Abstract
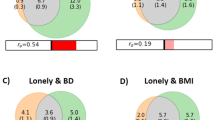
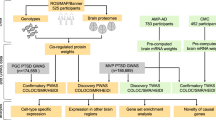
Enduring loneliness is associated with mental disorders and physical diseases. Although genome-wide association studies (GWAS) have identified risk loci associated with loneliness, how these loci confer the risk remains largely unknown. In the current study, we aimed to investigate key proteins underlying loneliness in the brain by integrating human brain proteomes and transcriptomes with loneliness GWAS to perform a discovery proteome-wide association study (PWAS), followed by a confirmatory PWAS, transcriptome-wide association analysis (TWAS), Mendelian randomization (MR), Steigering filtering analysis and Bayesian colocalization analysis. Moreover, given the fact that loneliness is associated with mental disorders, we explored the shared genetic architecture between loneliness and mental disorders. Totally, we identified 18 genes to be associated with loneliness via their cis-regulated brain protein abundance. Eleven of the 18 genes (61.1%) were replicated in the confirmatory PWAS, and mRNA levels of 4 genes were further validated to be associated with loneliness.MR and genetic colocalization analysis further confirmed that the increased protein abundance of ALDH2 and ICA1L was protective against loneliness, while the increased protein abundance of GPX1 was a risk for developing loneliness. Furthermore, we found genetic correlations, bidirectional causal associations and overlapping phenotype-associated protein profiles between loneliness and mental disorders including major depression and schizophrenia. In summary, our findings provided clues about the brain-related molecular basis underlying loneliness, which warrants further investigation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The data were obtained from the publicly available database (listed in Supplementary Table 1). The datasets analyzed during the current study are available from the corresponding author upon reasonable request.
References
Umberson D, Montez JK. Social relationships and health: a flashpoint for health policy. J Heal Soc Behav. 2010;51:S54–66.
HJ S, LK R, Debra U. Social Relationships and Health. Science (80-). 1988;241:540–5.
Russell D, Peplau LA, Cutrona CE. The revised UCLA Loneliness Scale: concurrent and discriminant validity evidence. J Pers Soc Psychol. 1980;39:472–80.
Cacioppo S, Grippo AJ, London S, Goossens L, Cacioppo JT. Loneliness: clinical import and interventions. Perspect Psychol Sci. 2015;10:238–49.
Malcolm M, Frost H, Cowie J. Loneliness and social isolation causal association with health-related lifestyle risk in older adults: a systematic review and meta-analysis protocol. Syst Rev. 2019;8:1–8.
Courtin E, Knapp M. Social isolation, loneliness and health in old age: a scoping review. Heal Soc Care Community. 2017;25:799–812.
Steptoe A, Shankar A, Demakakos P, Wardle J. Social isolation, loneliness, and all-cause mortality in older men and women. Proc Natl Acad Sci USA. 2013;110:5797–801.
Stokes AC, Xie W, Lundberg DJ, Glei DA, Weinstein MA. Loneliness, social isolation, and all-cause mortality in the United States. SSM - Ment Heal. 2021;1:100014.
Abdellaoui A, Sanchez-Roige S, Sealock J, Treur JL, Dennis J, Fontanillas P, et al. Phenome-wide investigation of health outcomes associated with genetic predisposition to loneliness. Hum Mol Genet. 2019;28:3853–65.
Elovainio M, Lahti J, Pirinen M, Pulkki-Råback L, Malmberg A, Lipsanen J, et al. Association of social isolation, loneliness and genetic risk with incidence of dementia: UK Biobank Cohort Study. BMJ Open. 2022;12:6–8.
Rødevand L, Bahrami S, Frei O, Lin A, Gani O, Shadrin A, et al. Polygenic overlap and shared genetic loci between loneliness, severe mental disorders, and cardiovascular disease risk factors suggest shared molecular mechanisms. Transl Psychiatry. 2021;11:3.
Hutten E, Jongen EMM, Hajema KJ, Ruiter RAC, Hamers F, Bos AER. Risk factors of loneliness across the life span. J Soc Pers Relat. 2022;39:1482–507.
Boomsma DI, Willemsen G, Dolan CV, Hawkley LC, Cacioppo JT. Genetic and environmental contributions to loneliness in adults: The Netherlands Twin Register study. Behav Genet. 2005;35:745–52.
Day FR, Ong KK, Perry JRB. Elucidating the genetic basis of social interaction and isolation. Nat Commun. 2018;9:1–6.
McLachlan AD. Protein structure and function. Annu Rev Phys Chem. 1972;23:165–92.
Robustelli BL, Newberry RE, Whisman MA, Mittal VA. Social relationships in young adults at ultra high risk for psychosis. Psychiatry Res. 2017;247:345–51.
Bennett DA, Buchman AS, Boyle PA, Barnes LL, Wilson RS, Schneider JA. Religious orders study and rush memory and aging project. J Alzheimers Dis. 2018;64:S161–89.
Wingo AP, Liu Y, Gerasimov ES, Gockley J, Logsdon BA, Duong DM, et al. Integrating human brain proteomes with genome-wide association data implicates new proteins in Alzheimer’s disease pathogenesis. Nat Genet. 2021;53:143–6.
Fromer M, Roussos P, Sieberts SK, Johnson JS, Kavanagh DH, Perumal TM, et al. Gene expression elucidates functional impact of polygenic risk for schizophrenia. Nat Neurosci. 2016;19:1442–53.
Wang D, Liu S, Warrell J, Won H, Shi X, Navarro FCP, et al. Comprehensive functional genomic resource and integrative model for the human brain. Science (80-). 2018;362:eaat8464.
Howard DM, Adams MJ, Clarke TK, Hafferty JD, Gibson J, Shirali M, et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat Neurosci. 2019;22:343–52.
Mullins N, Forstner AJ, O’Connell KS, Coombes B, Coleman JRI, Qiao Z, et al. Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nat Genet. 2021;53:817–29.
Trubetskoy V, Pardiñas AF, Qi T, Panagiotaropoulou G, Awasthi S, Bigdeli TB, et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature. 2022;604:502–8.
Gusev A, Ko A, Shi H, Bhatia G, Chung W, Penninx BWJH, et al. Integrative approaches for large-scale transcriptome-wide association studies. Nat Genet. 2016;48:245–52.
Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-base platform supports systematic causal inference across the human phenome. Elife. 2018;7:1–29.
Sanderson E, Glymour MM, Holmes MV, Kang H, Morrison J, Munafò MR, et al. Mendelian randomization. Nat Rev Methods Prim. 2022;2:6.
Hemani G, Tilling K, Smith GD. Orienting the causal relationship between imprecisely measured traits using genetic instruments. PLoS Genet. 2017;13:e1007081.
Giambartolomei C, Vukcevic D, Schadt EE, Franke L, Hingorani AD, Wallace C, et al. Bayesian test for colocalisation between pairs of genetic association studies using summary statistics. PLoS Genet. 2014;10:e1004383.
Timshel PN, Thompson JJ, Pers TH. Genetic mapping of etiologic brain cell types for obesity. Elife. 2020;9:1–45.
Bulik-Sullivan B, Finucane HK, Anttila V, Gusev A, Day FR, Loh PR, et al. An atlas of genetic correlations across human diseases and traits. Nat Genet. 2015;47:1236–41.
Chen CH, Ferreira JCB, Gross ER, Mochly-Rosen D. Targeting aldehyde dehydrogenase 2: new therapeutic opportunities. Physiol Rev. 2014;94:1–34.
Jin S, Cao Q, Yang F, Zhu H, Xu S, Chen Q, et al. Brain ethanol metabolism by astrocytic ALDH2 drives the behavioural effects of ethanol intoxication. Nat Metab. 2021;3:337–51.
Li K, Xu E. The role and the mechanism of γ-aminobutyric acid during central nervous system development. Neurosci Bull. 2008;24:195–200.
Longa Z, Medlockc C, Dzemidzic M, Shine Y-W, Goddard AWUD. Decreased GABA levels in anterior cingulate cortex/medial prefrontal cortex in panic disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2013;44:131–5.
Lydiard RB. The role of GABA in anxiety disorders. J Clin Psychiatry. 2003;64:21–7.
Paine TA, Swedlow N, Swetschinski L. Decreasing GABA function within the medial prefrontal cortex or basolateral amygdala decreases sociability. Behav Brain Res. 2017;317:542–52.
He J, Xia M, Tsang WH, Chow KL, Xia J. ICA1L forms BAR-domain complexes with PICK1 and is crucial for acrosome formation in spermiogenesis. J Cell Sci. 2015;128:3822–36.
Ou YN, Yang YX, Deng YT, Zhang C, Hu H, Wu BS, et al. Identification of novel drug targets for Alzheimer’s disease by integrating genetics and proteomes from brain and blood. Mol Psychiatry. 2021;26:6065–73.
Cullell N, Gallego-Fábrega C, Cárcel-Márquez J, Muiño E, Llucià-Carol L, Lledós M, et al. ICA1L is associated with small vessel disease: a proteome-wide association study in small vessel stroke and intracerebral haemorrhage. Int J Mol Sci. 2022;23:3161.
Zhang C, Qin F, Li X, Du X, Li T. Identification of novel proteins for lacunar stroke by integrating genome-wide association data and human brain proteomes. BMC Med. 2022;20:1–11.
Corbett AH. Post-transcriptional regulation of gene expression and human disease. Curr Opin Cell Biol. 2018;52:96–104.
Xiong Y, Shie FS, Zhang J, Lee CP, Ho YS. The protective role of cellular glutathione peroxidase against trauma-induced mitochondrial dysfunction in the mouse brain. J Stroke Cerebrovasc Dis. 2004;13:129–37.
Wong CHY, Bozinovski S, Hertzog PJ, Hickey MJ, Crack PJ. Absence of glutathione peroxidase-1 exacerbates cerebral ischemia-reperfusion injury by reducing post-ischemic microvascular perfusion. J Neurochem. 2008;107:241–52.
Hwang SN, Lee JS, Seo K, Lee H. Astrocytic regulation of neural circuits underlying behaviors. Cells. 2021;10:1–24.
Gao J, Davis LK, Hart AB, Sanchez-Roige S, Han L, Cacioppo JT, et al. Genome-wide association study of loneliness demonstrates a role for common variation. Neuropsychopharmacology. 2017;42:811–21.
Matthews T, Danese A, Wertz J, Odgers CL, Ambler A, Moffitt TE, et al. Social isolation, loneliness and depression in young adulthood: a behavioural genetic analysis. Soc Psychiatry Psychiatr Epidemiol. 2016;51:339–48.
Andreu-Bernabeu Á, Díaz-Caneja CM, Costas J, De Hoyos L, Stella C, Gurriarán X, et al. Polygenic contribution to the relationship of loneliness and social isolation with schizophrenia. Nat Commun. 2022;13:51.
Michalska Da Rocha B, Rhodes S, Vasilopoulou E, Hutton P. Loneliness in psychosis: a meta-analytical review. Schizophr Bull. 2018;44:114–25.
Eglit GML, Palmer BW, Martin AS, Tu X, Jeste DV. Loneliness in schizophrenia: construct clarification, measurement, and clinical relevance. PLoS One. 2018;13:1–20.
Grande I, Berk M, Birmaher B, Vieta E. Bipolar disorder. Lancet. 2016;387:1561–72.
Acknowledgements
We thank all the researchers who provided the original data publicly.
Funding
This study was supported by 1.3.5 projects for disciplines of excellence, West China Hospital, Sichuan University (Grant No. ZYJC21004), the National Natural Science Foundation of China (Grant No. 81871061), and the Postdoctoral Foundation of West China Hospital (Grant No. 2020HXBH041).
Author information
Authors and Affiliations
Contributions
XJG: conceptualization, formal analysis, methodology, writing—original draft; MD: data curation and methodology; MLY: writing—review & editing; WZ: conceptualization; writing—review & editing; supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gu, X., Dou, M., Yuan, M. et al. Identifying novel proteins underlying loneliness by integrating GWAS summary data with human brain proteomes. Neuropsychopharmacol. 48, 1087–1097 (2023). https://doi.org/10.1038/s41386-023-01536-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-023-01536-0