Abstract
Lithium is a common medication used to treat mania and bipolar disorder, but the mechanisms by which lithium stabilizes mood and modifies aggression are still not fully understood. Here we found that acute but not chronic lithium significantly suppresses aggression without affecting locomotion in Drosophila melanogaster. Male flies treated with acute lithium are also less competitive than control males in establishing dominance. We also provided evidence that glycogen synthase kinase-3 (GSK-3), a well-known target of lithium, plays an important role in the anti-aggressive effect of lithium in Drosophila. Our genetic data showed that acute knockdown of GSK-3 in neurons can mimic the inhibitory effect of acute lithium on aggression, while specific overexpression of GSK-3 in a subset of P1 neurons profoundly promotes aggression which can be partially rescued by acute lithium application. Thus, these findings revealed the inhibitory effect of lithium on aggression in Drosophila and laid a groundwork for using Drosophila as a powerful model to investigate the mechanisms by which lithium reduces aggression.
Similar content being viewed by others
Introduction
Lithium is one of the most commonly used medications for the treatment of bipolar disorder (BD), which is characterized by repetitive episodes of mania and depression. The beneficial effect of lithium on mood stabilization was first reported in 1949, when it was proposed to treat mania because of its sedative effect [1]. Since then, a number of studies successively demonstrated the inhibitory effect of lithium on aggressive behavior in humans, for example, lithium attenuates aggression in populations such as children and adolescents with conduct disorder [2,3,4,5,6], patients with mental handicaps [7,8,9,10,11], and aggressive prisoners [12,13,14]. Likewise, several studies have reported that lithium is effective in reducing aggression in rodents, including spontaneous aggression in the resident-intruder test [15] and aggression induced by isolation [15,16,17] or electrical shock [18,19,20,21,22,23]. Despite its widespread use and extensive research, the exact mechanisms by which lithium stabilizes mood and reduces aggression are still inconclusive.
Like humans and rodents, the fruit fly Drosophila melanogaster engages in aggressive behavior which usually occurs when male flies compete with each other for resources such as territory, food and mates [24,25,26,27,28,29,30]. In last decades, developments in genetic methods and neural technologies have made it feasible to manipulate genes of interest and target individual neurons in Drosophila [26, 27]. For these reasons, Drosophila has been established as a powerful model organism to understand aggressive behavior. Tremendous progress has been made in dissecting the neural circuits of aggression, ranging from the peripheral sensory systems [31,32,33] to neuromodulators [34,35,36,37,38,39,40] and neurons [41,42,43,44,45,46] in the central nervous system. In addition, many treatments that affect aggressive behavior in humans such as sleep deprivation and alcohol intake have also been shown to modulate aggression in flies [47, 48].
Drosophila is also an excellent model organism in pharmacology research [49,50,51]. In particular, an increasing number of studies are looking at Drosophila as a model to investigate the physiological effects and underlying mechanisms of lithium [49, 52,53,54,55,56,57,58,59,60]. Accumulating evidence has demonstrated that many of the actions and mechanisms of lithium in Drosophila are consistent with those of lithium in humans and rodents. For example, studies in both mice and Drosophila have shown that long-term treatment with lithium lengthens the period of circadian rhythms through GSK-3 pathway [55, 61, 62]. Moreover, the life-extending effect of lithium in humans has been demonstrated and further investigated in Drosophila [53, 63]. However, the effect of lithium on aggression in Drosophila has not been reported to date.
In this study, we investigated the effect of lithium on Drosophila aggression and found that acute lithium has a dose-dependent inhibitory effect on intermale aggression, while chronic lithium does not affect Drosophila aggression. We further found that short-term knockdown of glycogen synthase kinase-3 (GSK-3), a well-known target of lithium [64,65,66], significantly reduces aggression, while specific overexpression of GSK-3 in a subset of P1 neurons known to control social behavior in Drosophila [42, 67,68,69,70,71] profoundly promotes aggression. Thus, our work demonstrates the inhibitory effect of lithium on aggression in Drosophila and provides valuable insights into the underlying mechanisms by which lithium reduces aggression.
Materials and methods
Fly rearing and stocks
Flies were reared and maintained on standard medium at 25 °C, 60% humidity in a 12-h:12-h light-dark cycle. wild-type Canton-S (wtcs) and UAS-dcr2 flies were gifts from Prof. Yi Rao (Peking University). elav-GS line was provided by Prof. Yi Zhong (Tsinghua University). Dsk-GAL4 was generated in our lab as described previously [39]. The following stocks were obtained from Bloomington Drosophila Stock Center: UAS-sgg-RNAi-31308 (BL#31308), UAS-sgg-RNAi-31309 (BL#31309), UAS-sgg.B (BL#5435), R15A01-p65.AD (BL#68837), R71G01-GAL4.DBD (BL#69507), R71G01-GAL4 (BL#39599), R41A01-GAL4 (BL#39425), Tk-GAL4 (BL#51975). All experiments were approved by The Institute of Zoology for Animal Care and Use Committee.
Drug treatment
LiCl (Sigma Aldrich L4408) or NaCl (Sigma Aldrich S5886) was pre-dissolved in ddH2O at concentrations of 10 mM, 20 mM, 50 mM and 100 mM, and then diluted to 5 mM, 10 mM, 25 mM and 50 mM with equal amounts of melted medium, respectively. Li2CO3 (Sigma Aldrich 255823) and sodium valproate (Sigma Aldrich P4543) were diluted in the same way to different concentrations. An edible blue dye was added to the food containing drug to verify that flies had ingested the food. At last, 500 μl of the food was added to 2 ml tubes and cooled overnight for use the next day.
Aggression assays
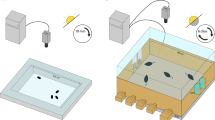
Aggression assays were performed as previously described with slight modifications [39]. Male flies were collected shortly after eclosion and reared in isolation for 5–7 days in 2 mL tubes containing 0.5 ml of food prior to aggression assays. All aggression assays were performed at 25 °C and 60% humidity between 11AM–14PM or 17PM–20PM (the incubator where flies were kept turns on the lights at 11AM and turns off the lights at 23PM). Two males were transferred into the fighting chamber depicted clearly in a previous study [39] by gentle aspiration. Fights between two males were recorded for 30 min using a video camera (Canon VIXIA HF R500). All aggression, except that in dominance assays, refers to aggression between two males in the same condition. In our study, lunging behavior was the primary pattern representing male aggression and was defined as the male suddenly accelerating and then pressing the front of his body against his opponent (Supplementary Video 1). The number of lunging during 30 min and the latency from the beginning of video to the first lunging were counted and used to assess the level of intermale aggression. All data were scored manually, and scorers were blind to the genotypes and conditions of flies.
Dominance assays
For the dominance assays, 2-3 days old male flies were anesthetized under light CO2 and marked with a small white or red dot of acrylic paint on their thorax, and then singly housed in food tubes. Males in different treatments were introduced into fighting chamber by gentle aspiration when they were 5–7 days old. In the dominance assays, we recorded 30 min of video, but only counted the number of lunging during the first 10 min for the flies in each treatment. The one that consistently dominated food and initiated attack from the first time establishing dominance to the end of video was judged as winner. Fights in group where dominance was not established or where the dominance relationship changed were judged to be draws.
Locomotion assays
Most of the experimental conditions in locomotion assays, such as rearing and age of flies, as well as time, humidity and temperature of experiments, are consistent with those in aggression assays. In locomotion assays, single male was transferred into the fighting chamber by gentle aspiration and the spontaneous movements of the flies were recorded with a camera (Canon VIXIA HF R500) for 30 min. The activity of flies during the middle 10 min was analyzed to calculate the average walking speed of flies using Ctrax software.
Climbing assays
Climbing assays were performed as previously described, with slight modifications [72]. In brief, ten 5–7 days old singly housed flies were placed in a plastic vial (length, 9.5 cm; diameter, 2 cm) without anesthesia. Flies were gently tapped to the bottom of the vial, and after 10 s, the number of flies that climbed above 8 cm were counted. Three trials were performed for each group with an interval of 20 seconds. The proportion of flies that climbed above 8 cm within 10 s to the total number of flies was calculated as the climbing index.
Statistical analysis
All statistical analyses were performed using GraphPad Prism 8. D’Agostino–Person normality test was used to verify the normal distribution of data. If the data were normally distributed, two-tailed Student’s t test was used to compare two sets of data, and one-way ANOVA followed by Tukey’s multiple comparisons test was used to compare multiple groups. If the data were non-normally distributed, two-tailed Mann–Whitney U-test was used to compare two sets of data, and Kruskal–Wallis test followed by Dunn’s multiple comparisons test was performed for comparisons of multiple groups. Fisher’s exact test was performed to compare fight outcomes in Figs. 1G, 2D, G, 3H, L and 4F. In comparisons of multiple groups, the reported p-values are p-values adjusted by Tukey’s multiple comparisons test or Dunn’s multiple comparisons test. All statistical analyses and the form in which the data are presented are indicated in the figure legends. The sample sizes are indicated in the figures.
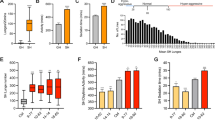
Wild-type males treated with LiCl at 10 mM to 50 mM for 1 hour showed reduced lunging frequency (A) and prolonged fighting latency (B) compared to control males fed with normal food. Walking speed (C) and climbing ability (D) of males treated with LiCl for 1 h were not significantly different from those of control males. Males treated with 25 mM NaCl for 1 h exhibited comparable lunges compared to control males (E) while males treated with 25 mM LiCl for 1 h showed less lunges than control males (F). G Percentage of males in each treatment establishing dominance in group (E) and group (F). H Recovery of aggression following prior 1 h of lithium treatment. *p < 0.05, ***p < 0.001, ****p < 0.0001, n.s. indicates no significant difference. Two-tailed Mann–Whitney U-test (A, C, F, H); two-tailed unpaired Student’s t test (B, D, E); Fisher’s exact test (G). For A–C, H, data are presented as box plots with medians; for D, data are presented as mean ± SEM.
A Lunging frequency and fighting latency of males treated with lithium at concentrations ranging from 0 mM to 50 mM for 4 h, 12 h, 20 h, 28 h, and 36 h. B, C Number of lunges during the first 10 min in the dominance test for males in each treatment when the administration time reached 12 hours. D Percentage of males in each treatment establishing dominance in group (B) and group (C). E, F Number of lunges during the first 10 min in the dominance test for males in each treatment when the treatment time reached 28 h. G Percentage of males in each treatment establishing dominance in group (E) and group (F) Treatment with valproate at concentrations ranging from 0.1 mM to 10 mM for 4 h did not affect lunge frequency (H) and fighting latency (I) in male flies. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, n.s. indicates no significant difference. Two-tailed Mann–Whitney U-test (A–C, F); two-tailed unpaired Student’s t test (E, H, I); Fisher’s exact test (D, G). For, A, H and I, data are presented as box plots with medians.
Acute knockdown of Sgg in neurons induced by feeding RU486 for 16 h reduced lunging frequency (A) and prolonged fighting latency (B) in males. Chronic knockdown of Sgg in neurons induced by feeding RU486 for 48 h did not affect lunging frequency (C) and fighting latency (D) in males. E–G Mutant males with acute knockdown of Sgg in neurons showed decreased number of lunges in dominance tests compare to uninduced males. H Percentages of males in each condition establishing dominance in group (E–G). I–K Mutant males with chronic knockdown of Sgg in neurons exhibited comparable fighting to uninduced males in dominance tests. L Percentages of males in each condition establishing dominance in group (I–K). ***p < 0.001, ****p < 0.0001, n.s. indicates no significant difference. Two-tailed unpaired Student’s t test (A, E–G, I–K); two-tailed Mann–Whitney U-test (B–D); Fisher’s exact test (H, L) For A–D, data are presented as box plots with medians.
A Lunging frequency in transgenic males overexpressing Sgg in a subset of P1 neurons, pCd neurons, Dsk neurons and TK neurons, respectively. B Specific overexpression of Sgg in a subset of P1 neurons by P1a-split-GAL4 driven UAS-sgg.B shortened fighting latency in males. C Specific overexpression of Sgg in a subset of P1 neurons promoted tussling behavior in males. D, E Transgenic males overexpressing Sgg in a subset of P1 neurons showed increased number of lunges in dominance tests compared to parental control males. F Percentages of males establishing dominance for each indicated genotype in D and E. Lunging frequency (G) and fighting latency (H) in transgenic males overexpressing Sgg in a subset of P1 neurons after acute treatment with 25 mM and 50 mM lithium for 4 h. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, n.s. indicates no significant difference. One-way ANOVA followed by Tukey’s multiple comparisons test (A); Kruskal–Wallis test followed by Dunn’s multiple comparisons test (B, C, G, H); two-tailed Mann–Whitney U-test (D, E); Fisher’s exact test (F) For A–C, G and H, data are presented as box plots with medians.
Results
Acute lithium suppresses aggression in Drosophila
To investigate how lithium regulates aggressive behavior in Drosophila, we treated adult wild-type Caton-S (wtcs) male flies with lithium chloride (LiCl) at concentrations ranging from 5 mM to 50 mM (doses that have been reported to be effective and harmless in flies [53, 55, 73]) prior to conducting aggression assays. We first examined the effect of acute lithium on Drosophila aggression. We found that treatment with lithium in the range of 10 mM to 50 mM for 1 h significantly suppressed aggression in male flies in a dose-dependent manner. Males treated with 10 mM to 50 mM of lithium showed reduced lunging frequency (Fig. 1A) and prolonged latency to initiate fighting (Fig. 1B) compared to control males fed with normal food (Supplementary Videos 1 and 2). However, we observed no significant difference in aggression between control males and males treated with lithium when we shortened the administration time to half an hour (Supplementary Figs. S1A and S1B), suggesting that the effect of lithium on suppressing aggression requires at least one hour to take place in Drosophila.
To ensure that the decrease of aggression induced by LiCl was dependent on the lithium cation rather than chloride anion, we treated males with equal concentrations of sodium chloride (NaCl) for 1 h and found that acute NaCl didn’t affect aggression (Supplementary Figs. S1C and S1D). Moreover, treatment with lithium carbonate (Li2CO3) at 5 mM to 25 mM for 1 h also had a significant inhibitory effect on intermale aggression (Supplementary Figs. S1E and S1F). Thus, these results exclude the effect of anions and suggest that it is indeed lithium that acts to suppress aggression.
It is of concern that the reduction in aggression is due to a defect in locomotion caused by acute lithium. Therefore, we examined walking speed and climbing behavior of flies to assess locomotion ability. There were no significant differences in walking speed (Fig. 1C) and climbing ability (Fig. 1D) between males treated with acute lithium and control males, suggesting that the decrease in aggression was due to the inhibition of aggressiveness by acute lithium rather than a result of impaired locomotion in flies.
It is well-known that dominance relationships could be established between male flies during fighting: the winner dominates food and initiates attacks on the loser, while the loser retreats from food and shows reduced aggression [39, 47, 74]. Since acute lithium suppresses aggression in males, we asked whether acute lithium affects the competitiveness of males in establishing dominance. For this purpose, we paired acute NaCl- or acute LiCl-treated males (treated at a dose of 25 mM for 1 h) with control males to compare the aggressiveness of males in each treatment and to count the outcome of the fights. Flies in different treatments were distinguished by the different colored dots on their thorax. We found that males treated with acute NaCl exhibited comparable fighting to control males (Fig. 1E) and were as competitive as control males in establishing dominance (Fig. 1G), whereas males treated with acute LiCl showed significantly less lunges than controls (Fig. 1F) and won a significantly lower percentage of fights than control males (Fig. 1G and Supplementary Video 3). Together, these results demonstrate that one-hour treatment with lithium is effective in suppressing aggression in Drosophila, thereby causing males to be less aggressive and competitive in establishing dominance.
To further clarify the effect of lithium on Drosophila aggression, we next tested how long the inhibitory effect of acute lithium can last. Flies were transferred to normal food for a period time as a recovery after one hour of acute lithium treatment and then subjected to aggression assays. We found that the aggression of males treated with 10 mM lithium had returned to a level comparable to that of controls after only two hours of recovery following lithium treatment (Fig. 1H). Aggression in males treated with 25 mM and 50 mM of lithium also returned to baseline levels after four hours of recovery (Fig. 1H). These results suggest that the inhibitory effect of acute lithium on aggression in Drosophila lasts for only a short period of time.
Chronic lithium treatment and valproate do not affect aggression in Drosophila
The administration of lithium is generally divided into acute lithium and chronic lithium, depending on the duration of administration. Previous studies have shown that both acute and chronic lithium reduce aggression in rodents [19,20,21,22,23]. Therefore, we next examined the effect of lithium on Drosophila aggression when administered for prolonged periods of time.
When we extended the duration of administration from 1 h to 4 h, we found that 5 mM of lithium also significantly reduced aggression in males (Fig. 2A), indicating an enhanced inhibitory effect of lithium on aggression. Intriguingly, the inhibitory effect of lithium on aggression began to diminish when males were treated with lithium for longer periods of time. When males were fed a lithium-containing diet continuously for 12 h, 5 mM of lithium no longer suppressed aggression (Fig. 2A). Thereafter, 10 mM and 25 mM of lithium also ceased to suppress aggression when the treatment time reached 20 h (Fig. 2A). Finally, when males were continuously treated with lithium for 28 h or longer, 5–50 mM of lithium had no significant effect on aggression (Fig. 2A). To further confirm that chronic lithium does not affect aggression, we fed males a diet containing lithium for several days (2 days, 4 days, and 6 days) and found no significant difference in aggression between lithium-treated males and control males (Supplementary Fig. S2A). Similarly, chronic treatment with 5 mM to 25 mM of Li2CO3 for 28 h did not affect aggression in males (Supplementary Figs. S2B and S2C).
We next tested the effect of long-term lithium on the performance of males in establishing dominance. We found that males treated with 25 mM lithium for 12 h were still less competitive than control males (Fig. 2B–D), while lithium-treated males were as competitive as control males in dominance assays when treatment time reached 28 h (Fig. 2E–G), consistent with the effect of 25 mM lithium on aggression. Thus, these results suggested that only acute lithium has an inhibitory effect on aggression, while chronic lithium does not affect aggression in Drosophila.
Valproate is another mood stabilizer commonly used to treat bipolar disorder. Previous research has shown that lithium and valproate share effects on the circadian behavior in Drosophila [55]. Therefore, we also tested the effect of valproate on Drosophila aggression and found that both acute (4 h) and chronic (48 h) treatments with 0.1 mM to 10 mM of valproate (the doses effective in flies [55]) did not affect aggression (Fig. 2H, I, Supplementary Figs. S2D and S2E), suggesting that lithium and valproate have different effects and mechanisms on aggressive behavior in Drosophila.
Short-term knockdown of Sgg in neurons suppresses aggression, while long-term knockdown does not
Glycogen synthase kinase-3 (GSK-3), whose ortholog in Drosophila is Shaggy (Sgg), is a well-known target of lithium [64,65,66]. It has been reported that lithium promotes longevity and lengthens the period of circadian rhythms in Drosophila through GSK-3 pathway [53, 55]. Therefore, we investigated whether GSK-3 is involved in the inhibition of Drosophila aggression by lithium.
Considering that only acute lithium suppresses aggression in males, we conditionally manipulated the activity of Sgg using a pan-neuronal Gene-Switch system (elav-GS) which is induced by RU486 [75]. With reference to the concentration and administration time of RU486 that have been reported to be effective in inducing gene expression [75], we acutely knocked down Sgg expression by feeding flies 0.5 mM RU486 for 16 h, while long-term knockdown was defined as continuous administration of 0.5 mM RU486 for 48 h. We found that acute knockdown of Sgg in neurons significantly inhibited aggression in males. Mutant males with acute knockdown of Sgg exhibited reduced lunging frequency (Fig. 3A) and prolonged fighting latency (Fig. 3B) compared to uninduced males.
Interestingly, there was no significant difference in aggression between mutant males with long-term knockdown of Sgg and uninduced males (Fig. 3C, D), consistent with the finding that chronic lithium does not affect aggression. Similarly, acute knockdown of Sgg using another dsRNA-expressing transgene also suppressed intermale aggression, whereas chronic knockdown did not (Supplementary Fig. S3A–D). Importantly, acute knockdown of Sgg using both of these two independent RNAi lines did not affect locomotion activity of flies (Supplementary Figs. S3E and S3F).
Furthermore, mutant males with acute knockdown of Sgg were less competitive than uninduced males in dominance tests (Fig. 3G, H). Data from parental controls suggest that treatment with RU486 per se did not affect the competitiveness of males to establish dominance (Fig. 3E, F, H). Mutant males with chronic knockdown of Sgg showed comparable fighting to uninduced males in dominance tests (Fig. 3I–L). Together, these results show that the effects of short-term and long-term knockdown of Sgg on aggression are consistent with the effects of acute and chronic lithium on aggression, respectively, suggesting that GSK-3 may be involved in the lithium-induced inhibition of aggression in Drosophila.
Specific overexpression of Sgg in a subset of P1 neurons promotes aggression
We further explored whether the overexpression of Sgg promotes aggression in males. Unexpectedly, we found that the overexpression of Sgg in neurons by elav-GAL4 driven UAS-sgg.B did not increase aggression (Supplementary Figs. S4A and S4B). Previous work reporting that lithium lengthens the period of circadian locomotor behavior in flies has shown that the overexpression of GSK-3 in the clock cells of Drosophila shortens the period of circadian locomotor activity [55]. Therefore, we overexpressed Sgg in several group of neurons that had been reported to modulate aggression in Drosophila including a subset of P1 neurons [42], pCd neurons [43], Tk neurons [36] and Dsk neurons [39] (Fig. 4A). We found that specific overexpression of Sgg in a subset of P1 neurons labeled by P1a split-GAL4 dramatically increased lunging frequency (Fig. 4A) and shortened fighting latency in males (Fig. 4B). Moreover, we observed that transgenic males overexpressing Sgg in P1a neurons exhibited increased tussling behavior, an escalating high-intensity fighting behavior that was rare in control males (Fig. 4C and Supplementary Video 4). Likewise, specific overexpression of Sgg in P1a neurons by R71G01-GAL4 driving UAS-sgg.B profoundly promotes intermale aggression (Supplementary Figs. S4C and S4D).
Intriguingly, we found that transgenic males with overexpression of Sgg in P1a neurons showed higher movement velocity than control males (Supplementary Figs. S4E and S4F), indicating that overexpression of Sgg in P1a neurons also induces hyperactivity in flies. When we paired transgenic males overexpressing Sgg in P1a neurons with two parental controls separately, we found that transgenic males were more aggressive in fighting with control males (Fig. 4D, E) and were overwhelmingly competitive in establishing dominance (Fig. 4F). Thus, these results demonstrate that overexpression of GSK-3, a target of lithium, in a subset of P1 neurons that control social behavior in Drosophila makes flies abnormally aggressive.
Importantly, we found that acute treatment with 25 mM lithium for 4 h failed to suppress aggression in transgenic males overexpressing Sgg in P1a neurons, while acute lithium at 50 mM still had an inhibitory effect on aggression in transgenic males (Fig. 4G, H), suggesting that overexpression of Sgg in P1a neurons could counteract the inhibitory effect of acute lithium on aggression to some extent. Considering that chronic lithium has been shown to be effective in suppressing aggressive behavior in prisoners [13, 14] and attenuating shock-induced aggression in rodents [19, 21, 22], we tested whether long-term treatment with low and medium concentrations of lithium would produce a inhibitory effect on the hyper-aggression in transgenic males overexpressing Sgg in P1 neurons. Unfortunately, we found that treatment with 5 mM, 10 mM and 25 mM lithium for 4 days and 6 days had no significant inhibitory effect on the abnormal aggression in transgenic males overexpressing Sgg in P1 neurons (Supplementary Fig. S4G–J). Together, these results show that chronic lithium affects neither normal aggression in wild-type Drosophila nor excessive aggression in transgenic males, which is a puzzling and intriguing issue in the study of lithium on Drosophila aggression.
Discussion
In recent years, tremendous progress has been made in studying aggressive behavior in the fruit fly Drosophila melanogaster, and this inspired us to use Drosophila as a research model to investigate the mechanisms by which lithium, a commonly used mood stabilizer, reduces aggression. In this study, we demonstrated that acute lithium significantly suppresses aggression in male flies, while chronic lithium does not affect Drosophila aggression. The suppression of aggression by acute lithium treatment is also manifested by making males less competitive in the fight for dominance. Furthermore, acute knockdown of GSK-3 in neurons significantly reduces aggression and specific overexpression of GSK-3 in a subset of P1 neurons profoundly promotes aggression, suggesting that GSK-3 is involved in the anti-aggressive effect of lithium in Drosophila.
Notably, our work focused on the effect of lithium on aggression in wild-type males, rather than on flies with mania-like state. Most research examining the effect of lithium on human aggression were conducted in populations with abnormally high levels of aggression, such as children and adolescents with conduct disorder [2,3,4,5,6], patients with mental handicap [7,8,9,10,11], and prisoners with extreme anger [12,13,14]. However, so far there are no good protocols for establishing stable manic states in Drosophila, although some manipulations, such as manipulating the activity of some neurons in flies can make flies more aggressive. For this reason, we could only examine the effect of lithium on the spontaneous aggression between two males reared in isolation in our study. Thus, the inhibitory effect of lithium on aggression in Drosophila that we observed is somewhat different from the reported effect of lithium on aggression in humans and rodents. The main difference lies in the different effects of acute and chronic lithium on aggression. There are studies showing that short-term lithium is effective in reducing aggression in prisoners [12], as well as in children and adolescents with conduct disorder [2, 4,5,6, 12]. However, several studies in violent prisoners have also shown that long-term lithium treatment has an inhibitory effect on aggressive behavior [13, 14], although this inhibition may be due to the placebo effect, which is an important factor in human pharmacology. Similarly, both acute and chronic lithium have been reported to attenuate shock-induced aggression in rodents, with slight difference in the effect [19,20,21,22,23]. Intriguingly, we found that only acute lithium has an inhibitory effect on Drosophila aggression, while chronic lithium does not affect aggression. In addition, only acute knockdown of GSK-3 induced by short-term RU486 administration reduces aggression in males. In this study, we did not delve into the reasons for the different effects of acute and chronic lithium on aggression in Drosophila. We suppose that this may be due to the tolerance to lithium in Drosophila, or a compensatory mechanism for the pathway through which lithium acts to suppress aggression. So far, our current results suggest that the duration of administration must be considered when studying the effects and mechanisms of lithium action, especially in fruit flies.
At the molecular and neural levels, we found that specific overexpression of GSK-3 in a subset of P1 neurons profoundly promotes aggression in males, consistent with the previously reported finding that lithium lengthens the period of circadian rhythms in Drosophila while overexpression of GSK-3 in clock neurons driven by pdf-GAL4 shortens the circadian period of flies [55]. P1a neurons are a cluster of ~20 male-specific interneurons per hemibrain that have been reported to modulate courtship and aggressive behavior in male flies [42]. Thermogenetic activation of P1a neurons in males can increase both lunging and wing extension. In addition, P1a neurons also promotes a persistent behavioral state that enhances both courtship and aggressive behavior. Strikingly, a small group of neurons (VMHvl neurons) in mice have also been identified as controlling mating and aggression, as well as being involved in promoting internal states [67]. Thus, our results that overexpression of GSK-3 in P1a neurons promotes aggression in males could provide novel insights into the establishment of mania models in mice and the exploration of the pathogenesis of mania or bipolar disorder. Furthermore, overexpression of GSK-3 in other group of neurons known to promote aggression when activated, including Tk neurons, Dsk neurons, and pCd neurons does not promote aggression, suggesting a unique role of P1a neurons in the lithium-induced inhibition of aggression in Drosophila.
In future studies, our main work will be to explain the different effects of acute and chronic lithium on aggression by comparing and analyzing the effects of acute and chronic lithium on flies at the molecular and neural levels. We are also interested in whether and how lithium affects sexual behaviors of flies, as a review that searched thirteen preclinical and clinical studies related to lithium suggests that lithium may cause sexual dysfunction in animal models and patients [76]. Considering the fact that P1a neurons also play an important role in controlling male courtship behavior, we speculate that lithium may have an inhibitory effect on male courtship behavior similar to that of lithium on intermale aggression, and that the underlying mechanism may also involve GSK-3 and P1a neurons.
References
Cade JF. Lithium salts in the treatment of psychotic excitement. Med J Aust. 1949;2:349–52.
Campbell M, Adams PB, Small AM, Kafantaris V, Silva RR, Shell J, et al. Lithium in hospitalized aggressive children with conduct disorder: a double-blind and placebo-controlled study. J Am Acad Child Adolesc Psychiatry. 1995;34:445–53.
Campbell M, Small AM, Green WH, Jennings SJ, Perry R, Bennett WG, et al. Behavioral efficacy of haloperidol and lithium carbonate. A comparison in hospitalized aggressive children with conduct disorder. Arch Gen Psychiatry. 1984;41:650–6.
Malone RP, Delaney MA, Luebbert JF, Cater J, Campbell M. A double-blind placebo-controlled study of lithium in hospitalized aggressive children and adolescents with conduct disorder. Arch Gen Psychiatry. 2000;57:649–54.
Malone RP, Luebbert J, Pena-Ariet M, Biesecker K, Delaney MA. The Overt Aggression Scale in a study of lithium in aggressive conduct disorder. Psychopharmacol Bull. 1994;30:215.
Rifkin A, Karajgi B, Dicker R, Perl E, Boppana V, Hasan N, et al. Lithium treatment of conduct disorders in adolescents. Am J Psychiatry. 1997;154:554–5.
Bellus SB, Stewart D, Vergo JG, Kost PP, Grace J, Barkstrom SR. The use of lithium in the treatment of aggressive behaviours with two brain-injured individuals in a state psychiatric hospital. Brain Inj. 1996;10:849–60.
Craft M, Ismail IA, Krishnamurti D, Mathews J, Regan A, Seth RV, et al. Lithium in the treatment of aggression in mentally handicapped patients. A double-blind trial. Br J Psychiatry J Ment Sci. 1987;150:685–9.
Glenn MB, Wroblewski B, Parziale J, Levine L, Whyte J, Rosenthal M. Lithium carbonate for aggressive behavior or affective instability in ten brain-injured patients. Am J Phys Med Rehabilit. 1989;68:221–6.
Luchins DJ, Dojka D. Lithium and propranolol in aggression and self-injurious behavior in the mentally retarded. Psychopharmacol Bull. 1989;25:372–5.
Micev V, Lynch DM. Letter: Effect of lithium on disturbed severely mentally retarded patients. Br J Psychiatry J Ment Sci. 1974;125:110.
Sheard M. Effect of lithium on human aggression. Nature. 1971;230:113–4.
Sheard MH, Marini JL, Bridges CI, Wagner E. The effect of lithium on impulsive aggressive behavior in man. Am J Psychiatry. 1976;133:1409–13.
Tupin JP, Smith DB, Clanon TL, Kim LI, Nugent A, Groupe A. The long-term use of lithium in aggressive prisoners. Compr Psychiatry. 1973;14:311–7.
Brain PF, Al-Maliki S. Effects of lithium chloride injections on rank-related fighting, maternal aggression and locust-killing responses in naive and experienced ‘TO’ strain mice. Pharm Biochem Behav. 1979;10:663–9.
Malick JG. Inhibition of fighting in isolated mice following repeated administration of lithium chloride. Pharm Biochem Behav. 1978;8:579–81.
Oehler J, Jähkel M, Schmidt J. The influence of chronic treatment with psychotropic drugs on behavioral changes by social isolation. Pol J Pharmacol Pharm. 1985;37:841–9.
Eichelman B, Thoa NB, Perez-Cruet J. Alkali metal cations: effects on aggression and adrenal enzymes. Pharm Biochem Behav. 1973;1:121–3.
Kovacsics CE, Gould TD. Shock-induced aggression in mice is modified by lithium. Pharm Biochem Behav. 2010;94:380–6.
Marini JL, Sheard MH, Kosten T. Study of the role of serotonin in lithium action using shock-elicited fighting. Commun Psychopharmacol. 1979;3:225–33.
Mukherjee BP, Pradhan SN. Effects of lithium on foot shock-induced aggressive behavior in rats. Arch internationales de pharmacodynamie et de therapie. 1976;222:125–31.
Prasad V, Sheard MH. Effect of lithium upon desipramine enhanced shock-elicited fighting in rats. Pharm Biochem Behav. 1982;17:377–8.
Sheard MH. Effect of lithium on foot shock aggression in rats. Nature. 1970;228:284–5.
Baier A, Wittek B, Brembs B. Drosophila as a new model organism for the neurobiology of aggression? J Exp Biol. 2002;205 (Pt 9):1233–40.
Chen S, Lee AY, Bowens NM, Huber R, Kravitz EA. Fighting fruit flies: a model system for the study of aggression. Proc Natl Acad Sci USA. 2002;99:5664–8.
Hoopfer ED. Neural control of aggression in Drosophila. Curr Opin Neurobiol. 2016;38:109–18.
Kravitz EA, Fernandez MP. Aggression in Drosophila. Behav Neurosci. 2015;129:549–63.
Kravitz EA, Huber R. Aggression in invertebrates. Curr Opin Neurobiol. 2003;13:736–43.
Nilsen SP, Chan YB, Huber R, Kravitz EA. Gender-selective patterns of aggressive behavior in Drosophila melanogaster. Proc Natl Acad Sci USA. 2004;101:12342–7.
Vrontou E, Nilsen SP, Demir E, Kravitz EA, Dickson BJ. fruitless regulates aggression and dominance in Drosophila. Nat Neurosci. 2006;9:1469–71.
Liu W, Liang X, Gong J, Yang Z, Zhang YH, Zhang JX, et al. Social regulation of aggression by pheromonal activation of Or65a olfactory neurons in Drosophila. Nat Neurosci. 2011;14:896–902.
Versteven M, Vanden Broeck L, Geurten B, Zwarts L, Decraecker L, Beelen M, et al. Hearing regulates Drosophila aggression. Proc Natl Acad Sci USA. 2017;114:1958–63.
Wang L, Anderson DJ. Identification of an aggression-promoting pheromone and its receptor neurons in Drosophila. Nature. 2010;463:227–31.
Alekseyenko OV, Chan YB, Fernandez MP, Bülow T, Pankratz MJ, Kravitz EA. Single serotonergic neurons that modulate aggression in Drosophila. Curr Biol. 2014;24:2700–7.
Alekseyenko OV, Chan YB, Li R, Kravitz EA. Single dopaminergic neurons that modulate aggression in Drosophila. Proc Natl Acad Sci USA. 2013;110:6151–6.
Asahina K, Watanabe K, Duistermars BJ, Hoopfer E, González CR, Eyjólfsdóttir EA, et al. Tachykinin-expressing neurons control male-specific aggressive arousal in Drosophila. Cell. 2014;156:221–35.
Dierick HA, Greenspan RJ. Serotonin and neuropeptide F have opposite modulatory effects on fly aggression. Nat Genet. 2007;39:678–82.
Hoyer SC, Eckart A, Herrel A, Zars T, Fischer SA, Hardie SL, et al. Octopamine in male aggression of Drosophila. Curr Biol. 2008;18:159–67.
Wu F, Deng B, Xiao N, Wang T, Li Y, Wang R, et al. A neuropeptide regulates fighting behavior in Drosophila melanogaster. Elife. 2020;9:e54229.
Zhou C, Rao Y, Rao Y. A subset of octopaminergic neurons are important for Drosophila aggression. Nat Neurosci. 2008;11:1059–67.
Duistermars BJ, Pfeiffer BD, Hoopfer ED, Anderson DJ. A brain module for scalable control of complex, multi-motor threat displays. Neuron. 2018;100:1474–90. e4.
Hoopfer ED, Jung Y, Inagaki HK, Rubin GM, Anderson DJ. P1 interneurons promote a persistent internal state that enhances inter-male aggression in Drosophila. Elife. 2015;4:e11346.
Jung Y, Kennedy A, Chiu H, Mohammad F, Claridge-Chang A, Anderson DJ. Neurons that function within an integrator to promote a persistent behavioral state in drosophila. Neuron. 2020;105:322–33. e5.
Koganezawa M, Kimura K, Yamamoto D. The neural circuitry that functions as a switch for courtship versus aggression in drosophila males. Curr Biol. 2016;26:1395–403.
Sengupta S, Chan YB, Palavicino-Maggio CB, Kravitz EA. GABA transmission from mAL interneurons regulates aggression in Drosophila males. Proc Natl Acad Sci USA. 2022;119:e2117101119.
Watanabe K, Chiu H, Pfeiffer BD, Wong AM, Hoopfer ED, Rubin GM, et al. A circuit node that integrates convergent input from neuromodulatory and social behavior-promoting neurons to control aggression in drosophila. Neuron. 2017;95:1112–28. e7.
Kayser MS, Mainwaring B, Yue Z, Sehgal A. Sleep deprivation suppresses aggression in Drosophila. Elife. 2015;4:e07643.
Park A, Tran T, Gutierrez L, Stojanik CJ, Plyler J, Thompson GA, et al. Alcohol-induced aggression in Drosophila. Addict Biol. 2021;26:e13045.
Jans K, Lüersen K, Rimbach G. Drosophila melanogaster as a Model Organism to Study Lithium and Boron Bioactivity. Int J Mol Sci. 2021;22:11710.
Narayanan AS, Rothenfluh AI. Believe i can fly!: use of drosophila as a model organism in neuropsychopharmacology research. Neuropsychopharmacology. 2016;41:1439–46.
Pandey UB, Nichols CD. Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacol Rev. 2011;63:411–36.
Berger Z, Ttofi EK, Michel CH, Pasco MY, Tenant S, Rubinsztein DC, et al. Lithium rescues toxicity of aggregate-prone proteins in Drosophila by perturbing Wnt pathway. Hum Mol Genet. 2005;14:3003–11.
Castillo-Quan JI, Li L, Kinghorn KJ, Ivanov DK, Tain LS, Slack C, et al. Lithium promotes longevity through GSK3/NRF2-dependent hormesis. Cell Rep. 2016;15:638–50.
Choi CH, McBride SM, Schoenfeld BP, Liebelt DA, Ferreiro D, Ferrick NJ, et al. Age-dependent cognitive impairment in a Drosophila fragile X model and its pharmacological rescue. Biogerontology. 2010;11:347–62.
Dokucu ME, Yu L, Taghert PH. Lithium- and valproate-induced alterations in circadian locomotor behavior in Drosophila. Neuropsychopharmacology. 2005;30:2216–24.
Hayward P. Lithium reverses tau pathology in Drosophila. Lancet Neurol. 2004;3:265.
Jia DD, Zhang L, Chen Z, Wang CR, Huang FZ, Duan RH, et al. Lithium chloride alleviates neurodegeneration partly by inhibiting activity of GSK3β in a SCA3 Drosophila model. Cerebellum. 2013;12:892–901.
Kaas GA, Kasuya J, Lansdon P, Ueda A, Iyengar A, Wu CF, et al. Lithium-responsive seizure-like hyperexcitability is caused by a mutation in the drosophila voltage-gated sodium channel gene paralytic. eNeuro. 2016;3:ENEURO.0221-16.2016.
Kasuya J, Kaas G, Kitamoto T. Effects of lithium chloride on the gene expression profiles in Drosophila heads. Neurosci Res. 2009;64:413–20.
Kasuya J, Kaas GA, Kitamoto T. A putative amino acid transporter of the solute carrier 6 family is upregulated by lithium and is required for resistance to lithium toxicity in Drosophila. Neuroscience 2009;163:825–37.
Iitaka C, Miyazaki K, Akaike T, Ishida N. A role for glycogen synthase kinase-3beta in the mammalian circadian clock. J Biol Chem. 2005;280:29397–402.
Padiath QS, Paranjpe D, Jain S, Sharma VK. Glycogen synthase kinase 3beta as a likely target for the action of lithium on circadian clocks. Chronobiol Int. 2004;21:43–55.
Zarse K, Terao T, Tian J, Iwata N, Ishii N, Ristow M. Low-dose lithium uptake promotes longevity in humans and metazoans. Eur J Nutr. 2011;50:387–9.
Eldar-Finkelman H, Martinez A. GSK-3 inhibitors: preclinical and clinical focus on CNS. Front Mol Neurosci. 2011;4:32.
Jope RS. Lithium and GSK-3: one inhibitor, two inhibitory actions, multiple outcomes. Trends Pharm Sci. 2003;24:441–3.
Phiel CJ, Klein PS. Molecular targets of lithium action. Annu Rev Pharm Toxicol. 2001;41:789–813.
Anderson DJ. Circuit modules linking internal states and social behaviour in flies and mice. Nat Rev Neurosci. 2016;17:692–704.
Kimura K, Hachiya T, Koganezawa M, Tazawa T, Yamamoto D. Fruitless and doublesex coordinate to generate male-specific neurons that can initiate courtship. Neuron 2008;59:759–69.
Kohatsu S, Koganezawa M, Yamamoto D. Female contact activates male-specific interneurons that trigger stereotypic courtship behavior in Drosophila. Neuron 2011;69:498–508.
Pan Y, Meissner GW, Baker BS. Joint control of Drosophila male courtship behavior by motion cues and activation of male-specific P1 neurons. Proc Natl Acad Sci USA. 2012;109:10065–70.
von Philipsborn AC, Liu T, Yu JY, Masser C, Bidaye SS, Dickson BJ. Neuronal control of Drosophila courtship song. Neuron 2011;69:509–22.
Hakim-Mishnaevski K, Flint-Brodsly N, Shklyar B, Levy-Adam F, Kurant E. Glial phagocytic receptors promote neuronal loss in adult drosophila brain. Cell Rep. 2019;29:1438–48. e3.
Ries AS, Hermanns T, Poeck B, Strauss R. Serotonin modulates a depression-like state in Drosophila responsive to lithium treatment. Nat Commun. 2017;8:15738.
Yurkovic A, Wang O, Basu AC, Kravitz EA. Learning and memory associated with aggression in Drosophila melanogaster. Proc Natl Acad Sci USA. 2006;103:17519–24.
Osterwalder T, Yoon KS, White BH, Keshishian H. A conditional tissue-specific transgene expression system using inducible GAL4. Proc Natl Acad Sci USA. 2001;98:12596–601.
Elnazer HY, Sampson A, Baldwin D. Lithium and sexual dysfunction: an under-researched area. Hum Psychopharmacol. 2015;30:66–9.
Acknowledgements
We thank Dr. Xianhui Wang (Chinese Academy of Sciences) and Dr. Yingxue Wang (Max Planck Florida Institute for Neuroscience) for their comments on the manuscript. We also thank Dr. Yufeng Pan (Southeast University) for his valuable comments on the revised manuscript. We thank the members of Zhou lab for their helpful discussion and their support on this study.
Funding
This research was supported by the Strategic Priority Research Program of the Chinese Academy of Science (No. XDB11010800) and National Natural Science Foundation of China (No. 31872280, 31622054). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
RW designed and performed the experiments. BM analyzed the majority of the data. KS analyzed the data from locomotion assays. RW and BM wrote the original draft. FW revised the article. CZ conceived and supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, R., Ma, B., Shi, K. et al. Effects of lithium on aggression in Drosophila. Neuropsychopharmacol. 48, 754–763 (2023). https://doi.org/10.1038/s41386-022-01475-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-022-01475-2