Abstract
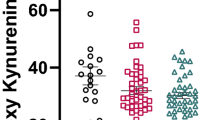
The tryptophan catabolite (TRYCAT) pathway is implicated in the pathophysiology of schizophrenia (SCZ) since the rate-limiting enzyme indoleamine-dioxygenase (IDO) may be induced by inflammatory and oxidative stress mediators. This systematic review searched PubMed, Web of Science, and Google Scholar for papers published from inception until August 2021 and meta-analyzed the association between SCZ and TRYCATs in the central nervous system (CNS) and peripheral blood. We included 61 studies comprising 2813 patients and 2948 healthy controls. In the CNS we found a significant (p < 0.001) increase in the kynurenine/tryptophan (KYN/TRP) (standardized mean difference, SMD = 0.769, 95% confidence interval, CI: 0.456; 1.082) and kynurenic acid (KA)/KYN + TRP (SMD = 0.697, CI: 0.478–0.917) ratios, KA (SMD = 0.646, CI: 0.422; 0.909) and KYN (SMD = 1.238; CI: 0.590; 1.886), while the 3OH-kynurenine (3HK) + KYN-3-monooxygenase (KMO)/KYN ratio was significantly reduced (SMD = −1.089, CI: −1.682; −0.496). There were significant differences between KYN/TRP, (KYN + KA)/TRP, (3HK + KMO)/KYN, KA, and KYN levels among the CNS and peripheral blood, and among serum and plasma KYN. The only useful peripheral marker of CNS TRYCATs findings was the increased KYN/TRP ratio in serum (SMD = 0.211, CI: 0.056; 0.366, p = 0.007), but not in plasma. There was no significant increase in a neurotoxic composite score based on KYN, 3HK, and picolinic, xanthurenic, and quinolinic acid. SCZ is accompanied by increased IDO activity in the CNS and serum, and reduced KMO activity and a shift towards KA production in the CNS. This CNS TRYCATs profile indicates neuroprotective, negative immunoregulatory and anti-inflammatory effects. Peripheral blood levels of TRYCATs are dissociated from CNS findings except for a modest increase in serum IDO activity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The dataset (excel file) generated during and/or analyzed during the current study will be available from MM upon reasonable request and once the dataset has been fully exploited by the authors.
References
Smith RS, Maes M. The macrophage-T-lymphocyte theory of schizophrenia: additional evidence. Med Hypotheses. 1995;45:135–41.
Kanchanatawan B, Sirivichayakul S, Ruxrungtham K, Carvalho AF, Geffard M, Ormstad H, et al. Deficit, but not nondeficit, schizophrenia is characterized by mucosa-associated activation of the tryptophan catabolite (TRYCAT) pathway with highly specific increases in IgA responses directed to picolinic, xanthurenic, and quinolinic acid. Mol Neurobiol. 2018;55:1524–36.
Noto C, Maes M, Ota VK, Teixeira AL, Bressan RA, Gadelha A, et al. High predictive value of immune-inflammatory biomarkers for schizophrenia diagnosis and association with treatment resistance. World J Biol Psychiatry. 2015;16:422–9.
Rubesa G, Gudelj L, Makovac D. Immunological characteristics of schizophrenia. Psychiatr Danubina. 2018;30 Suppl 4:180–7.
Al-Hakeim HK, Almulla AF, Maes M. The neuroimmune and neurotoxic fingerprint of major neurocognitive psychosis or deficit schizophrenia: a supervised machine learning study. Neurotox Res. 2020;37:753–71.
Almulla AF, Al-Hakeim HK, Abed MS, Carvalho AF, Maes M. Chronic fatigue and fibromyalgia symptoms are key components of deficit schizophrenia and are strongly associated with activated immune-inflammatory pathways. Schizophr Res. 2020;222:342–53.
Maes M, Sirivichayakul S, Matsumoto AK, Maes A, Michelin AP, de Oliveira Semeão L, et al. Increased levels of plasma tumor necrosis factor-α mediate schizophrenia symptom dimensions and neurocognitive impairments and are inversely associated with natural IgM directed to malondialdehyde and paraoxonase 1 activity. Mol Neurobiol. 2020;57:2333–45.
Noto MN, Maes M, Nunes SOV, Ota VK, Rossaneis AC, Verri WA Jr., et al. Activation of the immune-inflammatory response system and the compensatory immune-regulatory system in antipsychotic naive first episode psychosis. Eur Neuropsychopharmacol. 2019;29:416–31.
Roomruangwong C, Noto C, Kanchanatawan B, Anderson G, Kubera M, Carvalho AF, et al. The role of aberrations in the immune-inflammatory response system (IRS) and the compensatory immune-regulatory reflex system (CIRS) in different phenotypes of schizophrenia: the IRS-CIRS theory of schizophrenia. Mol Neurobiol. 2020;57:778–97.
Saito K, Markey SP, Heyes MP. Effects of immune activation on quinolinic acid and neuroactive kynurenines in the mouse. Neuroscience. 1992;51:25–39.
Reyes Ocampo J, Lugo Huitron R, Gonzalez-Esquivel D, Ugalde-Muniz P, Jimenez-Anguiano A, Pineda B, et al. Kynurenines with neuroactive and redox properties: relevance to aging and brain diseases. Oxid Med Cell Longev. 2014;2014:646909.
Anderson G, Maes M. Schizophrenia: linking prenatal infection to cytokines, the tryptophan catabolite (TRYCAT) pathway, NMDA receptor hypofunction, neurodevelopment and neuroprogression. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:5–19.
Maes M, Fišar Z, Medina M, Scapagnini G, Nowak G, Berk M. New drug targets in depression: inflammatory, cell-mediated immune, oxidative and nitrosative stress, mitochondrial, antioxidant, and neuroprogressive pathways. And new drug candidates—Nrf2 activators and GSK-3 inhibitors. Inflammopharmacology. 2012;20:127–50.
Maes M, Leonard BE, Myint AM, Kubera M, Verkerk R. The new ‘5-HT’ hypothesis of depression: Cell-mediated immune activation induces indoleamine 2,3-dioxygenase, which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), both of which contribute to the onset of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:702–21.
Maes M, Mihaylova I, Ruyter MD, Kubera M, Bosmans E. The immune effects of TRYCATs (tryptophan catabolites along the IDO pathway): relevance for depression—and other conditions characterized by tryptophan depletion induced by inflammation. Neuro Endocrinol Lett. 2007;28:826–31.
Krause D, Suh H-S, Tarassishin L, Cui QL, Durafourt BA, Choi N, et al. The tryptophan metabolite 3-hydroxyanthranilic acid plays anti-inflammatory and neuroprotective roles during inflammation: role of hemeoxygenase-1. Am J Pathol. 2011;179:1360–72.
Savitz J, Drevets WC, Smith CM, Victor TA, Wurfel BE, Bellgowan PS, et al. Putative neuroprotective and neurotoxic kynurenine pathway metabolites are associated with hippocampal and amygdalar volumes in subjects with major depressive disorder. Neuropsychopharmacology. 2015;40:463–71.
Baumann P. Relationships between plasma, CSF and brain tryptophan. Proceedings of the transport mechanisms of tryptophan in blood cells, nerve cells, and at the blood-brain barrier. Vienna: Springer; 1979.
Fernstrom JD, Larin F, Wurtman RJ. Correlation between brain tryptophan and plasma neutral amino acid levels following food consumption in rats. Life Sci. 1973;13:517–24.
Fukui S, Schwarcz R, Rapoport SI, Takada Y, Smith QR. Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J Neurochemistry. 1991;56:2007–17.
Saito K, Fujigaki S, Heyes MP, Shibata K, Takemura M, Fujii H, et al. Mechanism of increases in L-kynurenine and quinolinic acid in renal insufficiency. Am J Physiol Ren Physiol. 2000;279:F565–72.
Topczewska-Bruns J, Pawlak D, Tankiewicz A, Chabielska E, Buczko W. Kynurenine metabolism in central nervous system in experimental chronic renal failure. Adv Exp Med Biol. 2003;527:177–82.
Kita T, Morrison PF, Heyes MP, Markey SP. Effects of systemic and central nervous system localized inflammation on the contributions of metabolic precursors to the L-kynurenine and quinolinic acid pools in brain. J Neurochemistry. 2002;82:258–68.
Gál EM, Sherman AD. L-kynurenine: its synthesis and possible regulatory function in brain. Neurochem Res. 1980;5:223–39.
Linderholm KR, Skogh E, Olsson SK, Dahl ML, Holtze M, Engberg G, et al. Increased levels of kynurenine and kynurenic acid in the CSF of patients with schizophrenia. Schizophr Bull. 2010;38:426–32.
Schwarcz R, Rassoulpour A, Wu H-Q, Medoff D, Tamminga CA, Roberts RC. Increased cortical kynurenate content in schizophrenia. Biol Psychiatry. 2001;50:521–30.
Kindler J, Lim CK, Weickert CS, Boerrigter D, Galletly C, Liu D, et al. Dysregulation of kynurenine metabolism is related to proinflammatory cytokines, attention, and prefrontal cortex volume in schizophrenia. Mol Psychiatry. 2020;25:2860–72.
Bonaccorso S, Marino V, Puzella A, Pasquini M, Biondi M, Artini M, et al. Increased depressive ratings in patients with hepatitis C receiving interferon-alpha-based immunotherapy are related to interferon-alpha-induced changes in the serotonergic system. J Clin Psychopharmacol. 2002;22:86–90.
Lavebratt C, Olsson S, Backlund L, Frisén L, Sellgren C, Priebe L, et al. The KMO allele encoding Arg452 is associated with psychotic features in bipolar disorder type 1, and with increased CSF KYNA level and reduced KMO expression. Mol Psychiatry. 2014;19:334–41.
Plitman E, Iwata Y, Caravaggio F, Nakajima S, Chung JK, Gerretsen P, et al. Kynurenic acid in schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2017;43:764–77.
Morrens M, De Picker L, Kampen JK, Coppens V. Blood-based kynurenine pathway alterations in schizophrenia spectrum disorders: a meta-analysis. Schizophr Res. 2020;223:43–52.
Cao B, Chen Y, Ren Z, Pan Z, McIntyre RS, Wang D. Dysregulation of kynurenine pathway and potential dynamic changes of kynurenine in schizophrenia: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2021;123:203–14.
Marx W, McGuinness AJ, Rocks T, Ruusunen A, Cleminson J, Walker AJ, et al. The kynurenine pathway in major depressive disorder, bipolar disorder, and schizophrenia: a meta-analysis of 101 studies. Mol Psychiatry. 2021;26:4158–78.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med. 2021;18:e1003583.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane handbook for systematic reviews of interventions. 2nd ed. Chichester (UK): John Wiley & Sons; 2019.
Sathyasaikumar KV, Stachowski EK, Wonodi I, Roberts RC, Rassoulpour A, McMahon RP, et al. Impaired kynurenine pathway metabolism in the prefrontal cortex of individuals with schizophrenia. Schizophr Bull. 2011;37:1147–56.
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135.
Andrés-Rodríguez L, Borràs X, Feliu-Soler A, Pérez-Aranda A, Angarita-Osorio N, Moreno-Peral P, et al. Peripheral immune aberrations in fibromyalgia: a systematic review, meta-analysis and meta-regression. Brain, Behav Immun. 2019;87:881–9.
Cohen J. Statistical power analysis for the behavioral sciences. New York: Academic press; 2013.
Vasupanrajit A, Jirakran K, Tunvirachaisakul C, Solmi M, Maes M. Inflammation and nitro-oxidative stress in current suicidal attempts and current suicidal ideation: a systematic review and meta-analysis. Mol Psychiatry. 2022. [Epub ahead of print].
Vasupanrajit A, Jirakran K, Tunvirachaisakul C, Maes M. Suicide attempts are associated with activated immune-inflammatory, nitro-oxidative, and neurotoxic pathways: a systematic review and meta-analysis. J Affect Disord. 2021;295:80–92.
Wurfel BE, Drevets WC, Bliss SA, McMillin JR, Suzuki H, Ford BN, et al. Serum kynurenic acid is reduced in affective psychosis. Transl Psychiatry. 2017;7:e1115.
Szymona K, Zdzisinska B, Karakula-Juchnowicz H, Kocki T, Kandefer-Szerszen M, Flis M, et al. Correlations of kynurenic Acid, 3-hydroxykynurenine, sIL-2R, IFN-alpha, and IL-4 with clinical symptoms during acute relapse of schizophrenia. Neurotox Res. 2017;32:17–26.
Schwieler L, Larsson MK, Skogh E, Kegel ME, Orhan F, Abdelmoaty S, et al. Increased levels of IL-6 in the cerebrospinal fluid of patients with chronic schizophrenia−significance for activation of the kynurenine pathway. J Psychiatry Neurosci. 2015;40:126–33.
Nilsson LK, Linderholm KR, Engberg G, Paulson L, Blennow K, Lindstrom LH, et al. Elevated levels of kynurenic acid in the cerebrospinal fluid of male patients with schizophrenia. Schizophr Res. 2005;80:315–22.
Myint AM, Schwarz MJ, Verkerk R, Mueller HH, Zach J, Scharpe S, et al. Reversal of imbalance between kynurenic acid and 3-hydroxykynurenine by antipsychotics in medication-naive and medication-free schizophrenic patients. Brain Behav Immun. 2011;25:1576–81.
Huang X, Ding W, Wu F, Zhou S, Deng S, Ning Y. Increased plasma kynurenic acid levels are associated with impaired attention/vigilance and social cognition in patients with schizophrenia. Neuropsychiatr Dis Treat. 2020;16:263–71.
Huang J, Tong J, Zhang P, Zhou Y, Cui Y, Tan S, et al. Effects of neuroactive metabolites of the tryptophan pathway on working memory and cortical thickness in schizophrenia. Transl Psychiatry. 2021;11:198.
Holtze M, Saetre P, Engberg G, Schwieler L, Werge T, Andreassen OA, et al. Kynurenine 3-monooxygenase polymorphisms: relevance for kynurenic acid synthesis in patients with schizophrenia and healthy controls. J Psychiatry Neurosci. 2012;37:53–7.
Erhardt S, Blennow K, Nordin C, Skogh E, Lindström LH, Engberg G. Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci Lett. 2001;313:96–8.
De Picker L, Fransen E, Coppens V, Timmers M, de Boer P, Oberacher H, et al. Immune and neuroendocrine trait and state markers in psychotic illness: decreased kynurenines marking psychotic exacerbations. Front Immunol. 2019;10:2971.
Chiappelli J, Postolache TT, Kochunov P, Rowland LM, Wijtenburg SA, Shukla DK, et al. Tryptophan metabolism and white matter integrity in schizophrenia. Neuropsychopharmacology. 2016;41:2587–95.
Chiappelli J, Notarangelo FM, Pocivavsek A, Thomas MAR, Rowland LM, Schwarcz R, et al. Influence of plasma cytokines on kynurenine and kynurenic acid in schizophrenia. Neuropsychopharmacology. 2018;43:1675–80.
Cathomas F, Guetter K, Seifritz E, Klaus F, Kaiser S. Quinolinic acid is associated with cognitive deficits in schizophrenia but not major depressive disorder. Sci Rep. 2021;11:9992.
Barry S, Clarke G, Scully P, Dinan TG. Kynurenine pathway in psychosis: evidence of increased tryptophan degradation. J Psychopharmacol. 2009;23:287–94.
Cao B, Wang D, Brietzke E, McIntyre RS, Pan Z, Cha D, et al. Characterizing amino-acid biosignatures amongst individuals with schizophrenia: a case-control study. Amino Acids. 2018;50:1013–23.
Carl GF, Brogan MP, Young BK. Is plasma serine a marker for psychosis? Biol Psychiatry. 1992;31:1130–5.
Condray R, Dougherty GG Jr., Keshavan MS, Reddy RD, Haas GL, Montrose DM, et al. 3-Hydroxykynurenine and clinical symptoms in first-episode neuroleptic-naive patients with schizophrenia. Int J Neuropsychopharmacol. 2011;14:756–67.
Curto M, Lionetto L, Fazio F, Corigliano V, Comparelli A, Ferracuti S, et al. Serum xanthurenic acid levels: Reduced in subjects at ultra high risk for psychosis. Schizophr Res. 2019;208:465–6.
Fazio F, Lionetto L, Curto M, Iacovelli L, Cavallari M, Zappulla C, et al. Xanthurenic acid activates mGlu2/3 metabotropic glutamate receptors and is a potential trait marker for schizophrenia. Sci Rep. 2015;5:17799.
Fukushima T, Iizuka H, Yokota A, Suzuki T, Ohno C, Kono Y, et al. Quantitative analyses of schizophrenia-associated metabolites in serum: serum D-lactate levels are negatively correlated with gamma-glutamylcysteine in medicated schizophrenia patients. PLoS ONE. 2014;9:e101652.
Joaquim HPG, Costa AC, Gattaz WF, Talib LL. Kynurenine is correlated with IL-1beta in plasma of schizophrenia patients. J Neural Transm. 2018;125:869–73.
Kegel ME, Bhat M, Skogh E, Samuelsson M, Lundberg K, Dahl ML, et al. Imbalanced kynurenine pathway in schizophrenia. Int J Tryptophan Res. 2014;7:15–22.
Kim YK, Myint AM, Verkerk R, Scharpe S, Steinbusch H, Leonard B. Cytokine changes and tryptophan metabolites in medication-naive and medication-free schizophrenic patients. Neuropsychobiology. 2009;59:123–9.
Lee M, Jayathilake K, Dai J, Meltzer HY. Decreased plasma tryptophan and tryptophan/large neutral amino acid ratio in patients with neuroleptic-resistant schizophrenia: relationship to plasma cortisol concentration. Psychiatry Res. 2011;185:328–33.
Leppik L, Kriisa K, Koido K, Koch K, Kajalaid K, Haring L, et al. Profiling of amino acids and their derivatives biogenic amines before and after antipsychotic treatment in first-episode psychosis. Front Psychiatry. 2018;9:155.
Oxenkrug G, van der Hart M, Roeser J, Summergrad P. Peripheral kynurenine-3-monooxygenase deficiency as a potential risk factor for metabolic syndrome in schizophrenia patients. Integr Clin Med. 2017;1:1–3.
Paredes RM, Quinones M, Marballi K, Gao X, Valdez C, Ahuja SS, et al. Metabolomic profiling of schizophrenia patients at risk for metabolic syndrome. Int J Neuropsychopharmacol. 2014;17:1139–48.
Rao ML, Gross G, Strebel B, Bräunig P, Huber G, Klosterkötter J. Serum amino acids, central monoamines, and hormones in drug-naive, drug-free, and neuroleptic-treated schizophrenic patients and healthy subjects. Psychiatry Res. 1990;34:243–57.
Ravikumar A, Deepadevi KV, Arun P, Manojkumar V, Kurup PA. Tryptophan and tyrosine catabolic pattern in neuropsychiatric disorders. Neurol India. 2000;48:231–8.
Tortorella A, Monteleone P, Fabrazzo M, Viggiano A, De Luca L, Maj M. Plasma concentrations of amino acids in chronic schizophrenics treated with clozapine. Neuropsychobiology. 2001;44:167–71.
van der Heijden FM, Fekkes D, Tuinier S, Sijben AE, Kahn RS, Verhoeven WM. Amino acids in schizophrenia: evidence for lower tryptophan availability during treatment with atypical antipsychotics? J Neural Transm. 2005;112:577–85.
Zhang Z, Zhang M, Luo Y, Ni X, Lu H, Wen Y, et al. Preliminary comparative analysis of kynurenine pathway metabolites in chronic ketamine users, schizophrenic patients, and healthy controls. Hum Psychopharmacol. 2020;35:e2738.
Yao JK, Dougherty GG Jr., Reddy RD, Keshavan MS, Montrose DM, Matson WR, et al. Altered interactions of tryptophan metabolites in first-episode neuroleptic-naive patients with schizophrenia. Mol Psychiatry. 2010;15:938–53.
Wu F, Li H, Zhou Y, Huang Y. Relationship between kynurenine pathway metabolites and cognitive function in schizophrenia patients. Clin Med Eng. 2019;26:1153–4.
Wei J. Low concentrations of serum tyrosine in neuroleptic-free schizophrenics with an early onset. Schizophrenia Res. 1995;14:257–60.
van de Kerkhof NW, Fekkes D, van der Heijden FM, Hoogendijk WJ, Stober G, Egger JI, et al. Cycloid psychoses in the psychosis spectrum: evidence for biochemical differences with schizophrenia. Neuropsychiatr Dis Treat. 2016;12:1927–33.
Tang Y, Chen T, Zhang X, Luo X, Liu Y. Determination and clinical significance of serum kynurenine and kynurenic acid levels in schizophrenia %. J Chin J Behav Med Sci. 2009:103–4.
Steen NE, Dieset I, Hope S, Vedal TSJ, Smeland OB, Matson W, et al. Metabolic dysfunctions in the kynurenine pathway, noradrenergic and purine metabolism in schizophrenia and bipolar disorders. Psychol Med. 2020;50:595–606.
Sperner-Unterweger B, Miller C, Holzner B, Laich A, Widner B, Fleischhacker WW, et al. Immunologic alterations in schizophrenia: neopterin, L-kynurenine, tryptophan and T-cell subsets in the acute stage of illness. Pteridines. 2002;13:9–14.
Rao ML, Gross G, Strebel B, Halaris A, Huber G, Bräunig P, et al. Circadian rhythm of tryptophan, serotonin, melatonin, and pituitary hormones in schizophrenia. Biol Psychiatry. 1994;35:151–63.
Oxenkrug G, van der Hart M, Roeser J, Summergrad P, Anthranilic. Acid: a potential biomarker and treatment target for schizophrenia. Ann Psychiatry Ment Health. 2016;4:1059.
Noyan H, Erdag E, Tuzun E, Yaylim I, Kucukhuseyin O, Hakan MT, et al. Association of the kynurenine pathway metabolites with clinical, cognitive features and IL-1beta levels in patients with schizophrenia spectrum disorder and their siblings. Schizophr Res. 2021;229:27–37.
Nilsson-Todd LK, Nordin C, Jonsson EG, Skogh E, Erhardt S. Cerebrospinal fluid kynurenic acid in male patients with schizophrenia - correlation with monoamine metabolites. Acta Neuropsychiatr. 2007;19:45–52.
Manowitz P, Gilmour DG, Racevskis J. Low plasma tryptophan levels in recently hospitalized schizophrenics. Biol Psychiatry. 1973;6:109–18.
Koike S, Bundo M, Iwamoto K, Suga M, Kuwabara H, Ohashi Y, et al. A snapshot of plasma metabolites in first-episode schizophrenia: a capillary electrophoresis time-of-flight mass spectrometry study. Transl Psychiatry. 2014;4:e379.
Glassman M, Wehring HJ, Pocivavsek A, Sullivan KM, Rowland LM, McMahon RP, et al. Peripheral cortisol and inflammatory response to a psychosocial stressor in people with schizophrenia. J Neuropsychiatry. 2018;2;1–7.
Domino EF, Krause RR. Plasma tryptophan tolerance curves in drug free normal controls, schizophrenic patients and prisoner volunteers. J Psychiatr Res. 1974;10:247–61.
Brundin L, Sellgren CM, Lim CK, Grit J, Palsson E, Landen M, et al. An enzyme in the kynurenine pathway that governs vulnerability to suicidal behavior by regulating excitotoxicity and neuroinflammation. Transl Psychiatry. 2016;6:e865.
Oxenkrug G, Bernstein HG, Guest PC, van der Hart M, Roeser J, Summergrad P, et al. Plasma xanthurenic acid in a context of insulin resistance and obesity in schizophrenia. Schizophr Res. 2019;211:98–9.
Potkin SG, Cannon-Spoor HE, DeLisi LE, Neckers LM, Wyatt RJ. Plasma phenylalanine, tyrosine, and tryptophan in schizophrenia. Arch Gen Psychiatry. 1983;40:749–52.
Fekkes D, Bode WT, Zijlstra FJ, Pepplinkhuizen L. Eicosanoid and amino acid metabolism in transient acute psychoses with psychedelic symptoms. Prostaglandins leukot Essent Fat acids. 1996;54:261–4.
Miller CL, Llenos IC, Dulay JR, Weis S. Upregulation of the initiating step of the kynurenine pathway in postmortem anterior cingulate cortex from individuals with schizophrenia and bipolar disorder. Brain Res. 2006;1073-1074:25–37.
Pi G, Tang A, Mo X, Luo X, Ao X. Determination of kynurenic acid and tryptophan in serum by high performance liquid chromatography with fluorescence detection %. J Chin J Lab Med. 2007;30:1134–7.
Domingues DS, Crevelin EJ, de Moraes LA, Cecilio Hallak JE, de Souza Crippa JA, Costa Queiroz ME. Simultaneous determination of amino acids and neurotransmitters in plasma samples from schizophrenic patients by hydrophilic interaction liquid chromatography with tandem mass spectrometry. J Sep Sci. 2015;38:780–7.
Yang X, Yang Y, Lang W, Liu Y, Chen Z, Tao H. Determination and clinical significance of serum kynurenine and kynurenine 3 -monooxygenase levels in schizophrenia. J Hunan Norm Univ. 2016;13:18–9.
Shovestul BJ, Glassman M, Rowland LM, McMahon RP, Liu F, Kelly DL. Pilot study examining the relationship of childhood trauma, perceived stress, and medication use to serum kynurenic acid and kynurenine levels in schizophrenia. Schizophr Res. 2017;185:200–1.
Okamoto N, Natsuyama T, Igata R, Konishi Y, Tesen H, Ikenouchi A, et al. Associations between the kynurenine pathway, proinflammatory cytokines, and brain-derived neurotrophic factor in hospitalized patients with chronic schizophrenia: a preliminary study. Front Psychiatry. 2021;12:696059.
Anderson G, Maes M. How immune-inflammatory processes link CNS and psychiatric disorders: classification and treatment implications. CNS Neurol Disord Drug Targets. 2017;16:266–78.
Anderson G, Maes M. Interactions of tryptophan and its catabolites with melatonin and the alpha 7 nicotinic receptor in central nervous system and psychiatric disorders: role of the aryl hydrocarbon receptor and direct mitochondria regulation. Int J Tryptophan Res. 2017;10:1178646917691738.
Kanchanatawan B, Sirivichayakul S, Ruxrungtham K, Carvalho AF, Geffard M, Anderson G, et al. deficit schizophrenia is characterized by defects in IgM-mediated responses to tryptophan catabolites (TRYCATs): a paradigm shift towards defects in natural self-regulatory immune responses coupled with mucosa-derived TRYCAT pathway activation. Mol Neurobiol. 2018;55:2214–26.
Maes M, Galecki P, Verkerk R, Rief W. Somatization, but not depression, is characterized by disorders in the tryptophan catabolite (TRYCAT) pathway, indicating increased indoleamine 2,3-dioxygenase and lowered kynurenine aminotransferase activity. Neuroendocrinol Lett. 2011;32:264–73.
Nantachai G, Vasupanrajit A, Tunvirachaisakul C, Solmi M, Maes M. Oxidative stress and antioxidant defenses in mild cognitive impairment: a systematic review and meta-analysis. Preprint. 2021. https://doi.org/10.1101/2021.11.22.21266698.
Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci. 2012;13:465–77.
Reyes Ocampo J, Lugo Huitrón R, González-Esquivel D, Ugalde-Muñiz P, Jiménez-Anguiano A, Pineda B, et al. Kynurenines with neuroactive and redox properties: relevance to aging and brain diseases. Oxid Med Cell Longev. 2014;2014:646909.
Guillemin GJ, Kerr SJ, Smythe GA, Smith DG, Kapoor V, Armati PJ, et al. Kynurenine pathway metabolism in human astrocytes: a paradox for neuronal protection. J Neurochemistry. 2001;78:842–53.
Speciale C, Wu HQ, Cini M, Marconi M, Varasi M, Schwarcz R. (R,S)-3,4-dichlorobenzoylalanine (FCE 28833A) causes a large and persistent increase in brain kynurenic acid levels in rats. Eur J Pharmacol. 1996;315:263–7.
Röver S, Cesura AM, Huguenin P, Kettler R, Szente A. Synthesis and biochemical evaluation of N-(4-phenylthiazol-2-yl) benzenesulfonamides as high-affinity inhibitors of kynurenine 3-hydroxylase. 1997;40:4378–85.
Darlington LG, Forrest CM, Mackay GM, Smith RA, Smith AJ, Stoy N, et al. On the biological importance of the 3-hydroxyanthranilic acid: anthranilic acid ratio. Int J Tryptophan Res. 2010;3:51–9.
Guillemin GJ, Cullen KM, Lim CK, Smythe GA, Garner B, Kapoor V, et al. Characterization of the kynurenine pathway in human neurons. J Neurosci. 2007;27:12884–92.
Wirthgen E, Hoeflich A, Rebl A, Gunther J. Kynurenic acid: the janus-faced role of an immunomodulatory tryptophan metabolite and its link to pathological conditions. Front Immunol. 2017;8:1957.
Davies NW, Guillemin G, Brew BJ. Tryptophan, neurodegeneration and HIV-associated neurocognitive disorder. Int J Tryptophan Res. 2010;3:121–40.
Banerjee J, Alkondon M, Pereira EFR, Albuquerque EX. Regulation of GABAergic inputs to CA1 pyramidal neurons by nicotinic receptors and kynurenic acid. J Pharmacol Exp Ther. 2012;341:500–9.
Maes M, Vojdani A, Sirivichayakul S, Barbosa DS, Kanchanatawan B. Inflammatory and oxidative pathways are new drug targets in multiple episode schizophrenia and leaky gut, klebsiella pneumoniae, and C1q immune complexes are additional drug targets in first episode schizophrenia. Mol Neurobiol. 2021;58:3319–34.
Sotelo-Orozco J, Chen SY, Hertz-Picciotto I, Slupsky CM. A comparison of serum and plasma blood collection tubes for the integration of epidemiological and metabolomics data. Front Mol Biosci. 2021;8:682134.
Parvy PR, Bardet JI, Kamoun PP. EDTA in vacutainer tubes can interfere with plasma amino acid analysis. Clin Chem. 1983;29:735.
Bellmaine S, Schnellbaecher A, Zimmer A. Reactivity and degradation products of tryptophan in solution and proteins. Free Radic Biol Med. 2020;160:696–718.
Kulkarni P, Karanam A, Gurjar M, Dhoble S, Naik AB, Vidhun BH, et al. Effect of various anticoagulants on the bioanalysis of drugs in rat blood: implication for pharmacokinetic studies of anticancer drugs. Springerplus. 2016;5:2102.
Davidson DF. Effects of contamination of blood specimens with liquid potassium-EDTA anticoagulant. Ann Clin Biochem. 2002;39:273–80.
Baumann P, Perey M. The analysis of free tryptophan in human blood with the Ultrafiltrator: a comparison with other methods. Clin Chim Acta. 1977;76:223–31.
Bourgoin B, Faivre-Bauman A, Hery F, Ternaux JP, Hamon M. Characteristics of tryptophan binding in the serum of the newborn rat. Biol Neonate. 1977;31:141–54.
Dietrich-Muszalska A, Kwiatkowska A. Generation of superoxide anion radicals and platelet glutathione peroxidase activity in patients with schizophrenia. Neuropsychiatr Dis Treat. 2014;10:703–9.
Quintana J. Platelet serotonin and plasma tryptophan decreases in endogenous depression. Clinical, therapeutic, and biological correlations. J Affect Disord. 1992;24:55–62.
McMenamy RH, Watson F. Indole-albumin association: a comparative study. Comp Biochem Physiol. 1968;26:329–35.
Pardridge WM, Fierer G. Transport of tryptophan into brain from the circulating, albumin-bound pool in rats and in rabbits. J Neurochemistry. 1990;54:971–6.
Maes M, Jacobs MP, Suy E, Vandewoude M, Minner B, Raus J. Effects of dexamethasone on the availability of L-tryptophan and on the insulin and FFA concentrations in unipolar depressed patients. Biol Psychiatry. 1990;27:854–62.
Stoyanov D, Kandilarova S, Aryutova K, Paunova R, Todeva-Radneva A, Latypova A, et al. Multivariate analysis of structural and functional neuroimaging can inform psychiatric differential diagnosis. Diagnostics. 2020;11:1–17.
Stoyanov D, Kandilarova S, Paunova R, Barranco Garcia J, Latypova A, Kherif F. Cross-validation of functional MRI and paranoid-depressive scale: results from multivariate analysis. Front Psychiatry. 2019;10:1–8.
Uwai Y, Honjo H, Iwamoto K. Interaction and transport of kynurenic acid via human organic anion transporters hOAT1 and hOAT3. Pharmacol Res. 2012;65:254–60.
Funding
The study was funded by the C2F program, Chulalongkorn University.
Author information
Authors and Affiliations
Contributions
All authors contributed to the writing up of the paper. The work was designed by AA and MM. Data were collected by AA and AV. Statistical analyses were performed by AA and MM. All authors revised and approved the final draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests. MS received honoraria and has been a consultant for Angelini, Lundbeck.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Almulla, A.F., Vasupanrajit, A., Tunvirachaisakul, C. et al. The tryptophan catabolite or kynurenine pathway in schizophrenia: meta-analysis reveals dissociations between central, serum, and plasma compartments. Mol Psychiatry 27, 3679–3691 (2022). https://doi.org/10.1038/s41380-022-01552-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-022-01552-4
This article is cited by
-
Although serotonin is not a major player in depression, its precursor is
Molecular Psychiatry (2023)
-
Importance of the dysregulation of the kynurenine pathway on cognition in schizophrenia: a systematic review of clinical studies
European Archives of Psychiatry and Clinical Neuroscience (2023)
-
The tryptophan catabolite or kynurenine pathway in COVID-19 and critical COVID-19: a systematic review and meta-analysis
BMC Infectious Diseases (2022)