurotransmitter activity. Sci Rep. 2018;8:4074.
Xekardaki A, Rodriguez C, Montandon M-L, Toma S, Tombeur E, Herrmann FR, et al. Arterial spin labeling may contribute to the prediction of cognitive deterioration in healthy elderly individuals. Radiology. 2015;274:490–9.
Sas K, Csete K, Vécsei L, Papp JG. Effect of systemic administration of L-kynurenine on corticocerebral blood flow under normal and ischemic conditions of the brain in conscious rabbits. J Cardiovasc Pharm. 2003;42:403–9.
Varga DP, Menyhárt Á, Puskás T, Bari F, Farkas E, Kis Z, et al. Systemic administration of l-kynurenine sulfate induces cerebral hypoperfusion transients in adult C57Bl/6 mice. Microvasc Res. 2017;114:19–25.
Liu X-C, Holtze M, Powell SB, Terrando N, Larsson MK, Persson A, et al. Behavioral disturbances in adult mice following neonatal virus infection or kynurenine treatment–role of brain kynurenic acid. Brain Behav Immun. 2014;36:80–9.
Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III–the final common pathway. Schizophr Bull. 2009;35:549–62.
Vidal PM, Pacheco R. The cross-talk between the dopaminergic and the immune system involved in schizophrenia. Front Pharm. 2020;11:394.
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. Washington, DC: 1994.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: 2013.
First MB, Spitzer RL, Gibbon MG, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders — Patient Edition (SCID-I/P, 11/2002 revision). New York: Biometrics Research, New York State Psychiatric Institute; 2002.
Sathyasaikumar K, Notarangelo F, Kelly D, Rowland L, Hare S, Chen S, et al. Tryptophan challenge in healthy controls and people with schizophrenia: acute effects on plasma levels of kynurenine, kynurenic acid and 5-hydroxyindoleacetic acid. Pharmaceuticals (Basel). 2022;15:1003.
Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165:203–13.
Alsop DC, Detre JA, Golay X, Günther M, Hendrikse J, Hernandez-Garcia L, et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med. 2015;73:102–16.
Chappell MA, Groves AR, MacIntosh BJ, Donahue MJ, Jezzard P, Woolrich MW. Partial volume correction of multiple inversion time arterial spin labeling MRI data. Magn Reson Med. 2011;65:1173–83.
Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–41.
Eshaghzadeh Torbati M, Minhas DS, Ahmad G, O’Connor EE, Muschelli J, Laymon CM, et al. A multi-scanner neuroimaging data harmonization using RAVEL and ComBat. Neuroimage. 2021;245:118703.
Domino EF, Krause RR. Plasma tryptophan tolerance curves in drug free normal controls, schizophrenic patients and prisoner volunteers. J Psychiatr Res. 1974;10:247–61.
Kitzrow W, Uebelhack R. Tryptophan-loading in schizophrenia and endogenous depression. Psychiatr Neurol Med Psychol (Leipz). 1987;39:32–7.
Laurian S, Grasset F, Steck A, Baumann P, Gaillard JM. The influence of oral tryptophan on cortical evoked responses in normals and schizophrenics. J Neural Transm Suppl. 1979;15:177–88.
Gramsbergen JBP, Hodgkins PS, Rassoulpour A, Turski WA, Guidetti P, Schwarcz R. Brain-specific modulation of kynurenic acid synthesis in the rat. J Neurochem. 1997;69:290–8.
Chess AC, Landers AM, Bucci DJ. L-kynurenine treatment alters contextual fear conditioning and context discrimination but not cue-specific fear conditioning. Behav Brain Res. 2009;201:325–31.
Pershing ML, Bortz DM, Pocivavsek A, Fredericks PJ, Jørgensen CV, Vunck SA, et al. Elevated levels of kynurenic acid during gestation produce neurochemical, morphological, and cognitive deficits in adulthood: implications for schizophrenia. Neuropharmacology. 2015;90:33–41.
Giorgini F, Huang S-Y, Sathyasaikumar KV, Notarangelo FM, Thomas MAR, Tararina M, et al. Targeted deletion of kynurenine 3-monooxygenase in mice: a new tool for studying kynurenine pathway metabolism in periphery and brain. J Biol Chem. 2013;288:36554–66.
Sobczak S, Honig A, Schmitt JAJ, Riedel WJ. Pronounced cognitive deficits following an intravenous L-tryptophan challenge in first-degree relatives of bipolar patients compared to healthy controls. Neuropsychopharmacology. 2003;28:711–9.
Cowen MA. An electrophysiological study on the effects of tryptophan and cortisol on schizophrenic and other mentally ill patient groups and on normal subjects. Biol Psychiatry. 1976;11:389–401.
Morand C, Young SN, Ervin FR. Clinical response of aggressive schizophrenics to oral tryptophan. Biol Psychiatry. 1983;18:575–8.
Wang Y, Liu H, McKenzie G, Witting PK, Stasch J-P, Hahn M, et al. Kynurenine is a novel endothelium-derived relaxing factor produced during inflammation. Nat Med. 2010;16:279–85.
Sakakibara K, Feng G-G, Li J, Akahori T, Yasuda Y, Nakamura E, et al. Kynurenine causes vasodilation and hypotension induced by activation of KCNQ-encoded voltage-dependent K(+) channels. J Pharm Sci. 2015;129:31–7.
Stone TW, Darlington LG. The kynurenine pathway as a therapeutic target in cognitive and neurodegenerative disorders. Br J Pharm. 2013;169:1211–27.
Pollak TA, Drndarski S, Stone JM, David AS, McGuire P, Abbott NJ. The blood-brain barrier in psychosis. Lancet Psychiatry. 2018;5:79–92.
Ku H-L, Wang J-K, Lee H-C, Lane TJ, Liu I-C, Chen Y-C, et al. Cerebral blood flow autoregulation is impaired in schizophrenia: a pilot study. Schizophr Res. 2017;188:63–7.
JK Y, Gg D, CH G, Gl H, R C, JW K, et al. Prevalence and specificity of the abnormal niacin response: a potential endophenotype marker in schizophrenia. Schizophr Bull. 2016;42:369–76.
Uranova NA, Zimina IS, Vikhreva OV, Krukov NO, Rachmanova VI, Orlovskaya DD. Ultrastructural damage of capillaries in the neocortex in schizophrenia. World J Biol Psychiatry. 2010;11:567–78.
Thomsen MS, Hansen HH, Timmerman DB, Mikkelsen JD. Cognitive improvement by activation of alpha7 nicotinic acetylcholine receptors: from animal models to human pathophysiology. Curr Pharm Des. 2010;16:323–43.
Uno Y, Coyle JT. Glutamate hypothesis in schizophrenia. Psychiatry Clin Neurosci. 2019;73:204–15.
Wong DF, Kuwabara H, Horti AG, Roberts JM, Nandi A, Cascella N, et al. Brain PET imaging of α7-nAChR with [18F]ASEM: reproducibility, occupancy, receptor density, and changes in schizophrenia. Int J Neuropsychopharmacol. 2018;21:656–67.
Seillier C, Lesept F, Toutirais O, Potzeha F, Blanc M, Vivien D. Targeting NMDA receptors at the neurovascular unit: past and future treatments for central nervous system diseases. Int J Mol Sci. 2022;23:10336.
Acknowledgements
We thank the participants, especially those with schizophrenia, who graciously gave their time and effort to undergo the medical, imaging, and cognitive procedures in this project. We acknowledge the effort and contribution of many individuals to this project including Dr. Heather Adams, Pat Ball, Frank Blatt, Hongji Chen, Amber Earl, Frank Gaston, Matthew Glassman, Dr. David Gorelick, Katherine Kilday, Sharon Pugh, Marian Thomas, Samantha Trikeriotis, Dr. Gopal Vyas, and the Spring Grove Hospital Center Treatment Research Unit.
Funding
This study was supported by NIMH grant P50-MH103222 (Conte Center for Translational Neuroscience Research).
Author information
Authors and Affiliations
Contributions
Conceptualization: DLK, LMR, RWB and RS; funding acquisition: RWB and RS; methodology, data collection, quality control and analysis: SMH, BMA, SC, CM, SAW, CS, SKG, KVS, FMN, and LMR; writing (original draft preparation): SMH; writing (review and editing): SMH, BMA, CM, SC, SAW, CS, SKG, KVS, FMN, RS, LMR, DLK, and RWB; supervision: DLK, LMR, RWB and RS. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
RS is co-founder of KyNexis AB, which develops novel drugs targeting kynurenine pathway metabolism. RWB performs consultation for Boehringer-Ingelheim and serves on the Data Safety and Monitoring Boards of Roche, Merck and Newron; and on the Advisory Boards of Merck, Acadia, and Neurocrine. DLK is a consultant for Janseen, Alkermes and Sunovion. All other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hare, S.M., Adhikari, B.M., Mo, C. et al. Tryptophan challenge in individuals with schizophrenia and healthy controls: acute effects on circulating kynurenine and kynurenic acid, cognition and cerebral blood flow. Neuropsychopharmacol. 48, 1594–1601 (2023). https://doi.org/10.1038/s41386-023-01587-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-023-01587-3
This article is cited by
-
Kynurenic acid: translational perspectives of a therapeutically targetable gliotransmitter
Neuropsychopharmacology (2024)