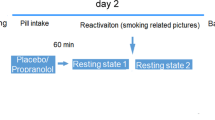
Abstract
The cholinergic system is a critical mediator of cognition in animals. People who smoke cigarettes exhibit cognitive deficits, especially during quit attempts. Few studies jointly examine the cholinergic system and cognition in people while trying to quit smoking. We used positron emission tomography (PET) brain imaging with the β2-subunit containing nicotinic acetylcholine receptor (β2*-nAChR) partial agonist radioligand (-)-[18F]flubatine and the acetylcholinesterase inhibitor physostigmine to jointly examine the cholinergic system, smoking status, and cognition. (-)-[18F]Flubatine scans and cognitive data were acquired from twenty people who recently stopped smoking cigarettes (aged 38 ± 11 years; 6 female, 14 male; abstinent 7 ± 1 days) and 27 people who never smoked cigarettes (aged 29 ± 8 years; 11 female, 16 male). A subset of fifteen recently abstinent smokers and 21 never smokers received a mid-scan physostigmine challenge to increase acetylcholine levels. Regional volume of distribution (VT) was estimated with equilibrium analysis at “baseline” and post-physostigmine. Participants completed a cognitive battery prior to (-)-[18F]flubatine injection and physostigmine administration assessing executive function (Groton Maze Learning test), verbal learning (International Shopping List test), and working memory (One Back test). Physostigmine significantly decreased cortical (-)-[18F]flubatine VT, consistent with increased cortical acetylcholine levels reducing the number of β2*-nAChR sites available for (-)-[18F]flubatine binding, at comparable magnitudes across groups (p values < 0.05). A larger magnitude of physostigmine-induced decrease in (-)-[18F]flubatine VT was significantly associated with worse executive function in people who recently stopped smoking (p values < 0.05). These findings underscore the role of the cholinergic system in early smoking cessation and highlight the importance of neuroscience-informed treatment strategies.
Similar content being viewed by others
Introduction
Tobacco cigarette smoking remains a leading cause of preventable disease and death [1]. Although 55% of people who smoked tobacco in 2018 made an attempt to quit, only 8% reported successful attempts [2]. Relapse during early quit attempts is often a consequence of smoking to relieve adverse effects of early abstinence, including cognitive deficits (e.g., adaptations in attention, inhibitory control, memory, etc.) [3,4,5]. Notably, cognitive dysfunction during quit attempts is predictive of poorer long-term smoking cessation success [6]. It is critical therefore to examine the brain during early abstinence and identify neural mechanisms associated with cognitive function to inform smoking cessation treatment strategies.
The cholinergic system is a critical mediator of cognitive function in animal models. Nicotine, a primary addictive constituent of cigarettes, can modulate this relationship in complex ways [7]. People who smoke cigarettes exhibit cognitive deficits compared to people who never smoked cigarettes and demonstrate worsened cognitive performance in early nicotine withdrawal [5, 8]. In the human brain, the frontal cortex is an important hub for cognitive function [9,10,11]. Aside from work on cognitive dysfunction in neurodegenerative disorders, there is a surprising dearth of molecular imaging studies that investigate relationships between the cholinergic system and cognition in people [12]. It is clinically imperative to examine this relationship to better understand how cholinergic interventions may influence cognition during smoking cessation.
Nicotine enacts its reinforcing properties by binding to and activating β2-subunit containing nicotinic acetylcholine receptors (β2*-nAChRs) on ventral tegmental area neurons to acutely facilitate downstream dopamine release in the ventral striatum [13,14,–15]. Nicotine also binds to β2*-nAChRs, and other related subtypes, across various subcortical and cortical brain regions [16]. Animal studies demonstrate that chronic nicotine can saturate, desensitize, and elevate the number of β2*-nAChRs [17, 18], and thus may disrupt activity of the endogenous β2*-nAChR ligand acetylcholine [19]. Human in vivo molecular imaging studies that utilize single photon emission computed tomography (SPECT) or positron emission tomography (PET) reveal that people who smoke cigarettes exhibit higher β2*-nAChR availability than people who never smoked cigarettes [20,21,22,23,–24], and that higher upregulation of β2*-nAChR availability is predictive of worse smoking cessation outcomes [25, 26]. As cognitive dysfunction and upregulated β2*-nAChR availability are each independently predictive of smoking cessation success, examination of links between the cholinergic system and cognition may lend insight into treatment strategies that decrease relapse likelihood [4, 25, 26].
In this study, PET imaging was conducted with the β2*-nAChR-specific partial agonist radioligand (-)-[18F]flubatine and combined with administration of physostigmine. Physostigmine inhibits acetylcholinesterase function, thereby elevating extracellular acetylcholine levels. Physostigmine administration in people reduces radiotracer VT estimates as shown in previous studies [27,28,–29], likely reflecting increased acetylcholine levels that compete with the radiotracer to reduce the number of β2*-nAChRs available for radiotracer binding. This work leveraged this study design to investigate relationships between cholinergic system function, smoking status, and cognition. We hypothesized that people who recently stopped smoking would exhibit altered physostigmine-induced reductions in (-)-[18F]flubatine VT compared to people who never smoked, and the magnitude of these changes in the frontal cortex would be associated with cognitive function in early abstinence.
Patients and methods
Study participants
Twenty people who recently stopped smoking cigarettes for 7 ± 1 days (abstinent smokers, AS; aged 38 ± 11 years; 6 female, 14 male; 10 individuals with some college education or higher) and 27 people who never smoked cigarettes (never smokers, NS; aged 29 ± 8 years; 11 female, 16 male; 23 individuals with some college education or higher) participated in one (-)-[18F]flubatine PET scan (data presented as mean ± standard deviation) (Table 1). Six never smokers and 7 people who recently stopped smoking reported cannabis use. Although people who recently stopped smoking were older and had higher body mass index (BMI) values than people who never smoked, these factors did not significantly influence the effect of smoking status on baseline β2*-nAChR availability (Supplementary Information). A subset of 15 people who recently stopped smoking cigarettes and 21 people who never smoked cigarettes also received physostigmine during the (-)-[18F]flubatine PET scan. Smokers received smoking cessation counselling and were imaged one week after their last cigarette. Structural magnetic resonance imaging (MRI) scans were acquired for delineation of regions of interest (ROIs). Cigarette use history was obtained prior to participants quitting smoking. Cognitive measures were collected early on PET scan day, prior to the (-)-[18F]flubatine injection and subsequent administration of physostigmine.
Participants were recruited from the community and administered the Structured Clinical Interview for DSM-IV or DSM-5 and a medical exam. Participants provided demographic information, substance use and psychiatric history, electrocardiogram, and urine samples for toxicology and pregnancy testing. Inclusion criteria for people who smoke cigarettes included smoking ≥5 cigarettes/day for ≥1 year, recent smoke inhalation and nicotine exposure indicated by carbon monoxide (CO) breath output >11 ppm [30] and urine cotinine (nicotine metabolite) concentration >150 ng/mL, respectively, and agreement to quit smoking for up to two weeks. Inclusion criteria for people who never smoked cigarettes included <100 cigarettes smoked in lifetime, CO < 8 ppm, and urine cotinine 0–30 ng/mL. Participants who never smoked cigarettes reported no other use of nicotine-containing products. Exclusion criteria for all participants included presence or history of serious medical or neurological illness, current major psychiatric diagnosis or non-nicotine substance use disorder, anxiolytic or antidepressant use, uncontrolled hypertension or electrocardiogram abnormalities, positive drug toxicology except for 11-nor-9-carboxy-Δ9-tetrahydrocannabinol (11-COOH-THC) reflecting recent cannabis use, MRI contraindications, and pregnancy or lactating. Participants provided written informed consent after review of the study protocols, approved by the Yale-New Haven Hospital Radiation Safety Committee and the Yale University Human Investigation Committee. This study was registered with ClinicalTrials.gov (Identifier: NCT02008292), and the analyses presented pertain to the registered study goal to examine the cholinergic system in people who either smoke or never smoked cigarettes.
Cigarette use characteristics, cessation counselling, and cognitive measures
Prior to quitting, people who smoked reported 16 ± 7 cigarettes per day for 16 ± 11 years or 14 ± 12 pack-years smoking, and moderate cigarette dependence (Fagerström Test for Cigarette Dependence, or FTCD, total score: 5.4 ± 2.5) [31, 32]. They also received daily cessation counselling for one week prior to PET scanning with the Smoking Cessation Clinical Practice Guideline [33] and were compensated $10 per daily visit contingent on breath CO < 11 ppm. On PET scan day, urine cotinine levels were acquired to confirm abstinence. Six people who recently stopped smoking had urine cotinine levels >600 ng/mL on scan day, indicative of a ‘lapse’ in smoking abstinence. Individuals with evidence of a “lapse” are referred to as “high-cotinine” participants, while remaining participants who recently stopped smoking for a least one week are referred to as “low-cotinine” participants.
On PET scan day, prior to (-)-[18F]flubatine injection and physostigmine administration, all participants completed a computerized cognitive battery (CogState Ltd.), except one person who recently stopped smoking was not able to complete the battery due to time constraints. A subset of three cognitive tasks were analyzed based on relevance in studies of cognition in cigarette use and withdrawal [3,4,5]. The three tasks were the Groton Maze Learning Test (executive function), the International Shopping List Test (verbal learning), and the One Back Test (working memory) [34]. One additional person who recently stopped smoking did not complete the working memory task. Statistical outliers in cognitive performance were defined as ≥1.5 standard deviations from the nearest neighbouring data point, were excluded from analyses, and are noted in the results.
PET and MR scanning and physostigmine administration
T1-weighted structural magnetic resonance (MR) scans were acquired from participants prior to PET scanning with a Siemens 3.0 T scanner and a 64-channel head coil to yield a high-resolution anatomical map for PET data coregistration. A sagittal gradient-echo MPRAGE sequence (FOV: 256 × 256 mm2, 176 slices (1 mm thickness), TE: 2.77 ms, TR: 2530 ms, TI: 1100 ms, FA: 7°) was used.
On PET scanning days, CO breath output and urine cotinine levels were measured in people who recently stopped smoking to confirm abstinence from cigarettes. Negative pregnancy and urine toxicology (except 11-COOH-THC) results were required to scan. PET scan data were acquired on the High Resolution Research Tomograph (Siemens/CTI). Participants wore a Vicra cap for motion correction and underwent a 6-min transmission scan for attenuation correction. (-)-[18F]Flubatine was synthesized at high molar activity as previously described [35] and administered with a computer-controlled pump (Harvard Apparatus) as a bolus plus constant infusion (B/I) (KBol = 360 min; NS: 248.6 ± 56.6 MBq, 0.09 ± 0.08 μg; AS: 258.9 ± 54.6 MBq, 0.09 ± 0.09 μg) for at least 120 min for baseline measurements, and up to 240 min for individuals who received a physostigmine challenge. Six people who never smoked (3 female, 3 male) and 5 people who recently stopped smoking (3 female, 2 male) did not participate in the physostigmine challenge due to a physostigmine supply shortage (n = 9), a sinus arrhythmia (n = 1), and an arterial line malfunction (n = 1). The remaining 21 people who never smoked and 15 people who recently stopped smoking received physostigmine. First, to reduce nausea symptoms 200 μg glycopyrrolate was administered intravenously over 1 min starting at 120 min of (-)-[18F]flubatine infusion. Next, 1.5 mg physostigmine was administered as a bolus plus infusion over 60 min, starting after 125 min of (-)-[18F]flubatine infusion as previously described [27]. There were no significant differences between participant groups in injected (-)-[18F]flubatine radioactivity or mass, independent of or relative to body weight. Emission data were acquired either 90–240 min or 90–120 min after B/I initiation for individuals who did or did not receive physostigmine, respectively. Arterial blood was collected in either 15-min intervals 90–240 min, or 10-min intervals 90–120 min, after (-)-[18F]flubatine injection to measure the metabolite-corrected (-)-[18F]flubatine parent input function in individuals with or without physostigmine administration, respectively, as previously described [27].
PET image processing
List-mode PET data were binned into 5 min frames and reconstructed with corrections for normalization, attenuation, randoms, scatter, deadtime, and head motion using the MOLAR algorithm [36] and an optical detector (Vicra, NDI Systems). Participants’ MR scans were transformed nonlinearly into Montreal Neurological Imaging template space, co-registered to early summed kinetic PET images generated with Vicra motion-corrected and transmission data, and parcellated into ROIs with Anatomic Automatic Labelling [37]. (-)-[18F]Flubatine PET time-activity curves were generated for a selection of grey matter subcortical and cortical cerebral regions involved in tobacco use and cognition and/or with measurable [18F]flubatine activity [27], including the caudate, frontal cortex, hippocampus, insula, occipital cortex, parietal cortex, putamen, and temporal cortex.
PET data analysis
(-)-[18F]Flubatine PET data were modelled with an equilibrium analysis to estimate volume of distribution (VT), the ratio of mean (-)-[18F]flubatine activity in tissue to parent (-)-[18F]flubatine activity in arterial plasma at equilibrium, proportional to β2*-nAChR availability [27, 38,39,40]. Baseline and post-physostigmine VT estimates were estimated during the established equilibrium periods of 90–120 min and 180–210 min post-injection, respectively [27]. VT estimates additionally featured a tissue clearance correction to account for potential inter-subject or -group variance in (-)-[18F]flubatine kinetics or metabolism and to reduce consequent bias in VT estimation [41]. The free plasma fraction of (-)-[18F]flubatine (fP) was only measured prior to physostigmine administration, and results from the baseline-only PET analyses were comparable with or without correction for fP. Therefore, regional VT was the selected PET outcome measure for both baseline-only and physostigmine-related analyses. Regional baseline (-)-[18F]flubatine VT estimates with and without correction for fP are summarized in Supplementary Table S1.
Physostigmine, as an acetylcholinesterase inhibitor, inhibits the breakdown of acetylcholine and thus increases levels of acetylcholine. Physostigmine administration in people reduces radiotracer VT estimates as shown in previous studies [27,28,–29], likely reflecting increased acetylcholine levels that compete with the radiotracer to reduce the number of β2*-nAChRs available for radiotracer binding. Consequently, physostigmine-induced percent change in regional (-)-[18F]flubatine VT was calculated as [1-(VT:Physostigmine/VT:Baseline)]*100 as an indirect measure of elevation in acetylcholine levels, such that higher, more positive values reflect higher elevation of acetylcholine levels after physostigmine.
Statistical analyses
Statistical analyses were performed with R (The R Foundation for Statistical Computing). Overall, two-sample tests (e.g., t tests, Welch’s t tests, or Wilcoxon rank sum tests) were selected according to data normality and variance and were conducted to evaluate differences between people who never smoked and people who recently stopped smoking in demographics, PET injection information, PET outcome measures, and PET scan day cognitive performance. Pearson’s correlation coefficients (r) were computed to assess relationships between PET outcome measures and cognitive performance metrics. All associative analyses focused on people who recently stopped smoking a priori as the clinical population of interest. A two-tailed α = 0.05 threshold was used to determine significance across analyses, and p values were adjusted with a false discovery rate (FDR) correction for multiple comparisons when appropriate and as described below.
Primary analyses examined the magnitude of, and group difference in, physostigmine-induced percent change in (-)-[18F]flubatine VT across regions. Statistical significance was FDR-corrected for multiple regions of interest (excluding the frontal cortex as our a priori region of interest; n = 7) within each diagnostic group. Primary analyses also examined relationships between cognitive performance metrics and frontal cortex estimates of physostigmine-induced percent change in (-)-[18F]flubatine VT in people who recently stopped smoking. Statistical significance results were FDR-corrected for multiple cognitive tasks (n = 3). Secondary analyses were conducted to investigate if this relationship was specific to the frontal cortex or specific to diagnosis. Here, associations between cognitive performance metrics and physostigmine-induced percent change in (-)-[18F]flubatine VT in the other 7 ROIs were computed, in both people who never smoked and people who recently stopped smoking. Statistical significance results for these secondary analyses were FDR-corrected for multiple cognitive tasks (n = 3).
Although primary analyses focused on physostigmine-induced percent change in (-)-[18F]flubatine VT, there was potential for insights to be gleaned from analysis of baseline (-)-[18F]flubatine VT in light of previous work examining the β2*-nAChR system [20,21,22,23,24,25,–26]. To build upon this work, secondary analyses were conducted to examine group differences in baseline (-)-[18F]flubatine VT and relationships of baseline (-)-[18F]flubatine VT with cognitive performance. Statistical significance results were FDR-corrected in the same manner as described for the physostigmine-related analyses. High-cotinine people who recently stopped smoking were excluded from baseline-related analyses, as nicotine was not cleared from the system and therefore may have affected (-)-[18F]flubatine binding to β2*-nAChR sites relative to low-cotinine people who recently stopped smoking [22]. High-cotinine participants were included for physostigmine-related analyses because the outcome of interest was a within-subject change, and baseline (-)-[18F]flubatine VT was not significantly associated with physostigmine-induced percent change in (-)-[18F]flubatine VT (Supplementary Table S2).
Results
Physostigmine administration lowers β2*-nAChR availability across groups
(-)-[18F]Flubatine VT estimates were significantly lower post-physostigmine than at baseline in the a priori frontal cortex of people who never smoked cigarettes (NS, or ‘never smokers’) (puncorr < 0.001), and “trend”-level lower post-physostigmine than at baseline in the frontal cortex of people who recently stopped smoking cigarettes (AS, or “abstinent smokers”) (puncorr = 0.068) (Fig. 1, Table 2). (-)-[18F]Flubatine VT estimates were significantly lower post-physostigmine than at baseline in both NS and AS in the occipital (NS: pcorr = 0.006, AS: pcorr = 0.04) and parietal (NS: pcorr = 0.004, AS: pcorr = 0.047) cortices. (-)-[18F]Flubatine VT estimates were significantly lower post-physostigmine than at baseline in the temporal cortex of NS (pcorr = 0.004), and “trend”-level lower post-physostigmine than at baseline in the temporal cortex of AS (pcorr = 0.076) (Fig. 1, Table 2). The magnitude of physostigmine’s effect on (-)-[18F]flubatine VT, calculated as the physostigmine-induced percent change in (-)-[18F]flubatine VT (%ΔVT), was on average lower in AS compared to NS, but group differences were not significantly different in any region (puncorr-values>0.05) (Fig. 1, Table 2). Additionally, no significant differences in physostigmine-induced percent change in (-)-[18F]flubatine VT were detected between high-cotinine and low-cotinine recently abstinent smokers (Supplementary Table S3).
In the frontal cortex, physostigmine significantly decreases estimates of β2*-nAChR availability ((-)-[18F]flubatine VT) in people who never smoked cigarettes (light circles) (puncorr < 0.001) and ‘trend’-level decreases estimates in people who recently stopped smoking cigarettes (dark squares) (puncorr = 0.068). Physostigmine significantly decreases estimates of β2*-nAChR availability in the occipital, parietal, and temporal cortices of people who never smoked cigarettes (pcorr-values<0.05), and in the occipital and parietal cortices of people who recently stopped smoking cigarettes (pcorr-values<0.05). The magnitude of physostigmine-induced percent change in β2*-nAChR availability is not significantly different between groups in any regions. Mean VT (bars) and group mean values of physostigmine-induced percent change in β2*-nAChR availability (percentages) are presented. n: sample size.
Worse executive function is associated with higher physostigmine-induced elevation of acetylcholine levels in people who recently stopped smoking
The number of errors made during the executive function ‘Groton Maze Learning’ task was not significantly different between groups on (-)-[18F]flubatine scan day, when including all task-completers (NS: 48 ± 15 errors; AS: 57 ± 32 errors; NS vs. AS pcorr = 0.46) or only task-completers who received physostigmine (NS: 49 ± 15 errors; AS: 48 ± 21 errors; NS vs. AS pcorr = 0.90) (Fig. 2). A higher number of errors made (i.e., worse executive function performance) was significantly associated with higher physostigmine-induced percent change in (-)-[18F]flubatine VT estimates in the frontal cortex of people who recently stopped smoking (r = 0.73, pcorr = 0.009) (Fig. 2), and not in people who never smoked (r = 0.16, pcorr = 0.73). Significant associations were also observed in the caudate, insula, and occipital and temporal cortices of people who recently stopped smoking (Supplementary Table S4). There were no other significant associations between physostigmine-induced changes in (-)-[18F]flubatine VT and cognitive performance.
People who recently stopped smoking cigarettes (‘AS,’ or ‘abstinent smokers,’ dark squares) do not significantly differ from people who never smoked cigarettes (‘NS,’ light circles) in number of errors made on the ‘Groton Maze Learning’ executive function task (NS: 49 ± 15 errors; AS: 48 ± 21 errors; pcorr = 0.90) (left panel). Higher number of errors made (i.e., worse executive function) is associated with higher physostigmine-induced percent change in (-)-[18F]flubatine VT (%ΔVT) estimates (i.e., higher magnitude increase in acetylcholine levels) in the frontal cortex of people who recently stopped smoking (r = 0.73, pcorr = 0.009) (right panel). Plots include task-completing individuals who received physostigmine. The plots are generated with distributions, sample sizes, and statistical analyses which exclude one statistical outlier who recently stopped smoking, included as a hollow marker for transparency. n: sample size. Horizontal bars (left panel): group mean task performance speed. r: Pearson correlation coefficient. Significance: *p < 0.05.
Worse working memory is associated with higher β2*-nAChR availability in people who recently stopped smoking
Baseline (-)-[18F]Flubatine VT estimates were significantly higher in low-cotinine people who recently stopped smoking compared to people who never smoked in all regions (pcorr-values<0.05) except the caudate (pcorr = 0.09) and hippocampus (pcorr = 0.15) (Supplementary Table S5). Performance speed on the working memory “One Back” task was not significantly different between groups on (-)-[18F]flubatine scan day, when including all task-completers recently abstinent from smoking (NS: 2.9 ± 0.1 log10 ms; AS: 2.9 ± 0.1 log10 ms; NS vs. AS pcorr = 0.46) or only task-completers who were low-cotinine and recently abstinent from smoking (NS: 2.9 ± 0.1 log10 ms; AS: 2.9 ± 0.1 log10 ms; NS vs. AS pcorr = 0.74) (Fig. 3). Slower performance speed (i.e., worse performance) was significantly associated with higher baseline (-)-[18F]flubatine VT estimates (i.e., higher β2*-nAChR availability) in the frontal cortex of low-cotinine people who recently stopped smoking (r = 0.59, puncorr = 0.045) (Fig. 3), and not in people who never smoked (r = 0.19, puncorr = 0.33), without correction for multiple cognitive tasks. After statistical correction for 3 cognitive tasks, the relationship observed in low-cotinine abstinent smokers was not significant (r = 0.59, pcorr = 0.14). Significant associations were also observed in the hippocampus and putamen of low-cotinine people who recently stopped smoking (Supplementary Table S6). There were no other significant associations between (-)-[18F]flubatine VT and cognitive performance.
Low-cotinine people who recently stopped smoking cigarettes (‘AS,’ or ‘abstinent smokers,’ dark squares) do not significantly differ from people who never smoked cigarettes (‘NS,’ light circles) in performance speed on the ‘One-Back’ working memory task (NS: 2.9 ± 0.1 log10 ms; AS: 2.9 ± 0.1 log10 ms; p = 0.49) (left panel). Slower task performance (i.e., worse working memory) is associated with higher (-)-[18F]flubatine VT estimates (i.e., higher β2*-nAChR availability) in the frontal cortex of low-cotinine people who recently stopped smoking (r = 0.59, puncorr = 0.045), without correction for multiple cognitive tasks (right panel). This relationship becomes statistically non-significant after correction for multiple cognitive tasks (pcorr = 0.14). n: sample size. Horizontal bars (left panel): group mean task performance speed. r: Pearson correlation coefficient. Significance: *p < 0.05.
Discussion
This study demonstrated that in people who recently stopped smoking, a larger reduction in cortical (-)-[18F]flubatine VT after physostigmine is significantly associated with worse executive function. These results suggest a link of cholinergic function with cognitive performance in people who recently stopped smoking. Given that cognitive deficits in early withdrawal can precipitate smoking relapse, these data highlight the cholinergic system as a target for early quit attempts to mitigate cognitive changes and promote long-term cessation.
Physostigmine administration in this study significantly decreased cortical (-)-[18F]flubatine VT, consistent with increased cortical acetylcholine levels reducing the number of β2*-nAChR sites available for (-)-[18F]flubatine binding, at comparable magnitudes across participant groups. This builds on previous work that established the physostigmine imaging protocol [27,28,–29] by innovatively extending this paradigm to a larger sample with a clinical focus on people who recently stopped smoking. Nicotine continuously saturates and desensitizes β2*-nAChRs in the brains of people who smoke [42], which can disrupt endogenous acetylcholine activity long-term and potentially contribute to cognitive dysfunction in early cigarette withdrawal. It was therefore surprising to not detect a significant difference between groups in physostigmine-induced elevation of acetylcholine levels. Detection of group differences may have been precluded by the small response to physostigmine (approximate average of 4–7% change in cortical β2*-nAChR availability) across groups as measured with (-)-[18F]flubatine VT. However, if an estimated (-)-[18F]flubatine VND of ~6.5 mL/cm3 is assumed uniform throughout the brain [43, 44], a 4-7% reduction in (-)-[18F]flubatine VT translates to 10–20% reduction in (-)-[18F]flubatine BPND, comparable to differences observed with amphetamine challenge [45, 46]. The physostigmine effects on (-)-[18F]flubatine VT were most robust in cortical brain regions but more muted in hippocampus and striatum (see Table 2). Interestingly, this regional pattern matches regions with relatively higher basal levels of acetylcholine that occupy a larger fraction of β2*-nAChRs [47, 48], potentially altering the physostigmine effect in these regions. This study also reproduced results from prior studies that demonstrated higher global baseline β2*-nAChR availability in people who recently stopped smoking than in people who never smoked [20,21,22,23,–24]. Since β2*-nAChR upregulation is detected at one week of abstinence and most individuals resume use within two weeks of quit attempt initiation [49], it is clinically imperative for smoking cessation success to mitigate withdrawal effects through the weeks or months needed for β2*-nAChR normalization [22]. Ultimately, this study innovatively combined physostigmine administration with (-)-[18F]flubatine PET imaging to investigate cholinergic system function in early cigarette withdrawal.
The a priori region of interest, the frontal cortex, as well as the cholinergic system broadly, have demonstrated roles in cognitive function [50,51,52,–53]. Indeed, 3–4 week interventions that either increase acetylcholine levels or target the β2*-nAChR system specifically demonstrated pro-cognitive effects in people who smoke [54, 55]. The present work builds on these findings with evidence that a larger magnitude of acute physostigmine-induced decrease in (-)-[18F]flubatine VT (consistent with higher magnitude elevation of acetylcholine levels) was significantly associated with worse executive function in people who recently stopped smoking cigarettes, but were otherwise untreated. Taken together, this raises the hypothesis that recently abstinent smokers with larger underlying cholinergic dysfunction may exhibit a larger neurobiological response to an acute pro-cholinergic physostigmine challenge. Relatedly, individuals with larger underlying cholinergic dysfunction would be expected to exhibit worsened cognitive performance, given the role of acetylcholine in cognitive function. This could indicate a brain-behaviour phenotype most likely to reap the pro-cognitive benefits of extended acetylcholinesterase inhibitor treatment. While additional studies would be needed to test this hypothesis, the current study may lend mechanistic understanding to pro-cognitive effects of acetylcholinesterase inhibitors.
Limitations of this study include no evaluation of cognitive performance after physostigmine, which precluded our ability to capture whether acute pro-cognitive effects of physostigmine were linked to pre-treatment measures of the cholinergic system. Second, the groups in this study did not differ in cognitive performance, despite larger studies repeatedly showing links between worse cognitive performance and cigarette use and withdrawal [5, 8, 56, 57]. It is possible, therefore, that we did not recruit a representative sample of cigarette smokers in early withdrawal. Finally, the clinical significance of these findings may be limited by the modest sample sizes of individuals who completed all study tasks, and the uneven distribution of cannabis use across diagnostic groups, both of which may impact (-)-[18F]flubatine VT and cognitive performance. In contrast, strengths of this study include use of the radiotracer (-)-[18F]flubatine, a second-generation PET radioligand targeting β2*-nAChRs with fast kinetics, favourable metabolism [44]. PET imaging also occurred at the optimal timepoint of one week of abstinence: early enough to observe persistent neurobiological adaptations, and late enough to ensure nicotine clearance from the brain [21, 22, 58]. Most innovatively, this study uniquely investigated and detected associations between physostigmine-induced effects on (-)-[18F]flubatine VT, baseline (-)-[18F]flubatine VT, and cognitive performance in healthy people who recently stopped smoking cigarettes. Few in vivo human PET imaging studies have jointly examined cholinergic and cognitive function, most of which focus on populations with neurodegenerative diseases [12, 59]. Given that cognitive dysfunction in withdrawal predicts poorer smoking cessation outcomes [7], these results highlight the importance of further investigating pro-cognitive effects of cholinergic smoking cessation aids.
In summary, this study jointly examined the cholinergic system and cognitive performance during early abstinence from cigarette use. The results revealed evidence for significant associations between larger physostigmine-induced elevation of acetylcholine levels and worse executive function in people who recently stopped smoking. Altogether, these findings underscore the importance of normalization of the cholinergic system and the development of neuroscience-informed treatment strategies to mitigate the consequences of cigarette withdrawal and promote smoking cessation success.
References
West R. Tobacco smoking: health impact, prevalence, correlates and interventions. Psychol Health. 2017;32:1018–36.
Creamer M, Wang T, Babb S, Cullen K, Day H, Willis G, et al. Tobacco product use and cessation indicators among adults—United States, 2018. MMWR Morb Mortal Wkly Rep. 2019;68:1013–9.
Leventhal AM, Waters AJ, Moolchan ET, Heishman SJ, Pickworth WB. A quantitative analysis of subjective, cognitive, and physiological manifestations of the acute tobacco abstinence syndrome. Addict Behav. 2010;35:1120–30.
Powell JH, Pickering AD, Dawkins L, West R, Powell JF. Cognitive and psychological correlates of smoking abstinence, and predictors of successful cessation. Addict Behav. 2004;29:1407–26.
Ashare RL, Falcone M, Lerman C. Cognitive function during nicotine withdrawal: Implications for nicotine dependence treatment. Neuropharmacology 2014;76:581–91.
Patterson F, Jepson C, Loughead J, Perkins K, Strasser AA, Siegel S, et al. Working memory deficits predict short-term smoking resumption following brief abstinence. Drug Alcohol Depend. 2010;106:61–4.
Valentine G, Sofuoglu M. Cognitive effects of nicotine: recent progress. Curr Neuropharmacol. 2018;16:403–14.
Conti AA, McLean L, Tolomeo S, Steele JD, Baldacchino A. Chronic tobacco smoking and neuropsychological impairments: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2019;96:143–54.
Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–7.
Friedman NP, Robbins TW. The role of prefrontal cortex in cognitive control and executive function. Neuropsychopharmacology 2022;47:72–89.
Badre D, Nee DE. Frontal cortex and the hierarchical control of behavior. Trends Cogn Sci. 2018;22:170–88.
Jasinska AJ, Zorick T, Brody AL, Stein EA. Dual role of nicotine in addiction and cognition: a review of neuroimaging studies in humans. Neuropharmacology. 2014;84:111–22.
Picciotto MR, Zoli M, Rimondini R, Léna C, Marubio LM, Pich EM, et al. Acetylcholine receptors containing the β2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173.
Pontieri FE, Tanda G, Orzi F, Di Chiara G. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature 1996;382:255–7.
Imperato A, Mulas A, Di Chiara G. Nicotine preferentially stimulates dopamine release in the limbic system of freely moving rats. Eur J Pharm. 1986;132:337–8.
Wills L, Kenny PJ. Addiction-related neuroadaptations following chronic nicotine exposure. J Neurochem. 2021;157:1652–73.
Picciotto MR, Addy NA, Mineur YS, Brunzell DH. It is not “either/or”: Activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol. 2008;84:329–42.
Picciotto MR, Higley MJ, Mineur YS. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron. 2012;76:116–29.
Nees F. The nicotinic cholinergic system function in the human brain. Neuropharmacology. 2015;96:289–301.
Mukhin AG, Kimes AS, Chefer SI, Matochik JA, Contoreggi CS, Horti AG, et al. Greater nicotinic acetylcholine receptor density in smokers than in nonsmokers: a PET study with 2-18F-FA-85380. J Nucl Med. 2008;49:1628–35.
Staley JK, Krishnan-Sarin S, Cosgrove KP, Krantzler E, Frohlich E, Perry E, et al. Human tobacco smokers in early abstinence have higher levels of beta2* nicotinic acetylcholine receptors than nonsmokers. J Neurosci. 2006;26:8707.
Cosgrove KP, Batis J, Bois F, Maciejewski PK, Esterlis I, Kloczynski T, et al. β2-nicotinic acetylcholine receptor availability during acute and prolonged abstinence from tobacco smoking. JAMA Psychiatry. 2009;66:666–76.
Wüllner U, Gündisch D, Herzog H, Minnerop M, Joe A, Warnecke M, et al. Smoking upregulates α4β2* nicotinic acetylcholine receptors in the human brain. Neurosci Lett. 2008;430:34–7.
Mamede M, Ishizu K, Ueda M, Mukai T, Iida Y, Kawashima H, et al. Temporal change in human nicotinic acetylcholine receptor after smoking cessation: 5IA SPECT study. J Nucl Med. 2007;48:1829–35.
Brody AL, Mukhin AG, Mamoun MS, Luu T, Neary M, Liang L, et al. Brain nicotinic acetylcholine receptor availability and response to smoking cessation treatment: a randomized trial. JAMA Psychiatry. 2014;71:797–805.
Brody AL, Mukhin AG, Stephanie S, Mamoun MS, Kozman M, Phuong J, et al. Treatment for Tobacco Dependence: Effect on Brain Nicotinic Acetylcholine Receptor Density. Neuropsychopharmacology. 2013;38:1548–56.
Hillmer AT, Esterlis I, Gallezot JD, Bois F, Zheng MQ, Nabulsi N, et al. Imaging of cerebral α4β2* nicotinic acetylcholine receptors with (−)-[18F]Flubatine PET: Implementation of bolus plus constant infusion and sensitivity to acetylcholine in human brain. Neuroimage. 2016;141:71–80.
Esterlis I, Hannestad JO, Bois F, Sewell RA, Tyndale RF, Seibyl JP, et al. Imaging changes in synaptic acetylcholine availability in living human subjects. J Nucl Med. 2013;54:78–82.
Gallezot J-D, Esterlis I, Bois F, Zheng M-Q, Lin S-F, Kloczynski T, et al. Evaluation of the sensitivity of the novel α4β2* nicotinic acetylcholine receptor PET radioligand 18F-(-)-NCFHEB to increases in synaptic acetylcholine levels in rhesus monkeys. Synapse. 2014;68:556–64.
Sandberg A, Sköld CM, Grunewald J, Eklund A, Wheelock ÅM. Assessing recent smoking status by measuring exhaled carbon monoxide levels. PLoS One. 2011;6:e28864–e28864.
Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The fagerstrom test for nicotine dependence: a revision of the fagerstrom tolerance questionnaire. Br J Addict. 1991;86:1119–27.
Fagerström K. Determinants of tobacco use and renaming the FTND to the fagerström test for cigarette dependence. Nicotine Tob Res. 2012;14:75–78.
Fiore MC, Wetter DW, Bailey WC, Bennett G, Cohen SJ, Dorfman SF, et al. The agency for health care policy and research smoking cessation clinical practice guideline. JAMA 1996;275:1270–80.
Computerized Cognitive Assessment. Clinical Trials: CogState Ltd.
Bois F, Gallezot JD, Zheng MQ, Lin SF, Esterlis I, Cosgrove KP, et al. Evaluation of [(18)F]-(-)-norchlorofluorohomoepibatidine ([(18)F]-(-)-NCFHEB) as a PET radioligand to image the nicotinic acetylcholine receptors in non-human primates. Nucl Med Biol. 2015;42:570–7.
Carson RE, Barker WC, Jeih-San L, Johnson CA. Design of a motion-compensation OSEM list-mode algorithm for resolution-recovery reconstruction for the HRRT. 2003 IEEE Nucl Sci Symp Conf Rec (IEEE Cat No03CH37515). 2003;3285:3281–5.
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89.
Lassen NA. Neuroreceptor quantitation in vivo by the steady-state principle using constant infusion or bolus injection of radioactive tracers. J Cereb Blood Flow Metab. 1992;12:709–16.
Carson RE, Channing MA, Blasberg RG, Dunn BB, Cohen RM, Rice KC, et al. Comparison of bolus and infusion methods for receptor quantitation: application to [18F]cyclofoxy and positron emission tomography. J Cereb Blood Flow Metab. 1993;13:24–42.
Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–9.
Hillmer AT, Carson RE. Quantification of PET infusion studies without true equilibrium: A tissue clearance correction. J Cereb Blood Flow Metab. 2019;40:860–74.
Brody AL, Mandelkern MA, London ED, Olmstead RE, Farahi J, Scheibal D, et al. Cigarette smoking saturates brain α4β2 nicotinic acetylcholine receptors. JAMA Psychiatry. 2006;63:907–14.
Bhatt S, Hillmer AT, Nabulsi N, Matuskey D, Lim K, Lin S-F, et al. Evaluation of (-)-[18F]Flubatine-specific binding: Implications for reference region approaches. Synapse. 2018;72:e22016.
Sabri O, Becker G-A, Meyer PM, Hesse S, Wilke S, Graef S, et al. First-in-human PET quantification study of cerebral α4β2* nicotinic acetylcholine receptors using the novel specific radioligand (−)-[18F]Flubatine. Neuroimage. 2015;118:199–208.
Shotbolt P, Tziortzi AC, Searle GE, Colasanti A, van der Aart J, Abanades S, et al. Within-subject comparison of [11C]-(+)-PHNO and [11C]raclopride sensitivity to acute amphetamine challenge in healthy humans. J Cereb Blood Flow Metab 2011;32:127–36.
Narendran R, Frankle WG, Mason NS, Rabiner EA, Gunn RN, Searle GE, et al. Positron emission tomography imaging of amphetamine-induced dopamine release in the human cortex: A comparative evaluation of the high affinity dopamine D2/3 radiotracers [11C]FLB 457 and [11C]fallypride. Synapse. 2009;63:447–61.
Smart K, Naganawa M, Baldassarri SR, Nabulsi N, Ropchan J, Najafzadeh S, et al. PET imaging estimates of regional acetylcholine concentration variation in living human brain. Cereb Cortex. 2021;31:2787–98.
Naganawa M, Nabulsi N, Henry S, Matuskey D, Lin S-F, Slieker L, et al. First-in-human assessment of (11)C-LSN3172176, an M1 muscarinic acetylcholine receptor PET radiotracer. J Nucl Med. 2021;62:553–60.
Hughes JR, Gulliver SB, Fenwick JW, Valliere WA, Cruser K, Pepper S, et al. Smoking cessation among self-quitters. Health Psychol. 1992;11:331–4.
Petrides M, Alivisatos B, Meyer E, Evans AC. Functional activation of the human frontal cortex during the performance of verbal working memory tasks. Proc Natl Acad Sci USA. 1993;90:878.
Lara AH, Wallis JD. The role of prefrontal cortex in working memory: a mini review. Front Syst Neurosci. 2015;9:173.
Levin ED. Complex relationships of nicotinic receptor actions and cognitive functions. Biochem Pharm. 2013;86:1145–52.
Koukouli F, Changeux J-P. Do nicotinic receptors modulate high-order cognitive processing? Trends Neurosci. 2020;43:550–64.
Ashare RL, Ray R, Lerman C, Strasser AA. Cognitive effects of the acetylcholinesterase inhibitor, donepezil, in healthy, non-treatment seeking smokers: a pilot feasibility study. Drug Alcohol Depend. 2012;126:263–7.
Patterson F, Jepson C, Strasser AA, Loughead J, Perkins KA, Gur RC, et al. Varenicline improves mood and cognition during smoking abstinence. Biol Psychiatry. 2009;65:144–9.
Chamberlain SR, Odlaug BL, Schreiber LR, Grant JE. Association between tobacco smoking and cognitive functioning in young adults. Am J Addict. 2012;21Suppl 1:S14–19.
Durazzo TC, Meyerhoff DJ, Nixon SJ. A comprehensive assessment of neurocognition in middle-aged chronic cigarette smokers. Drug Alcohol Depend. 2012;122:105–11.
Piper ME, Schlam TR, Cook JW, Sheffer MA, Smith SS, Loh W-Y, et al. Tobacco withdrawal components and their relations with cessation success. Psychopharmacol (Berl). 2011;216:569–78.
Bohnen NI, Grothe MJ, Ray NJ, Müller MLTM, Teipel SJ. Recent advances in cholinergic imaging and cognitive decline—revisiting the cholinergic hypothesis of dementia. Curr Geriatr Rep. 2018;7:1–11.
Acknowledgements
We thank the Yale PET Centre and research assistants Elizabeth Yanac and Ryan Cool for scan management and MRI analysis. We thank Dr. Nii Addy, Dr. Richard Carson, and Dr. Stephanie O’Malley for their insightful scientific contributions.
Funding
This work was supported by the National Institute on Drug Abuse (Grants R01 DA038832, K02 DA031750) and the National Institute on Alcohol Abuse and Alcoholism (Grant K01 AA024788). KCC and SRB were also supported by the National Institute of Neurological Disorders and Stroke (Grant T32 NS041228) and the National Institute on Drug Abuse (Grant K23 DA045957), respectively.
Author information
Authors and Affiliations
Contributions
KPC designed the study. KCC, ATH, JA, BL, SRB, GAA, DM, and KPC supervised recruitment and participation of human volunteers. SRB, GAA, and DM were study physicians. MK, MZ, and YH were study radiochemists. ATH performed image analysis. KCC analyzed clinical data and performed statistical analyses of imaging and clinical data. KCC and KPC drafted the initial manuscript. All authors contributed to editing this article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Calakos, K.C., Hillmer, A.T., Anderson, J.M. et al. Cholinergic system adaptations are associated with cognitive function in people recently abstinent from smoking: a (-)-[18F]flubatine PET study. Neuropsychopharmacol. 48, 683–689 (2023). https://doi.org/10.1038/s41386-023-01535-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-023-01535-1