Abstract
Chronic exposure to Δ-9-tetrahydrocannabinol (THC) during adolescence is associated with long-lasting cognitive impairments and enhanced susceptibility to anxiety and mood disorders. Previous evidence has revealed functional and anatomical dissociations between the posterior vs. anterior portions of the hippocampal formation, which are classified as the dorsal and ventral regions in rodents, respectively. Notably, the dorsal hippocampus is critical for cognitive and contextual processing, whereas the ventral region is critical for affective and emotional processing. While adolescent THC exposure can induce significant morphological disturbances and glutamatergic signaling abnormalities in the hippocampus, it is not currently understood how the dorsal vs. ventral hippocampal regions are affected by THC during neurodevelopment. In the present study, we used an integrative combination of behavioral, molecular, and neural assays in a neurodevelopmental rodent model of adolescent THC exposure. We report that adolescent THC exposure induces long-lasting memory deficits and anxiety like-behaviors concomitant with a wide range of differential molecular and neuronal abnormalities in dorsal vs. ventral hippocampal regions. In addition, using matrix-assisted laser desorption/ionization imaging mass spectrometry (MALDI-IMS), we show for the first time that adolescent THC exposure induces significant and enduring dysregulation of GABA and glutamate levels in dorsal vs. ventral hippocampus. Finally, adolescent THC exposure induced dissociable dysregulations of hippocampal glutamatergic signaling, characterized by differential glutamatergic receptor expression markers, profound alterations in pyramidal neuronal activity and associated oscillatory patterns in dorsal vs. ventral hippocampal subregions.
Similar content being viewed by others
Introduction
Clinical and preclinical evidence has demonstrated that adolescent cannabis consumption can be associated with long-term cognitive impairments and increase vulnerability for schizophrenia, anxiety and mood disorders, in some individuals [1,2,3,4].
Δ-9-tetrahydrocannabinol (THC), the main psychoactive component of marijuana, acts as a partial CB1 receptor (CB1R) agonist, exerting a profound impact on mammalian brain maturation. Given that CB1Rs critically modulate neuronal excitability and synaptic communication via multiple complex signaling mechanisms, dynamically increasing density of CB1Rs in the prefrontal cortex (PFC), hippocampus (Hipp) and striatum during adolescent neurodevelopment [5], underscores the vulnerability of these corticolimbic circuits to cannabis-induced developmental disturbances. Indeed, considerable evidence indicated that chronic THC exposure can induce profound morphological abnormalities in the Hipp similar to those observed in schizophrenia patients, such as a reduction of gray matter, volumetric changes [6, 7], and impairments in axonal connectivity [8]. In addition, preclinical studies demonstrated long-lasting dysregulation in various hippocampal neurotransmitter signaling and protein expression patterns [9,10,11,12], along with astrocytic activation and pro-inflammatory states [11] following adolescent THC exposure.
While aberrations in hippocampal functions have been primarily associated with memory impairments, increasing attention has been focused on the functional dissociation between the posterior and anterior hippocampus [13, 14]. These two regions, identified as dorsal hippocampus (dHipp) and ventral hippocampus (vHipp) in rodents, are preferentially linked to cognitive memory-related processing vs. affective processing, respectively [15, 16]. In fact, several studies demonstrated that lesions of dHipp, but not of vHipp, impair spatial learning performance [17,18,19,20,21], whereas lesions of vHipp were associated with impaired conditioned freezing and anxiety-related behaviors [22,23,24,25].
These functional dissociations may reflect their differences in anatomical and neural connectivity patterns as well as distinctive functional gene expression profiles [16, 26]. Moreover, electrophysiological studies demonstrate higher concentrations of ‘place cells’ in dHipp vs. vHipp, supporting its specialized role in spatial learning and playing a key role in memory and navigation processing [27]. In contrast, the vHipp is more strongly connected with structures mediating fear and anxiety behaviors [15, 16, 22], like the amygdala, bed nucleus of the stria terminalis (BNST) and the hypothalamic-pituitary-adrenal (HPA) axis.
Pathological dysregulation of either the dHipp or vHipp is associated with the emergence of schizophrenia-like symptoms, such as impairments in prepulse inhibition (PPI) and reduced latent inhibition (LI) [28,29,30], as well as alterations in extracellular dopamine (DA) levels in the nucleus accumbens (NAc) [31]. In addition, acute hyperstimulation of vHipp CB1Rs has been shown to disrupt social and anxiety-related behaviors, dysregulate reward-related processing, and alter DA and non-DAergical neural activity patterns in mesocorticolimbic pathways [32, 33], all of which are core schizophrenia-related endophenotypes. We have previously reported that acute intra-vHipp THC can induce profound dysregulation of mesolimbic activity states and induce behavioral phenotypes resembling core schizophrenia-related affective and cognitive symptoms [32]. However, the effects of neurodevelopmental THC exposure on these hippocampal phenomena are not well understood.
Using an established rodent model of adolescent THC exposure, we investigated how chronic cannabinoid exposure during adolescence may lead to acute and/or long-term pathophysiology in the hippocampal formation, and whether these effects may differentially affect dorsal vs. ventral hippocampal regions. We used an integrative combination of behavioral assays for anxiety and memory processing, in vivo electrophysiology, MALDI-IMS and molecular protein expression analyses. We report that adolescent THC exposure strongly impairs cognitive and affective processing. Remarkably, we found substantial and significant differences in terms of how adolescent THC exposure impacts dorsal vs. ventral hippocampal regions, revealing important new neuropathological mechanisms that may differentially underlie cognitive vs. affective symptoms linked to cannabinoid-induced neurodevelopmental pathologies.
Materials and methods
Animals and housing
Male Sprague-Dawley rats were obtained at postnatal day (PND) 28 from Charles River Laboratories (Quebec, Canada). Rats were pair-housed in controlled conditions (constant temperature and humidity, 12 h light/dark cycle) with free access to food and water. All procedures and protocols were approved by appropriate Governmental and Institutional guidelines.
Adolescent THC exposure protocol
THC (Cayman Chemical) was dissolved in ethanol, cremophor, and saline (1:1:18). Ethanol was evaporated using nitrogen gas to remove it from the final THC solution. Our adolescent THC exposure protocol has been previously shown to induce profound pathological phenotypes during adolescent neurodevelopment [34,35,36]. Rats were treated twice daily from postnatal day (PND) 35 to 45 with escalating doses of THC (2.5 mg/kg; PND 35-37; 5 mg/kg; PND 38-41; 10 mg/kg, PND 42-45) or vehicle. This increasing dosing schedule was chosen to overcome the potential development of tolerance to THC. Experimental procedures started after a 30-day-drug free washout period (PND 75). A subset of rats’ brains for matrix-assisted laser desorption/ionization imaging mass spectrometry (MALDI-IMS) was collected at PND 45, 2 h after the last THC injection. See Fig. 1a for a schematic representation of the experimental procedures.
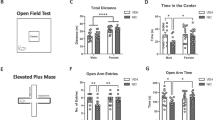
a Experimental timeline including the number of subjects used for each procedure. b Schematic representation of light–dark box apparatus for anxiety test. c Adolescent THC-treated rats re-emerged later from the dark environment than vehicle group (n = 16 for both groups), while the time spent in the light and dark environments (d, e) as well as the number of entries in both sides (f, g) were similar between vehicle- and THC-treated rats. h Representative open-field activity traces for vehicle- and THC-exposed rats. i–l Both groups (veh vs. THC: n = 16 vs. 14) exhibited larger locomotor activity and higher rearing behavior in the outer zone vs. the inner zone of the OF arena at any of the timepoints examined, however in the first 5 min of the test, THC-treated rats spent less time in the inner zone of the arena (m) and did less transitions between inner vs. outer zones (n) compared to their vehicle counterparts. o Schematic protocol representation for object location test. p THC-treated rats exhibited spatial memory impairments after 5-h delay but not after 1 h delay, when compared to their vehicle counterparts (n = 16 for both groups). q Schematic protocol representation for object in context test. r Adolescent THC exposure altered rats’ ability to recognize a familiar object in a novel context (veh vs. THC: n = 14 vs. 13). Mann–Whitney U test, Student’s t test, two-way or three-way ANOVAs, *P < 0.05, **P < 0.01, ***P < 0.001.
Behavioral tasks
Open field (OF)
OF apparatus was used to assess locomotion and anxiety. The test details are described in Supplementary Materials.
Light–Dark box (LD)
LD test measures anxiety level, based upon the innate aversion of rodents to brightly illuminated environments. The test was carried out as previously described [34, 36]. See Supplementary Materials for details.
Object-location (OL)
OL is a validated memory task to assess spatial memory and discrimination. See Supplementary Materials for details.
Object-in-context (OIC)
OIC test is used to assess rodents’ ability to recognize a familiar object in a novel context. See Supplementary Materials for details.
Western blots
Rats received an overdose of sodium pentobarbital (240 mg/kg, i.p., EuthanylTM) and brains were removed and flash frozen. The methods used to carry out protein analyses in dHipp/vHipp are described in detail in Supplementary Materials.
In vivo electrophysiology
In vivo single-unit recordings of putative dHipp/vHipp glutamatergic neurons were performed on urethane anesthetized (1.4 g/kg, i.p.) rats. The methods used to perform in vivo electrophysiology are described in detail in Supplementary Materials.
Matrix-assisted laser desorption/ionization imaging mass spectrometry (MALDI-IMS)
Brains from vehicle and THC-treated rats at PND 45 and PND 75 were randomly paired, and relative quantification of GABA and glutamate levels were examined. The methods used to perform MALDI-IMS are based on previously published paper [37], and described in detail in Supplementary Materials.
Statistical analysis
Experimental data are presented as mean ± SEM. Statistical analyses were performed using GraphPad Prism 9 (San Diego, CA, USA). All datasets were tested for outliers. In vivo electrophysiology and behavioral datasets were tested for normality using D’Agostino–Pearson normality test (significance level set to P < 0.05). Western blots and MALDI-IMS datasets were tested for normality using Kolmogorov–Smirnov normality tests (significance level set to P < 0.05). Comparisons between groups were determined using two-way ANOVA or three-way ANOVA followed by Fisher’s least significant difference (LSD) test. Mann–Whitney U test or Student’s t-test were used for non-normally or normally distributed samples, respectively. MALDI-IMS data were analyzed using one-sample t-test. The significance level was established at P < 0.05. Effect sizes are reported using Cohen’s d test or partial eta squared, where appropriate.
Results
Adolescent THC exposure induces long-lasting anxiety-like phenotypes
THC exposure during adolescence can lead to affective dysregulation, including increased anxiety [34]. Therefore, we first evaluated anxiety-like behaviors using the LD test. Statistical analysis revealed that THC-exposed rats showed a longer latency to re-emerge from the dark to the light environment compared to vehicle controls (U = 81.50; P = 0.0406; d = 0.75; Fig. 1c). No differences between groups were observed in the time spent in the light (t(30) = 0.88; P = 0.3858; Fig. 1d) or dark (t(30) = 0.88; P = 0.3863; Fig. 1e) environments, as well as in the number of entries (light side: t(30) = 1.08; P = 0.2900; Fig. 1f; dark side: t(30) = 1.38; P = 0.1784; Fig. 1g). We next examined motor activity and thigmotaxis index in the OF test. Three-way ANOVA repeated measures analysis of the distance traveled in the apparatus revealed significant effects of zone (F(1,28) = 186; P < 0.0001; ηp2 = 0.869; Fig. 1i), time (F(2,56) = 46.57; P < 0.0001; ηp2 = 0.625; Fig. 1i) and interaction zone x time (F(2,56) = 4.04; P = 0.0229; ηp2 = 0.126; Fig. 1i). Post hoc comparisons demonstrated that both vehicle and THC-treated rats had greater locomotory activity in the outer zone compared to the inner zone of the arena at all the time points examined (P < 0.0001 for all; Fig. 1i), while no differences were observed between groups (veh vs. THC: P > 0.05 for all zones and time points; Fig. 1I). Accordingly, three-way ANOVA analysis of rearing behavior revealed a main effect of zone (F(1,28) = 169.6; P < 0.0001; ηp2 = 0.858; Fig. 1l), time (F(2,56) = 107.1; P < 0.0001; ηp2 = 0.793; Fig. 1l) and their interaction (zone x time: F(2,56) = 11.91; P < 0.0001; ηp2 = 0.298; Fig. 1l). Post hoc analyses revealed that both groups showed a higher rearing behavior in the outer zone of the arena vs. the inner zone at each time point examined (P < 0.0001 for all; Fig. 1l). No differences were found between vehicle and THC-treated rats (P > 0.05 for all zones and time points; Fig. 1l). Notably, time-specific thigmotaxis behaviors (i.e., wall-hugging) were observed in THC-treated rats. Statistical analysis of the time spent in the inner zone of the open field arena revealed a significant main effect of time (F(2,56) = 27.96; P < 0.0001; ηp2 = 0.500; two-way ANOVA; Fig. 1m) and treatment (F(1,28) = 4.40; P = 0451; ηp2 = 0.136; two-way ANOVA; Fig. 1m). Post hoc comparisons revealed that the THC-treated rats spent significantly less time in the inner zone of the arena compared their vehicle counterpart only in the first 5 min of the test (P = 0.0096; Fig. 1m). In addition, analysis of the number of entries in the inner vs. outer zones of the arena revealed a main effect of time (F(2,56) = 39.61; P < 0.0001; ηp2 = 0.586; three-way ANOVA; Fig. 1n) and interaction of time x treatment (F(2,56) = 3.75; P = 0.0296; ηp2 = 0.118; three-way ANOVA; Fig. 1n). Post hoc comparisons demonstrated the THC-treated rats did significantly less transitions compared to their vehicle counterparts exclusively on the first 5 min of the test (veh vs. THC entries in inner: P = 0.0066; veh vs. THC entries in outer: P = 0.0069; Fig. 1n).
THC exposure during adolescence induces long-term memory impairments
Chronic THC is linked to lasting memory deficits and alterations in hippocampal function [38]. Thus, using the OL memory task we investigated the effects of adolescent THC exposure on spatial memory after 1-h and 5-h-delay. Two-way ANOVA revealed a significant effect of adolescent THC exposure (F(1,60) = 6.26; P = 0.0151; ηp2 = 0.095; Fig. 1p). Post hoc analyses revealed that THC-exposed rats exhibited significant impairments in the recognition of the object in the novel location after 5 h compared to their vehicle counterparts (P = 0.0201; Fig. 1p). We next assessed rats’ ability to associate an object with the context where it had been previously encountered. Adolescent THC exposure strongly reduced the object/context discrimination index compared to vehicle counterparts (t(25) = 3.05; P = 0.0054; d = 1.18; Fig. 1r).
Adolescent THC exposure induces long-lasting molecular changes in dHipp and vHipp
We next investigated protein expression levels in dHipp and vHipp of selected molecular biomarkers known to be associated with neurodevelopmental THC exposure and neuropsychiatric disorders. Western blot analysis in dHipp revealed that THC exposure during adolescence induced a significant decrease in NMDA2BR (t(12) = 2.29; P = 0.0406; d = 1.23; Fig. 2a) and NMDA2AR (t(12) = 3.27; P = 0.0067; d = 1.75; Fig. 2a), while no difference were observed in the ratio of NMDA2AR/NMDA2BR (t(12) = 2.02; P = 0.0666; Fig. 2a). No significant changes were observed in mGluR2/3 (t(11) = 0.71; P = 0.4930; Fig. 2b), GAD67 (t(12) = 2.01; P = 0.0672; Fig. 2c), dopamine receptors (D1R: t(12) = 0.25; P = 0.8082; D2R: t(12) = 0.03; P = 0.9756; Fig. 2d, e) or mTOR levels (p-mTOR: U = 12; P = 0.1282; t-mTOR: t(12) = 0.67; P = 0.5157; p-mTOR/t-mTOR: t(12) = 1.40; P = 0.1876; Fig. 2f). In contrast, analysis in vHipp revealed that adolescent THC exposure significantly increased NMDA2BR (t(13) = 2.17; P = 0.0495; d = 1.13; Fig. 2g) and mGluR2/3 (t(14) = 2.16; P = 0.0488; d = 1.15; Fig. 2h), but did not alter NMDA2AR (t(12) = 1.66; P = 0.1228; Fig. 2g) or the ratio of NMDA2AR/NMDA2BR (t(12) = 1.75; P = 0.1063; Fig. 2g). No differences were observed in GAD67 (t(16) = 0.55; P = 0.5872; Fig. 2i), dopamine receptor expression levels (D1R: U = 11; P = 0.3434; D2R: t(9) = 0.23; P = 0.8262 Fig. 2l, m) or mTOR expression levels (p-mTOR: t(10) = 0.81; P = 0.4376; t-mTOR: t(10) = 1.15; P = 0.2752; p-mTOR/t-mTOR: t(10) = 0.14; P = 0.8910; Fig. 2n).
a–f, insets on the left side of the bar graphs, Representative western blots for NMDA2AR, NMDA2BR, mGluR2/3, GAD67, D1R, D2R and mTOR in dHipp. a, right, Densitometry analysis revealed a reduction in NMDA2AR and NMDA2BR levels induced by adolescent THC exposure, but not in their ratio of NMDA2A/NMDA2B (n = 7 for both groups). b–f, right, No differences were observed between groups in mGluR2/3 (veh vs. THC: n = 6 vs. 7), GAD67 (n = 7 for both groups), D1R (n = 7 for both groups), D2R (n = 7 for both groups) and mTOR (n = 7 for both groups) expression. g–n, insets on the left side of the bar graphs, Representative western blots for NMDA2AR, NMDA2BR, mGluR2/3, GAD67, D1R, D2R and mTOR in vHipp (left). g, h, right, Adolescent THC treatment induced a long-lasting increase in NMDA2BR (veh vs. THC: n = 7 vs. 8) and mGluR2/3 (veh vs. THC: n = 7 vs. 9) levels, but not in NMDA2AR or in the ratio of NMDA2A/NMDA2B (veh vs. THC: n = 6 vs. 8 for both). i–n, right, Chronic THC treatment did not affect GAD67 (n = 9 for both groups), D1R (veh vs. THC: n = 5 vs. 7), D2R (veh vs. THC: n = 6 vs. 5) and mTOR (n = 6 for both groups) expression. Mann–Whitney U test or Student’s t test, *P < 0.05, **P < 0.01.
THC exposure during adolescence differentially disrupts neuronal activity patterns in dHipp vs. vHipp
Dysregulation of intra-Hipp inhibitory/excitatory signaling substrates have been previously reported in schizophrenia [39, 40]. Thus, we investigated the long-term effects of adolescent THC exposure on spontaneous pyramidal neural activity in both, dHipp and vHipp. Statistical analysis revealed that THC-treated rats exhibited a significant decrease in the firing frequency of dHipp glutamatergic cells (U = 765; P = 0.0069; d = 0.65; Fig. 3b). Moreover, examination of bursting cells pointed out a THC-induced reduction in bursting rate (U = 405.5; P = 0.0341; d = 0.53; Fig. 3c). On the other hand, no changes were observed in vHipp firing frequency (U = 1354; P = 0.3040; Fig. 3e) or bursting rate (U = 305; P = 0.1641; Fig. 3f).
a Traces and rate histograms of representative dHipp glutamatergic cells recorded from vehicle and THC group. b, c THC-treated rats showed a reduction in the firing rate (veh vs. THC: n = 41 cells/8 rats vs. 55 cells/13 rats) and bursting activity (veh vs. THC: n = 33 cells/8 rats vs. 35 cells/13 rats) in of pyramidal neurons in dHipp. d Traces and rate histograms of representative vHipp glutamatergic cells recorded from vehicle and THC group. e, f No differences were observed between groups in the firing rate (veh vs. THC: n = 45 cells/9 rats vs. 68 cells/9 rats) and bursting activity (veh vs. THC: n = 23 cells/9 rats vs. 34 cells/9 rats) of pyramidal neurons in vHipp. Mann–Whitney U test, *P < 0.05, **P < 0.01.
Subsequently, we investigated dHipp and vHipp oscillation patterns. LFP analyses revealed that adolescent THC exposure induced an increase in theta power in vHipp (t(40) = 2.28; P = 0.0282; d = 0.88; Fig. 4g) but not in dHipp (U = 468; P = 0.7678; Fig. 4c). Moreover, THC-treated rats exhibited higher beta power in dHipp (t(61) = 2.16; P = 0.0343; d = 0.56; Fig. 4d), while it was decreased in vHipp (U = 88; P = 0.0174; d = 0.98; Fig. 4h). No differences between groups were observed in the other oscillatory bands examined in this study (data not shown).
a Representative spectrogram of a 5-min recording in dHipp. b Average normalized LFP power spectra in dHipp of vehicle and THC groups. c, d THC exposure during adolescence did not affect the theta waves in dHipp, while it significantly enhanced the beta oscillations (veh vs. THC: n = 28 recording site/5 rats vs. 35 cells/9 rats). e Representative spectrogram of a 5-min recording in vHipp. f Average normalized LFP power spectra in vHipp of vehicle and THC groups. g, h The THC-treated group exhibited an increase in theta oscillations and a decrease in beta oscillation (veh vs. THC: n = 11 recording site/4 rats vs. 31 cells/4 rats). Student’s t test or Mann–Whitney U test, *P < 0.05.
Chronic adolescent THC profoundly alters neurotransmitter levels in dHipp and vHipp
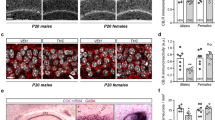
Given our above-described results showing profound alterations in glutamatergic networks, we next performed MALDI-IMS relative quantification and spatial distribution of GABA and glutamate neurotransmitters in dHipp and vHipp at adolescence and adulthood. The results of MALDI-IMS relative quantification in adolescent brains (PND 45) revealed that THC exposure significantly reduced GABA (t(5) = 5.94; P = 0.0019; d = 3.43; Fig. 5b, c) and glutamate (t(5) = 5.08; P = 0.0038; d = 2.93; Fig. 5b, c) levels in dHipp, while only GABA (t(5) = 10.07; P = 0.0002; d = 5.81; Fig. 5d, e), but not glutamate levels (t(5) = 0.31; P = 0.7698; Fig. 5d, e), were decreased in vHipp. Interestingly, decreased dHipp GABA levels persisted into adulthood (PND 75) (t(7) = 4.84; P = 0.0019; d = 2.42; Fig. 5f, g), while other changes observed at PND 45 dissipated over time (dHipp glutamate: t(7) = 1.87; P = 0.1030; Fig. 5f, g; vHipp GABA: (t(7) = 2.08; P = 0.0764; Fig. 5h, i; vHipp glutamate: t(7) = 1.17; P = 0.2788; Fig. 5h, i).
a MALDI-IMS images of representative vehicle and THC sections at PND 45 and 75. Representative ion-mass spectra in dHipp (b, f) and vHipp (d, h) of vehicle and THC groups at PND 45 (b, d) and 75 (f, h) for [GABA + K]+ at m/z 142 (top) and [glutamic acid + K]+ at m/z 186 (bottom). c MALDI-IMS relative quantification revealed a short-term reduction in GABA and glutamate levels (n = 6 for both groups and neurotransmitters) in dHipp of THC-exposed rat. e GABA levels were decreased in vHipp, while no differences were observed in glutamate (n = 6 for both groups and neurotransmitters). g MALDI-IMS relative quantification revealed a long-lasting reduction in GABA, but not glutamate levels (n = 8 for both groups and neurotransmitters), in dHipp of rats exposed to THC during adolescence. i No differences were observed in GABA and glutamate levels (n = 8 for both groups and neurotransmitters) in vHipp. One-sample t-test, *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
Clinical and preclinical evidence has demonstrated that adolescent THC exposure can lead to long-lasting cognitive impairments and psychiatric-like endophenotypes, concomitant with a wide array of neural maladaptations [1,2,3, 34, 35]. The present study examined the effects of adolescent THC exposure on hippocampal-dependent cognitive functions and affective behaviors with specific molecular and electrophysiological measures, targeting distinct subdivisions of the hippocampal formation. We hypothesized that chronic THC exposure during adolescence will induce selective and dissociable abnormalities in the dorsal vs. ventral portions of the hippocampus, which might underlie THC-induced cognitive and emotional processing behaviors respectively associated with these two distinct subregions.
In line with previous findings [34, 36, 41, 42], we observed an enduring increase in anxiety-like behaviors following adolescent THC exposure. Moreover, THC-treated rats exhibited significant impairments in spatial memory and discrimination performance, core features of chronic THC exposure reported in humans and rodents [43,44,45]. Notably, we report that adolescent THC exposure impacts the ability to discriminate the novel location of an object after 5 h, but not after 1 h. Although these results are in contrast with previous studies which reported spatial memory impairments already after a 1-h-delay [9, 10], the differences in THC dosing regimens as well as in OL protocols may account for this discrepancy.
The roles of the hippocampus in spatial and discrimination learning and memory are well established. In particular, lesions of the entire hippocampal formation in rodents were found to selectively impact both object location and context-specific memory processing [46, 47]. In contract, adolescent THC exposure has been associated with long-lasting dysregulation in hippocampal plasticity, neurotransmitter signaling, and neurogenesis [9,10,11,12]. Importantly, THC-induced cognitive impairments observed in the present study were associated with anatomically dissociable abnormalities in the hippocampal formation at the molecular, neuronal and circuit level.
Although the hippocampal formation is generally considered to represent a monolithic memory processing region, emerging evidence has revealed the anatomically heterogeneous nature of the region and specialized functions. Indeed, remarkable differences between the posterior/dorsal and anterior/ventral portions of the hippocampus have been observed [16, 26], with several preclinical investigations highlighting the neural connections of dHipp → cortical pathways being more specialized for learning and memory functions vs. the vHipp → sub-cortical pathways (e.g., mesolimbic, amygdala targets) being more specialized for processing anxiety and affective-related information [15, 17, 18, 21, 22]. Nevertheless, the neurochemical and neuronal mechanisms underlying these hippocampal specializations are not entirely understood.
Such functional and anatomical dissociations in the hippocampus have been largely explored for the purpose of increasing knowledge about the multidimensional nature of neuropsychiatric disorders. Indeed, investigations across the longitudinal axis of the hippocampal formation have revealed that, in an early stage of psychosis, some structural abnormalities firstly occur in the anterior portion of the hippocampus [48], and are negatively correlated with the positive symptoms of schizophrenia [49]. In addition, functional dissociations between these two hippocampal subregions have been reported in spatial memory processing, indicating decreased activity of the posterior hippocampus and hyperactivity of the anterior portion in schizophrenia [14]. Lastly, analysis of the brain proteome in the anterior vs. posterior hippocampus of schizophrenia subjects revealed that molecular abnormalities were more pathological in the anterior subregions, and were associated with dysfunctions in glutamate and GABA neurotransmission [50].
Imbalances between excitatory and inhibitory substrates within the hippocampal formation are commonly reported in clinical and preclinical studies examining schizophrenia-like neuropathology [51,52,53]. Specifically, alterations in the expression of the NMDA2BR and mGluR2/3 are linked to schizophrenia-related phenotypes and affective and cognitive processing abnormalities [54,55,56,57]. In the present study, molecular analyses in the dHipp and vHipp revealed long-term THC-induced dissociable effects in glutamatergic receptor expression patterns. In particular, NMDA2AR and NMDA2BR expression were decreased in dHipp, while NMDA2BR was increased in vHipp, concomitant with a higher expression of mGluR2/3. The glutamatergic system has been found to undergo important changes and rearrangements throughout brain development, with NMDARs playing a crucial role [58]. Notably, the widespread distribution of NMDARs in both hippocampal pyramidal cells and interneurons highlights the complexity of the glutamatergic system and paves the way for various potential mechanisms underlying our results. For example, it has been demonstrated that either pharmacological inactivation of glutamatergic system in dHipp or activation in vHipp leads to disruption of prepulse inhibition, a common schizophrenia-related phenotype [59, 60]. Moreover, dysregulation of NMDARs expression in dHipp and vHipp has been reported following chronic stress exposure [61]. Therefore, it is plausible that adolescent THC exposure induces a pathophysiological development of the glutamatergic system in dHipp/vHipp, leading to abnormal communication patterns between efferent/afferent pathways and pathological affective and cognition phenotypes. In addition, hypo-functionality of NMDARs has been previously associated with memory and learning deficits [62], whereas their overactivity has been related to anxiety-like behaviors [63]. In particular, while intra-dHipp infusions of NMDAR antagonists have been shown to strongly disrupt memory processing [64], they had no effects on anxiety-related behaviors [65], which were instead observed following intra-vHipp administration [65]. These findings are concordant with our results showing a reduction in NMDAR expression in dHipp vs. the increase in vHipp following adolescent THC treatment and associated THC-induced memory impairments and anxiety-like behaviors, respectively.
In contrast to our previously reported findings of adolescent THC-induced dysregulation of excitatory/inhibitory balance in the rodent PFC [35], in the present study, we did not observe any difference in GAD67 expression between vehicle vs. THC-treated rats, in either dHipp or vHipp regions. Although to our knowledge, no study has quantified hippocampal GAD67 levels following adolescent THC exposure, previous investigations in the hippocampus of schizophrenia patients did not reveal any alterations in GAD67 expression [66]. Moreover, electrophysiological analyses in hippocampus demonstrated that THC effects on GABA release are not mediated by changes in GABAA receptors or in GABA uptake [67], suggesting that the role of intra-hippocampal GAD67 expression might be functionally secondary compared to other signaling pathways. Furthermore, while mTOR expression in the PFC was significantly affected by adolescent THC exposure [34], hippocampal mTOR levels were found unaltered. Similar results have been reported in a rodent model of schizophrenia as well as following adolescent stress exposure [68, 69]. In both conditions, changes in hippocampal mTOR expression were not detected or limited to a selective region of the hippocampus. Lastly, in line with previous findings in a neurodevelopmental rodent model of schizophrenia [70], adolescent THC exposure did not alter D1R and D2R expression levels in the hippocampus, suggesting that dopaminergic signaling mechanisms are unlikely to contribute to the behavioral deficits reported in the present study.
Concomitant with decreased NMDAR expression, we also observed that chronic THC exposure caused significant reductions in the firing and bursting activity states of dHipp glutamatergic neurons. Hypoactivity in the posterior hippocampus has been previously observed in schizophrenia patients during a recognition test [14], highlighting the crucial involvement of this structure in processing spatial information. However, the mechanisms underlying region-specific effects of glutamatergic transmission in the hippocampus remain unclear. The progressive downregulation of the glutamatergic system that we observed in the dHipp might result from some neuro-adaptative process during development in response to overactive glutamatergic drive induced by THC during adolescent neurodevelopment. Alternatively, the reduction in firing rate might be linked to astrocytic dysfunction, which has been shown to impact the neural activity states of hippocampal pyramidal cells [71]. Notably, adolescent THC exposure leads to reduced GFAP levels in hippocampus, including dorsal and ventral regions [72], with intra-dHipp GFAP levels selectively associated with spatial memory deficits [73]. Thus, adolescent THC exposure may selectively affect glial cells in dHipp inducing a reduction in pyramidal neuron activity and in turn, lead to the cognitive impairments reported in the present study. Indeed, it has been previously reported that acute administration of cannabinoids attenuates both firing and bursting activity of hippocampal neurons [74] and induces behavioral memory impairments [75].
In direct contrast, adolescent THC exposure did not affect either the firing rate or bursting activity states of pyramidal cells recorded in the vHipp. These findings are inconsistent with previous reports demonstrating hyperactivity of the anterior hippocampus in schizophrenia subjects [14] and observed in the rodent vHipp using the methyazoxymethanol acetate (MAM) neurodevelopmental model of schizophrenia [76]. Nevertheless, a recent study demonstrated that administration of a mGluR2/3 agonist normalized the enhanced vHipp glutamatergic activity induced by MAM [77], pointing out the crucial role of mGluR2/3 dysregulation in the pathophysiology of schizophrenia-related neurodevelopmental disorders. Thus, one possibility is that the THC-induced increase in mGluR2/3 expression levels in vHipp observed in the present study (Fig. 2h), may serve as a developmental compensatory mechanism to restore glutamatergic homeostasis, and preventing excitotoxic increases in hippocampal glutamate signaling. Future studies are required to more fully explore these questions.
Aberrant hippocampal oscillatory states have been previously reported in various neuropsychiatric disorders and following chronic cannabinoid exposure [78, 79]. In the present study, we report for the first time that adolescent THC exposure augmented beta wave activity in dHipp whilst significantly attenuated beta activity in the vHipp. Interestingly, elevated beta oscillation states in schizophrenia subjects have been associated with poorer performance in memory and learning tasks [80], thus alterations of beta oscillatory in dHipp may underlie our observed THC-related memory impairments, highlighting the role of the dHipp in these cognitive functions. In addition, the observed opposing THC-induced alterations in dHipp/vHipp beta oscillations may be related to the concomitant opposing levels of NMDA2BR expression in the dHipp vs. vHipp, respectively, with significantly decreased levels in dHipp vs. increased levels in the vHipp. Indeed, excessive beta wave activity has been reported in patients with anti-NMDAR encephalitis, a neurological disease in which antibodies are produced against NMDARs [81], whereas acute administration of a NMDAR positive allosteric modulator has been reported to decrease beta oscillatory states [82]. Concomitant with the reduction in beta waves, we also reported an increase in theta oscillatory power selectively in vHipp. This region-specific effect might be a result of the theta rhythm spectrum analyzed in our study, as we selectively examined Type-2 theta oscillations (frequencies: 4–7 Hz). Interestingly, investigations in the human hippocampus have demonstrated that theta waves propagate along a posterior-to-anterior axis, with slow theta oscillations, comparable to Type-2 theta oscillations in rodents, being more prevalent in the anterior portion of the hippocampus [83]. In addition, theta oscillations in vHipp are associated with elevated anxiety, and persistent theta wave hyperactivity has been observed following chronic stress exposure [84]. Thus, our hyper-theta wave phenotypes selectively detected in the vHipp may underlie our observed anxiogenic behavioral phenotypes following adolescent THC exposure. Although we have previously reported that adolescent THC exposure induces persistent disruptions in gamma band oscillations in the PFC [36], we did not observe such alterations in the hippocampal formation. The lack of changes in hippocampal GAD67 observed in the present study might potentially explain these results. Indeed, THC exposure during adolescence has been found to significantly decrease the expression levels of GAD67 in PFC, inducing persistent GABAergic hypofunction, which in turn causes an increase in pyramidal firing frequency and abnormalities in gamma oscillations [35]. Nevertheless, it is plausible that our THC-induced hippocampal oscillatory phenotypes are functionally linked to PFC oscillatory dysregulation. In particular, given the important interconnectivity in the vHipp-PFC network, the alterations in vHipp theta waves might exert a control onto the cortical gamma network [85, 86]. Future studies are required to investigate these possibilities.
Using neurotransmitter selective MALDI imaging, we observed short and long-term alterations in the levels of GABA and glutamate in both the dHipp and vHipp of THC-exposed adolescent and adult rats. Remarkably, we observed profound THC-induced neurotransmitter downregulation that occurred in adolescence immediately following THC exposure and that persisted into adulthood, with more pronounced pathophysiological effects observed in the dHipp. Although the mechanisms underlying these effects are not yet clearly elucidated, cannabinoids have been found to acutely inhibit GABA and glutamate release in the hippocampus [87, 88], likely driven by their agonistic activity on CB1 presynaptic receptors and consequent inhibitory modulation of N- and P/Q-type calcium channel [89]. Notably, reductions in dHipp glutamate and vHipp GABA levels were observed immediately after adolescent THC exposure but not later in life, suggesting that these dysregulation patterns may normalize over time. Alternatively, such short-term observations of neurotransmitter alterations may represent an acute effect of residual THC or its metabolites in the system [90], sampled at this particular time point.
In contrast, we observed significant reductions in dHipp GABA concentration persisting into adulthood. Low GABA levels have previously been observed in subjects with cognitive impairments [91], while regulated hippocampal GABA concentrations are required for normal associative learning processing [92]. Thus, the observed selective decreases in dHipp GABA levels may underlie the memory impairments reported in this study. Although these reductions in GABA levels may appear inconsistent with the hypo-glutamatergic states we observed in dHipp, the sustained stimulation of the endocannabinoid system via THC exposure during adolescence may impact the normal homeostatic balance between these inhibitory/excitatory substrates. In addition, MALDI-IMS enables the relative quantification of total neurotransmitter levels, which may reflect not only local release patterns, but also the sum effects of afferent inputs to these regions. Indeed, recent evidence has shown long-range inhibitory projections from PFC to dHipp that can modulate hippocampal activity states [93]. Since we have previously demonstrated that adolescent THC exposure induces profound reductions in PFC GABAergic neurotransmission [35, 94], but does not interfere with hippocampal GAD67 (Fig. 2c,i) one possibility is that our observed THC-induced decreases in dHipp GABA levels may result from dysregulated PFC→dHipp inhibitory control mechanisms, impacting dHipp neural activity states and cognitive performance. Future studies are required to investigate these possibilities.
There are several caveats to the present study. First, our investigations were limited to male rats and thus cannot speak to potential sex differences related to cannabis exposure during adolescence. Second, to precisely control for cannabinoid exposure, THC was administered intraperitoneally which, unlike human cannabis smoking behaviors, may not reflect translational levels of THC exposure experiences in the human context. Third, the dosing regimen used in the present study is designed to mimic heavy marijuana consumption, given that our doses (2.5–5 and 10 mg/kg) are approximately the translational equivalent of one, two or four cannabis cigarettes, respectively [35]. Currently, such a dosing protocol translates to a limited sample population, given that only ~17% of 16 year-old youth reported having consumed cannabis frequently over the past 30 days [95]. Of those, 54% reported using cannabis daily, 20% twice daily, and 11% five or more times a day [95]. Regardless, frequent cannabis use is increasingly common among youth and is associated with a wide range of potentially detrimental outcomes [96]. While these precise experimental administration protocols are important for control in preclinical studies, human cannabis consumption patterns during adolescence may vary widely and have differential impacts on individuals due to pre-existing genetic and/or physiological phenotypes.
In conclusion, we demonstrate that sustained THC exposure during adolescence can cause profound and dissociable pathophysiological impacts on the dorsal vs. ventral regions of the hippocampal formation which may account for the differential impacts on cognitive and affective phenotypes linked to these distinct neural regions. In addition, using MALDI imaging, we report for the first time that adolescent THC exposure can induce both acute and long-term disruptions in GABA and glutamate signaling directly in these hippocampal subregions. Together, these findings have important implications for understanding the respective impacts of neurodevelopmental cannabinoid exposure on hippocampal subregions and identifying several novel molecular and neuronal biomarkers that may lead to long-term neuropsychiatric risks following chronic adolescent cannabis consumption.
References
Andréasson S, Allebeck P, Engström A, Rydberg U. Cannabis and schizophrenia. A longitudinal study of Swedish conscripts. Lancet. 1987;2:1483–6.
Krebs MO, Kebir O, Jay TM. Exposure to cannabinoids can lead to persistent cognitive and psychiatric disorders. Eur J Pain. 2019;23:1225–33.
Murray RM, Englund A, Abi-Dargham A, Lewis DA, Di Forti M, Davies C, et al. Cannabis-associated psychosis: Neural substrate and clinical impact. Neuropharmacology 2017;124:89–104.
Gobbi G, Atkin T, Zytynski T, Wang S, Askari S, Boruff J, et al. Association of cannabis use in adolescence and risk of depression, anxiety, and suicidality in young adulthood: a systematic review and meta-analysis. JAMA Psychiatry 2019;76:426–34.
Mato S, Del Olmo E, Pazos A. Ontogenetic development of cannabinoid receptor expression and signal transduction functionality in the human brain. Eur J Neurosci. 2003;17:1747–54.
Yücel M, Solowij N, Respondek C, Whittle S, Fornito A, Pantelis C, et al. Regional brain abnormalities associated with long-term heavy cannabis use. Arch Gen Psychiatry. 2008;65:694–701.
Cousijn J, Wiers RW, Ridderinkhof KR, Van den Brink W, Veltman DJ, Goudriaan AE. Grey matter alterations associated with cannabis use: Results of a VBM study in heavy cannabis users and healthy controls. Neuroimage. 2012;59:3845–51.
Zalesky A, Solowij N, Yücel M, Lubman DI, Takagi M, Harding IH, et al. Effect of long-term cannabis use on axonal fibre connectivity. Brain. 2012;135:2245–55.
Poulia N, Delis F, Brakatselos C, Polissidis A, Koutmani Y, Kokras N, et al. Detrimental effects of adolescent escalating low-dose Δ9-tetrahydrocannabinol leads to a specific bio-behavioural profile in adult male rats. Br J Pharm. 2021;178:1722–36.
Poulia N, Delis F, Brakatselos C, Lekkas P, Kokras N, Dalla C, et al. Escalating low-dose Δ9-tetrahydrocannabinol exposure during adolescence induces differential behavioral and neurochemical effects in male and female adult rats. Eur J Neurosci. 2020;52:2681–93.
Zamberletti E, Gabaglio M, Grilli M, Prini P, Catanese A, Pittaluga A, et al. Long-term hippocampal glutamate synapse and astrocyte dysfunctions underlying the altered phenotype induced by adolescent THC treatment in male rats. Pharm Res. 2016;111:459–70.
Stringfield SJ, Torregrossa MM. Intravenous self-administration of delta-9-THC in adolescent rats produces long-lasting alterations in behavior and receptor protein expression. Psychopharmacology. 2021;238:305–19.
Avery SN, Rogers BP, Heckers S. Hippocampal network modularity is associated with relational memory dysfunction in schizophrenia. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3:423–32.
Ragland JD, Layher E, Hannula DE, Niendam TA, Lesh TA, Solomon M, et al. Impact of schizophrenia on anterior and posterior hippocampus during memory for complex scenes. NeuroImage Clin. 2017;13:82–88.
Bannerman DM, Rawlins JNP, McHugh SB, Deacon RMJ, Yee BK, Bast T, et al. Regional dissociations within the hippocampus-Memory and anxiety. Neurosci Biobehav Rev. 2004;28:273–83.
Fanselow MS, Dong H-W. Are The Dorsal and Ventral Hippocampus functionally distinct structures? Neuron. 2010;14:7–19.
Moser MB, Moser EI, Forrest E, Andersen P, Morris RGM. Spatial learning with a minislab in the dorsal hippocampus. Proc Natl Acad Sci USA. 1995;92:9697–701.
Moser E, Moser MB, Andersen P. Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. J Neurosci. 1993;13:3916–25.
Pothuizen HHJ, Zhang WN, Jongen-Rêlo AL, Feldon J, Yee BK. Dissociation of function between the dorsal and the ventral hippocampus in spatial learning abilities of the rat: A within-subject, within-task comparison of reference and working spatial memory. Eur J Neurosci. 2004;19:705–12.
Hock BJ, Bunsey MD. Differential effects of dorsal and ventral hippocampal lesions. J Neurosci. 1998;18:7027–32.
Bannerman DM, Good MA, Yee BK, Heupel MJ, Iversen SD, Rawlins JNP. Double dissociation of function within the hippocampus: A comparison of dorsal, ventral, and complete hippocampal cytotoxic lesions. Behav Neurosci. 1999;113:1170–88.
Bannerman DM, Grubb M, Deacon RMJ, Yee BK, Feldon J, Rawlins JNP. Ventral hippocampal lesions affect anxiety but not spatial learning. Behav Brain Res. 2003;139:197–213.
Maren S. Neurotoxic or electrolytic lesions of the ventral subiculum produce deficits in the acquisition and expression of Pavlovian fear conditioning in rats. Behav Neurosci. 1999;113:283–90.
Maren S, Holt WG. Hippocampus and Pavlovian fear conditioning in rats: muscimol infusions into the ventral, but not dorsal, hippocampus impair the acquisition of conditional freezing to an auditory conditional stimulus. Behav Neurosci. 2004;118:97–110.
Kjelstrup KG, Tuvnes FA, Steffenach HA, Murison R, Moser EI, Moser MB. Reduced fear expression after lesions of the ventral hippocampus. Proc Natl Acad Sci USA. 2002;99:10825–30.
Strange BA, Witter MP, Lein ES, Moser EI. Functional organization of the hippocampal longitudinal axis. Nat Rev Neurosci. 2014;15:655–69.
Jung MW, Wiener SI, McNaughton BL. Comparison of spatial firing characteristics of units in dorsal and ventral hippocampus of the rat. J Neurosci. 1994;14:7347–56.
Howland JG, MacKenzie EM, Yim TT, Taepavarapruk P, Phillips AG. Electrical stimulation of the hippocampus disrupts prepulse inhibition in rats: Frequency- and site-dependent effects. Behav Brain Res. 2004;152:187–97.
Pouzet B, Zhang WN, Weiner I, Feldon J, Yee BK. Latent inhibition is spared by N-methyl-D-aspartate (NMDA)-induced ventral hippocampal lesions, but is attenuated following local activation of the ventral hippocampus by intracerebral NMDA infusion. Neuroscience. 2004;124:183–94.
Zhang WN, Bast T, Feldon J. Prepulse inhibition in rats with temporary inhibition/inactivation of ventral or dorsal hippocampus. Pharm Biochem Behav. 2002;73:929–40.
Peleg-Raibstein D, Feldon J. Effects of dorsal and ventral hippocampal NMDA stimulation on nucleus accumbens core and shell dopamine release. Neuropharmacology. 2006;51:947–57.
Hudson R, Renard J, Norris C, Rushlow WJ, Laviolette SR. Cannabidiol counteracts the psychotropic side-effects of Δ-9-tetrahydrocannabinol in the ventral hippocampus through bidirectional control of ERK1-2 phosphorylation. J Neurosci. 2019;39:8762–77.
Loureiro M, Renard J, Zunder J, Laviolette SR. Hippocampal cannabinoid transmission modulates dopamine neuron activity: impact on rewarding memory formation and social interaction. Neuropsychopharmacology. 2015;40:1436–47.
Renard J, Rosen LG, Loureiro M, De Oliveira C, Schmid S, Rushlow WJ, et al. Adolescent cannabinoid exposure induces a persistent sub-cortical hyper-dopaminergic state and associated molecular adaptations in the prefrontal cortex. Cereb Cortex. 2017;27:1297–310.
Renard J, Szkudlarek HJ, Kramar CP, Jobson CEL, Moura K, Rushlow WJ, et al. Adolescent THC exposure causes enduring prefrontal cortical disruption of GABAergic inhibition and dysregulation of sub-cortical dopamine function. Sci Rep. 2017;7:1–14.
De Felice M, Renard J, Hudson R, Szkudlarek HJ, Pereira BJ, Schmid S, et al. L-theanine prevents long-term affective and cognitive side effects of adolescent Δ-9-tetrahydrocannabinol exposure and blocks associated molecular and neuronal abnormalities in the mesocorticolimbic circuitry. J Neurosci. 2021;41:739–50.
Chen C, Laviolette SR, Whitehead SN, Renaud JB, Yeung KKC. Imaging of neurotransmitters and small molecules in brain tissues using laser desorption/ionization mass spectrometry assisted with zinc oxide nanoparticles. J Am Soc Mass Spectrom. 2021;32:1065–79.
Quinn HR, Matsumoto I, Callaghan PD, Long LE, Arnold JC, Gunasekaran N, et al. Adolescent rats find repeated Δ9-THC less aversive than adult rats but display greater residual cognitive deficits and changes in hippocampal protein expression following exposure. Neuropsychopharmacology. 2008;33:1113–26.
Heckers S, Konradi C. Hippocampal neurons in schizophrenia. J Neural Transm. 2002;109:891–905.
Moghaddam B, Javitt D. From revolution to evolution: The glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology. 2012;37:4–15.
De Gregorio D, Dean Conway J, Canul M-L, Posa L, Bambico FR, Gobbi G. Effects of chronic exposure to low-dose delta-9-tetrahydrocannabinol in adolescence and adulthood on serotonin/norepinephrine neurotransmission and emotional behavior. Int J Neuropsychopharmacol. 2020;23:751–61.
Llorente-berzal A, Puighermanal E, Burokas A. Sex-dependent psychoneuroendocrine effects of THC and MDMA in an animal model of adolescent drug consumption. PLoS One. 2013;8:e78386.
Viravathana P, Marr DWM. Differential effects of Δ9-THC on spatial reference and working memory in mice. Psychopharmacology. 2001;157:142–50.
Bhattacharyya S, Schoeler T. The effect of cannabis use on memory function: an update. Subst Abuse Rehabil. 2013;4:11–27.
Noorbakhsh S, Afzali MH, Boers E, Conrod PJ. Cognitive function impairments linked to alcohol and cannabis use during adolescence: a study of gender differences. Front Hum Neurosci. 2020;14:1–11.
Barker GRI, Warburton EC. When is the hippocampus involved in recognition memory? J Neurosci. 2011;31:10721–31.
Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: Memory for objects, places, and contexts. Learn Mem. 2002;9:49–57.
Lieberman JA, Girgis RR, Brucato G, Moore H, Provenzano F, Kegeles L, et al. Hippocampal dysfunction in the pathophysiology of schizophrenia: a selective review and hypothesis for early detection and intervention. Mol Psychiatry. 2018;23:1764–72.
Kalmady SV, Shivakumar V, Arasappa R, Subramaniam A, Gautham S, Venkatasubramanian G, et al. Clinical correlates of hippocampus volume and shape in antipsychotic-naïve schizophrenia. Psychiatry Res Neuroimaging. 2017;263:93–102.
Nesvadeni M, Matsumoto I, Sivagnanasundaram S. Anterior hippocampus in schizophrenia pathogenesis: molecular evidence from a proteome study. Aust N Z J Psychiatry. 2009;43:310–22.
Ma YN, Sun YX, Wang T, Wang H, Zhang Y, Su YA, et al. Subchronic MK-801 treatment during adolescence induces long-term, not permanent, excitatory-inhibitory imbalance in the rat hippocampus. Eur J Pharmacol. 2020;867:172807.
Gao R, Penzes P. Common mechanisms of excitatory and inhibitory imbalance in schizophrenia and autism spectrum disorders. Curr Mol Med. 2015;15:146–67.
Liu Y, Ouyang P, Zheng Y, Mi L, Zhao J, Ning Y, et al. A selective review of the excitatory-inhibitory imbalance in schizophrenia: underlying biology, genetics, microcircuits, and symptoms. Front Cell Dev Biol. 2021;9:1–15.
Dogra S, Conn PJ. Metabotropic glutamate receptors as emerging targets for the treatment of schizophrenia. Mol Pharm. 2022;101:275–85.
Adell A. Brain NMDA receptors in schizophrenia and depression. Biomolecules. 2020;10:244.
Balu DT. The NMDA receptor and schizophrenia: from pathophysiology to treatment. Adv Pharm. 2016;76:351–82.
Hashimoto K, Malchow B, Falkai P, Schmitt A. Glutamate modulators as potential therapeutic drugs in schizophrenia and affective disorders. Eur Arch Psychiatry Clin Neurosci. 2013;263:367–77.
Hsia AY, Malenka RC, Nicoll RA. Development of excitatory circuitry in the hippocampus. J Neurophysiol. 1998;79:2013–24.
Bakshi VP, Geyer MA. Multiple limbic regions mediate the disruption of prepulse inhibition produced in rats by the noncompetitive NMDA antagonist dizocilpine. J Neurosci. 1998;18:8394–401.
Zhang W, Pouzet B, Jongen-Rêlo AL, Weiner I, Feldon J. Disruption of prepulse inhibition following N-methyl-D-aspartate infusion into the ventral hippocampus is antagonized by clozapine but not by haloperidol: A possible model for the screening of atypical antipsychotics. Neuroreport. 1999;10:2533–8.
Pacheco A, Aguayo FI, Aliaga E, Muñoz M, García-Rojo G, Olave FA, et al. Chronic stress triggers expression of immediate early genes and differentially affects the expression of AMPA and NMDA subunits in dorsal and ventral hippocampus of rats. Front Mol Neurosci. 2017;10:244.
Newcomer JW, Farber NB, Jevtovic-Todorovic V, Selke G, Melson AK, Hershey T, et al. Ketamine-induced NMDA receptor hypofunction as a model of memory impairment and psychosis. Neuropsychopharmacology. 1999;20:106–18.
Lorigooini Z, Nasiri boroujeni S, Balali-Dehkordi S, Ebrahimi L, Bijad E, Rahimi-Madiseh M, et al. Possible involvement of NMDA receptor in the anxiolytic-like effect of caffeic acid in mice model of maternal separation stress. Heliyon. 2020;6:e04833.
Jafari-Sabet M. NMDA receptor blockers prevents the facilitatory effects of post-training intra-dorsal hippocampal NMDA and physostigmine on memory retention of passive avoidance learning in rats. Behav Brain Res. 2006;169:120–7.
Nascimento Häckl LP, Carobrez AP. Distinct ventral and dorsal hippocampus AP5 anxiolytic effects revealed in the elevated plus-maze task in rats. Neurobiol Learn Mem. 2007;88:177–85.
Heckers S, Stone D, Walsh J, Shick J, Koul P, Benes FM. Differential hippocampal expression of glutamic acid decarboxylase 65 and 67 messenger RNA in bipolar disorder and schizophrenia. Arch Gen Psychiatry. 2002;59:521–9.
Laaris N, Good CH, Lupica CR. Δ9-tetrahydrocannabinol is a full agonist at CB1 receptors on GABA neuron axon terminals in the hippocampus. Neuropharmacology. 2010;59:121–7.
Suo L, Zhao L, Si J, Liu J, Zhu W, Chai B, et al. Predictable chronic mild stress in adolescence increases resilience in adulthood. Neuropsychopharmacology. 2013;38:1387–1400.
Xie R, Xie J, Ye Y, Wang X, Chen F, Yang L, et al. mTOR expression in hippocampus and prefrontal cortex is downregulated in a rat model of schizophrenia induced by chronic administration of ketamine. J Mol Neurosci. 2020;70:269–75.
Zhao T, Gao X, Huang G-B. Effects of chronic social defeat stress on behavior and dopamine receptors in adolescent mice with 6-hydroxydopamine lesions of the medial prefrontal cortex. Front Behav Neurosci. 2021;15:731373.
Angulo MC, Kozlov AS, Charpak S, Audinat E. Glutamate released from glial cells synchronizes neuronal activity in the hippocampus. J Neurosci. 2004;24:6920–7.
Rubino T, Realini N, Braida D, Guidi S, Capurro V, Guidali C, et al. Changes in hippocampal morphology and neuroplasticity induced by adolescent thc treatment are associated with cognitive impairment in adulthood. Hippocampus. 2009;772:763–72.
Sampedro-Piquero P, De Bartolo P, Petrosini L, Zancada-Menendez C, Arias JL, Begega A. Astrocytic plasticity as a possible mediator of the cognitive improvements after environmental enrichment in aged rats. Neurobiol Learn Mem. 2014;114:16–25.
Goonawardena AV, Riedel G, Hampson RE. Cannabinoids alter spontaneous firing, bursting and cell synchrony of hippocampal principal cells. Hippocampus. 2011;5:520–31.
Robinson L, Goonawardena AV, Pertwee RG, Hampson RE, Riedel G. The synthetic cannabinoid HU210 induces spatial memory deficits and suppresses hippocampal firing rate in rats. Br J Pharm. 2007;151:688–700.
Lodge DJ, Grace AA. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J Neurosci. 2007;27:11424–30.
Sonnenschein SF, Grace AA. Peripubertal mGluR2/3 agonist treatment prevents hippocampal dysfunction and dopamine system hyperactivity in adulthood in MAM model of schizophrenia. Schizophr Bull. 2021;47:1806–14.
Speers LJ, Bilkey DK. Disorganization of oscillatory activity in animal models of schizophrenia. Front Neural Circuits. 2021;15:1–25.
Skosnik PD, Cortes-Briones JA, Hajós M. It’s all in the rhythm: The role of cannabinoids in neural oscillations and psychosis. Biol Psychiatry. 2016;79:568–77.
Sargent K, Chavez-Baldini UY, Master SL, Verweij KJH, Lok A, Sutterland AL, et al. Resting-state brain oscillations predict cognitive function in psychiatric disorders: A transdiagnostic machine learning approach. NeuroImage Clin. 2021;30:102617.
Jeannin-Mayer S, André-Obadia N, Rosenberg S, Boutet C, Honnorat J, Antoine JC, et al. EEG analysis in anti-NMDA receptor encephalitis: Description of typical patterns. Clin Neurophysiol. 2019;130:289–96.
Hanson JE, Ma K, Elstrott J, Weber M, Saillet S, Khan AS, et al. GluN2A NMDA receptor enhancement improves brain oscillations, synchrony, and cognitive functions in dravet syndrome and Alzheimer’s disease models. Cell Rep. 2020;30:381–.e4.
Goyal A, Miller J, Qasim SE, Watrous AJ, Zhang H, Stein JM, et al. Functionally distinct high and low theta oscillations in the human hippocampus. Nat Commun. 2020;11:2469.
Jacinto LR, Reis JS, Dias NS, Cerqueira JJ, Correia JH, Sousa N. Stress affects theta activity in limbic networks and impairs novelty-induced exploration and familiarization. Front Behav Neurosci. 2013;7:1–11.
Sirota A, Montgomery S, Fujisawa S, Isomura Y, Zugaro M, Buzsáki G. Entrainment of neocortical neurons and gamma oscillations by the Hippocampal theta rhythm. Neuron. 2008;60:683–97.
Cass DK, Flores-Barrera E, Thomases DR, Vital WF, Caballero A, Tseng KY. CB1 cannabinoid receptor stimulation during adolescence impairs the maturation of GABA function in the adult rat prefrontal cortex. Mol Psychiatry. 2014;19:536–43.
Katona I, Sperlágh B, Sík A, Käfalvi A, Vizi ES, Mackie K, et al. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–58.
Shen M, Piser TM, Seybold VS, Thayer SA. Cannabinoid receptor agonists inhibit glutamatergic synaptic transmission in rat hippocampal cultures. J Neurosci. 1996;16:4322–34.
Sullivan JM. Cellular and molecular mechanisms underlying learning and memory impairments produced by cannabinoids. Learn Mem. 2000;7:132–9.
Baglot SL, Hume C, Petrie GN, Aukema RJ, Lightfoot SHM, Grace LM, et al. Pharmacokinetics and central accumulation of delta-9-tetrahydrocannabinol (THC) and its bioactive metabolites are influenced by route of administration and sex in rats. Sci Rep. 2021;11:23990.
Oeltzschner G, Wijtenburg SA, Mikkelsen M, Edden RAE, Barker PB, Joo JH, et al. Neurometabolites and associations with cognitive deficits in mild cognitive impairment: a magnetic resonance spectroscopy study at 7 Tesla. Neurobiol Aging. 2019;73:211–8.
Spurny B, Seiger R, Moser P, Vanicek T, Reed MB, Heckova E, et al. Hippocampal GABA levels correlate with retrieval performance in an associative learning paradigm. Neuroimage. 2020;204:116244.
Malik R, Li Y, Schamiloglu S, Sohal VS. Top-down control of hippocampal signal-to-noise by prefrontal long-range inhibition. Cell. 2022;185:1602-1617.e17.
Renard J, Rushlow WJ, Laviolette SR. Effects of adolescent THC exposure on the prefrontal GABAergic system: Implications for schizophrenia-related psychopathology. Front Psychiatry. 2018;9:1–13.
Canadian Cannabis Survey 2021: Summary. Gov Canada. https://www.canada.ca/en/health-canada/services/drugs-medication/cannabis/research-data/canadian-cannabis-survey-2021-summary.html. Accessed 23 Dec 2021.
Shanahan L, Steinhoff A, Bechtiger L, Copeland WE, Ribeaud D, Eisner M, et al. Frequent teenage cannabis use: Prevalence across adolescence and associations with young adult psychopathology and functional well-being in an urban cohort. Drug Alcohol Depend. 2021;228:109063.
Funding
This work was supported by Canadian Institute of Health Research (CIHR; MOP-123378); Natural Sciences and Engineering Research Council (NSERC); MITACS Canada; Canada First Research Excellence Fund (CFREF) awarded to BrainsCAN at Western University.
Author information
Authors and Affiliations
Contributions
MDF and SRL designed the study. MDF performed the behavioral and electrophysiological experiments with help from HJS. MRR performed and analyzed Western Blot experiments with help from SS. SS performed histology. CC and ML performed and analyzed MALDI-IMS experiments under the supervision of KKCY. SRL, WJR, KKCY, and SNW contributed reagents and equipment. MDF and SRL prepared the figures and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
De Felice, M., Chen, C., Rodríguez-Ruiz, M. et al. Adolescent Δ-9-tetrahydrocannabinol exposure induces differential acute and long-term neuronal and molecular disturbances in dorsal vs. ventral hippocampal subregions. Neuropsychopharmacol. 48, 540–551 (2023). https://doi.org/10.1038/s41386-022-01496-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-022-01496-x