Abstract
Persons at risk for developing alcohol use disorder (AUD) differ in their sensitivity to acute alcohol intoxication. Alcohol effects are complex and thought to depend on multiple mechanisms. Here, we explored whether acid-sensing ion channels (ASICs) might play a role. We tested ASIC function in transfected CHO cells and amygdala principal neurons, and found alcohol potentiated currents mediated by ASIC1A homomeric channels, but not ASIC1A/2 A heteromeric channels. Supporting a role for ASIC1A in the intoxicating effects of alcohol in vivo, we observed marked alcohol-induced changes on local field potentials in basolateral amygdala, which differed significantly in Asic1a–/– mice, particularly in the gamma, delta, and theta frequency ranges. Altered electrophysiological responses to alcohol in mice lacking ASIC1A, were accompanied by changes in multiple behavioral measures. Alcohol administration during amygdala-dependent fear conditioning dramatically diminished context and cue-evoked memory on subsequent days after the alcohol had cleared. There was a significant alcohol by genotype interaction. Context- and cue-evoked memory were notably worse in Asic1a–/– mice. We further examined acute stimulating and sedating effects of alcohol on locomotor activity, loss of righting reflex, and in an acute intoxication severity scale. We found loss of ASIC1A increased the stimulating effects of alcohol and reduced the sedating effects compared to wild-type mice, despite similar blood alcohol levels. Together these observations suggest a novel role for ASIC1A in the acute intoxicating effects of alcohol in mice. They further suggest that ASICs might contribute to intoxicating effects of alcohol and AUD in humans.
Similar content being viewed by others
Introduction
Alcohol use disorder (AUD) affects hundreds of millions of people worldwide [1]. Costs related to excessive alcohol use, including lost productivity and increased healthcare needs, total hundreds of billions of dollars per year in the U.S. alone [2]. For individuals, AUD causes numerous adverse health and social outcomes [3, 4]. Risk for AUD is not well understood but is thought to be reflected in an individual’s acute response to the intoxicating effects of alcohol, with increased risk associated with reduced sedation and/or increased stimulation [5,6,7,8]. Thus, understanding the neurobiology of alcohol intoxication may provide critical insight into AUD.
Alcohol intoxication is thought to lie along a spectrum that depends on increasing blood alcohol level (BAL). This spectrum ranges from mild intoxication, characterized by euphoria and disinhibition, to severe intoxication which progresses from slowed reaction time to loss of coordination, impaired cognition, stupor, and even coma or death [9]. Alcohol readily interacts with proteins through hydrogen bonding and is known to bind to a diverse array of receptors and channels [10]. However, how alcohol exerts its varied intoxicating effects is complex and incompletely understood. A number of important molecular targets are well-established [11], and even more are likely to be discovered. Here, we investigated whether acid-sensing ion channels (ASICs) might play a role in alcohol’s complex effects. No previous studies have tested if these channels contribute to the neurophysiological and behavioral effects of alcohol.
ASICs are cation channels of the degenerin/epithelial Na+ channel (DEG/ENaC) family that are sensitive to extracellular pH [12, 13]. Extracellular acidosis produces a large inward cation current through ASICs with greater acidosis producing greater current [14]. ASICs are comprised of trimeric assemblies of subunits (e.g. ASIC1A, ASIC2A, and ASIC2B) into homo- and heterotrimeric complexes [12, 14,15,16,17,18,19]. Different subunit combinations can influence diverse channel properties including subcellular localization, kinetics, and pH sensitivity. In brain neurons, functioning, endogenous channels are thought to be comprised largely of ASIC1A homotrimers and ASIC1A/2A-containing heterotrimers [15, 18, 19]. The ASIC1A subunit is required for normal channel function within a physiologically relevant pH range (from below pH 7.4 to pH 5), as deleting ASIC1A eliminates currents evoked by acidosis in this range [20,21,22,23,24]. ASICs have been implicated in synaptic plasticity [20, 21, 23, 25] as well as in learning and memory [20, 23, 25,26,27,28]. Furthermore, ASIC1A influences the effects of other substances of abuse, including cocaine and morphine [20, 29]. Thus, ASIC1A is well-positioned to modify synaptic function and influence behavioral outcomes, and might therefore contribute to effects of alcohol. Here we conducted a series of experiments to test the novel hypothesis that ASIC1A plays a role in acute alcohol intoxication.
Materials and methods
CHO cell electrophysiology
Rat ASIC1A and ASIC2A were transiently expressed in Chinese Hamster Ovary-K1 (CHO) cells using rASIC1a-IRES2-DsRed plasmid (received from Francois Rugiero, University College London, UK) and pcDNA3.1-rASIC2A (received from Peter McNaughton, University of Cambridge, UK) with lipofectamine (Invitrogen, CA, USA). The holding potential was -60 mV for all cells. Acid-evoked current was assessed as previously described [30] with pH = 6.0 applied in the absence or presence of alcohol (10–100 mM). Acid and alcohol were applied using a custom-made gravity-driven fast perfusion system [30, 31]. One dose was tested per cell. Potentiation was calculated as ((current after alcohol – control current)/control current) * 100 percent. Percent potentiation by alcohol dose was analyzed by linear regression.
Mice
Both male and female Asic1a+/+, Asic1a−/− [25], and Asic2−/− [32] mice were generated and bred in-house, and maintained on a congenic C57BL6/J background. Although effects of alcohol on these mice have never been previously tested, they have been extensively characterized and phenotyped in a number of physiological and behavioral assays, with relevant testing including fear conditioning, locomotion, locomotor stimulation by cocaine, and synaptic plasticity in amygdala and nucleus accumbens [20, 21, 25, 26, 28, 29, 32, 33]. Mice were housed on a 12-hour light-dark cycle with same-sex littermates in groups of 2–5, except for mice with chronic amygdala electrode implants, which were singly-housed. Brain activity and behaviors were tested in mice >10 weeks of age. Electrophysiological slice recordings were obtained at 8–12 weeks of age. Different mice were used for each behavioral assay except the intoxication scale and loss of righting reflex, in which the same mice were used with a one-week gap between experiments. All animal experiments were approved by the University of Iowa IACUC.
Basolateral amygdala (BLA) neuron electrophysiology
Coronal BLA slices (300 μm) were obtained and voltage-clamp recordings were made from visually identified principal neurons, using protocols and solutions as previously described [20]. Recordings were made with gluconate-based internal solution in the presence of 100 μM picrotoxin, 20 μM CNQX, and 50 μM d-APV. Acidic ACSF (buffered with 5 mM HEPES and 5 mM MES and titrated to pH 6.3 with NaOH) was applied from a recording pipette positioned ~10–30 μM from the cell body. Acidic ASCF containing no alcohol vs indicated alcohol dose were applied to each cell with a Femtojet 5247 (Eppendorf) 1–2 psi for 3 s. Three doses were tested: 5, 50, or 100 mM alcohol. Order of solution presentation was counterbalanced between cells. The holding potential was −70 mV. Alcohol (100 mM) did not change membrane resistance (t-test, t (11) = 1.679, p = 0.121).
Local field potential (LFP) recording
Mice were implanted with a 16-channel microelectrode array (MicroProbes, Gaithersburg, MD) targeting the basolateral amygdala (bregma + /−3.4 mm ML, −1.4 mm AP; −3.9 mm DV from brain surface). On day 1, mice were connected to the recording equipment (Plexon, Dallas, TX) and placed in a custom-built plexiglass chamber (approx. 20 cm × 20 cm × 33 cm tall) for acclimation and adjustment of recording parameters. On days 2 and 3, the mice were recorded for a 10-minute baseline and then for 30 min following intraperitoneal (i.p.) injection (0.0125 ml/g body weight) with 0.9% saline (day 2) or EtOH 1.5 g/kg (day 3). LFPs were recorded at 1000 Hz.
LFP data processing
Data were extracted from raw recordings using NeuroExplorer software (Plexon, Dallas, TX) and processed with MATLAB. LFP signal was averaged across 16 channels per mouse. Signal power was obtained by wavelet convolution across 30 frequency steps [34] and averaged across 60 s bins. Power data were normalized to a 6-minute window during the baseline period (minutes −8 to −2 prior to injection). Frequency bands were defined as delta (1–4 Hz), theta (5–8 Hz), alpha (9–12 Hz), beta (14–28 Hz), gamma (32–48 Hz), and high gamma (72–110 Hz). LFP frequency band power was analyzed with mixed effects models incorporating time, genotype, treatment, and their interactions as fixed effects, and mouse and treatment as random effects (R/RStudio).
Pavlovian fear conditioning
Mice were injected with saline (0 g/kg) or alcohol (0.25, 0.75, or 1.5 g/kg), returned to homecage for 5 min, then placed in fear conditioning chambers (Med Associates, VT). Fear conditioning was performed [35,36,37,38,39]. Briefly, on day 1 mice were habituated for 3 min followed by 5 tone (90 dB, 20 s)-footshock (0.75 mA, 1 s) pairings co-terminating, with an interstimulus interval of 120 s. On day 2, mice were placed back into the training context and freezing was assessed for 5 min. On day 3, freezing to conditioned stimulus (tone) was assessed in novel context. Two animals were excluded due to technical problems and 4 intra-group outliers were excluded (ROUT test). Freezing data was analyzed as a dose-response via linear regression (R/RStudio).
Open field test
Mice were injected with alcohol (0.25 to 2.5 g/kg) or saline (i.p.) and immediately placed into the center of the open field chamber (San Diego Instruments, San Diego, CA) [33, 40]. Activity was recorded by infrared beam breaks for 15 min. One intra-group outlier (ROUT test) was excluded. A best-fit regression model accounting for genotype and dose was identified by Akaike Information Criterion in the statistical software package R, and included a quadratic term for alcohol dose.
Blood alcohol levels (BALs)
Mice were injected with either high-dose alcohol (3.0 g/kg, i.p.), medium-dose alcohol (1.5 g/kg), or saline. Trunk blood was collected 6 min post-injection using K2 EDTA Microvette CB300 sampling tubes (Sarstedt Inc.). Plasma was analyzed using the Enzychrom kit (BioAssay Systems). The standard curve was calculated from alcohol-naïve mouse plasma spiked with known alcohol concentrations. BALs in alcohol-injected mice were compared by two-way ANOVA.
Intoxication scale
Mice were injected with alcohol (2.5, 3.0, and 3.5 g/kg, i.p.), placed into a plexiglass enclosure, and behavior was filmed for 30 min. Videos were scored by blinded observer. Total time in and initial latency to the following intoxication levels were quantified: (level 0) No observable effect on locomotion; (level 1) stumbling gait with upright posture, (level 2) stumbling gait/organized movements with frequent loss of posture (level 3) largely immobile with loss of posture, but with small movements, and (level 4) completely immobile and without maintained posture. These endpoints were previously used in similar sedation scale for rats [41, 42]. Mice were tested at each dose in a counterbalanced design with a 1-week interval between dosing. Total time spent in each level and latency to reach each level were analyzed by 2-way repeated measures ANOVAs, with planned t-test comparisons between genotypes.
Loss of righting reflex (LORR)
LORR duration following alcohol injection (3.5 g/kg i.p.) was assessed by placing mice supine in a V-shaped trough, modified from previous work [43]. Righting reflex was defined the ability to turn over 3 times in 30 s, or altogether resisting the supine position. Animals were tested every 5 min post-injection, and LORR duration was calculated as total time required to recover righting response. Mice had been previously tested for intoxication level, with a 1-week interval between assays. A t-test was used to compare LORR duration between genotypes.
Statistics
Data analysis was performed with Microsoft Excel, Graphpad Prism, R/RStudio [44, 45], and Mathworks MATLAB according to the needs of each experiment as described above. Third-party packages in R included the tidyverse [46], car [47], MASS [48], coin [49], lme4 [50], optimx [51], lmerTest [52], extrafont [53], and their dependencies. Details on all statistical tests can be found in Supplementary Table 1. In graphs, all error bars represent standard error of the mean (S.E.M.).
Results
Effects of alcohol on ASIC-mediated acid-evoked current
To test if ASICs might mediate effects of alcohol, we examined whether ASIC function was directly impacted by alcohol. We tested effects of a range of alcohol concentrations (10–100 mM) on ASIC1A homomeric channels by transfecting the ASIC1A subunit into CHO cells, which do not endogenously express these channels or have acid-evoked currents [54,55,56]. In ASIC1A-transfected CHO cells, alcohol (100 mM) by itself induced no current at pH 7.4, suggesting it does not activate ASIC1A homomeric channels on its own, or any other ionotropic receptors in these cells (Supplementary Fig. 1). However, when combined with acidic pH (pH = 6.0), alcohol increased the amplitude of acid-evoked currents in a dose-dependent manner, with up to 34% potentiation at 100 mM of alcohol (Fig. 1A, B) (linear regression, effect of alcohol dose, p < 0.0001). We next tested whether alcohol similarly affected ASIC1A/2 A heteromeric channels expressed in CHO cells. Interestingly, alcohol had no effect on these channels when combined with extracellular pH 6.0 (Fig. 1C, D) (linear regression, p = 0.7078), suggesting alcohol may increase the function of ASIC1A homomeric channels but not of ASIC1A/2 A heteromeric channels.
A Representative traces of acid-evoked currents (pH = 6.0) in ASIC1A-expressing CHO cells, with and without alcohol (100 mM). B Increasing alcohol concentration enhanced acid-evoked current in a dose-dependent manner (effect of dose, p < 0.0001, n = 3–6). C Representative traces of acid-evoked currents (pH = 6.0) in ASIC1A/2A-expressing CHO cells, with and without alcohol (100 mM). D Alcohol did not dose-dependently potentiate acid-evoked current of ASIC1A/2 A heteromers (no effect of dose, p = 0.7078, n = 5–9). E Representative traces of acid-evoked currents (pH = 6.3) in BLA principal neurons of Asic1a+/+ mice, with and without alcohol (100 mM). F Representative traces showing no acid-evoked currents (pH = 6.3) in BLA neurons of Asic1a−/− mice, with and without alcohol (100 mM). G Representative traces showing acid-evoked current in BLA neurons of Asic2−/− mice, with and without alcohol (100 mM). H Alcohol increased acid-evoked currents in BLA neurons of both Asic1a+/+ (t-test, *p = 0.0422, n = 14) and Asic2−/− mice (t-test, *p = 0.0415, n = 8).
To determine if endogenous ASICs in mouse brain neurons would be similarly affected by alcohol, we tested effects on basolateral amygdala (BLA) principal neurons. The BLA experiences physiological changes in response to alcohol exposure [57,58,59,60,61,62,63], is implicated in alcohol-related behaviors [64,65,66], abundantly expresses the ASIC1A subunit [15, 23, 26, 28], and is predicted to contain a substantial proportion of ASIC1A homomers [15]. Principal neurons were selected because they comprise the majority of neurons in the BLA [67] and have been found to have ASIC1A-dependent neuroplasticity [21]. As previously described [68], acidic pH (pH = 6.3) evoked large inward currents in Asic1a+/+mice (Fig. 1E). Moreover, co-application of 100 mM alcohol significantly enhanced these currents (Fig. 1E, H) (t-test, p = 0.0422), while lower doses (5 and 50 mM) produced less potentiation with mean values consistent with those observed in CHO cells. We also tested Asic1a−/− mice and saw no acid-evoked current in either the presence or absence alcohol, suggesting the effects depend on ASIC1A (Fig. 1F). To isolate ASIC1A homomeric channels, we similarly tested BLA principal neurons from Asic2−/− mice, which lack both ASIC2A and ASIC2B subunits [32]. We again found that 100 mM alcohol potentiated the acid-evoked currents (Fig. 1G, H) (t-test, p = 0.0415). These results were consistent with the effects of alcohol in CHO cells, and suggest that alcohol can directly enhance activation of ASIC1A homomeric channels in BLA neurons. Interestingly, the normal expression of ASIC2 subunits in wild-type mice did not preclude these effects of alcohol, presumably because a major proportion of the acid-evoked current in BLA principal neurons is mediated by ASIC1A homomeric channels.
Effects of alcohol and ASIC1A on neural activity in the amygdala in vivo
The above-described effects of alcohol on ASIC1A function in amygdala neurons in vitro raised the possibility that alcohol would affect brain function in vivo in an ASIC1A-dependent manner. To test this hypothesis, we implanted microelectrode arrays into the basolateral amygdala of Asic1a+/+ and Asic1a−/− mice. Local field potentials (LFPs) were recorded before and after saline or alcohol injections (Fig. 2A). We chose an alcohol dose (1.5 g/kg) previously found to alter LFPs in rodents [69,70,71] but not cause loss of consciousness. We computed power spectrograms normalized to pre-injection baseline (Fig. 2B–E) and compared specific frequency bands between groups (Fig. 2F–K). Compared to saline, alcohol had profound effects in both genotypes. In Asic1a+/+ mice, alcohol suppressed power across multiple frequency bands, including delta, alpha, beta, gamma, and high gamma, while leaving theta relatively unaffected (Fig. 2F–K). In Asic1a−/− mice, alcohol also suppressed alpha, beta, gamma, and high gamma power. There were several genotype-dependent effects of alcohol. While alcohol suppressed delta in Asic1a+/+ mice, in Asic1a−/− mice delta was largely unchanged (Fig. 2F) (genotype*treatment*time interaction, p = 0.0268). Alcohol also produced greater suppression of gamma and high gamma in Asic1a−/− mice compared to Asic1a+/+ mice (Fig. 2J, K) (genotype*treatment interactions, p < 0.0001 and p = 0.0454). Most strikingly, alcohol transiently increased theta in the Asic1a−/− mice, but not in the Asic1a+/+ mice (Fig. 2G) (genotype*treatment*time interaction, p < 0.0001). These results suggest alcohol exerts substantial effects on amygdala function in vivo, and that at least some of these effects depend on ASIC1A.
A Diagram of recording timeline (n = 6–7 mice per genotype). B–E Power spectrograms for four groups: (B) Asic1a+/+ with saline, (C) Asic1a+/+ with alcohol 1.5 g/kg (EtOH), (D) Asic1a−/− with saline, and (E) Asic1a−/− with alcohol. Data is normalized to the baseline period from −8 to −2 min (F–J) Normalized power over time across six frequency bands: (F) delta 1–4 Hz; (G) theta 5-8 Hz; (H) alpha 9–12 Hz; (I) beta 14–28 Hz; (J) gamma 32–48 Hz; and (K) high gamma 72–110 Hz. Dotted lines represent saline, solid lines represent EtOH. Black lines represent Asic1a+/+ (+/+), and red lines indicate Asic1a−/− (−/−). Data from each frequency band were analyzed from minutes 2–15 post-injection in a mixed-effects model. Only the highest-order significant effect involving alcohol treatment is indicated. There was a time*genotype*treatment interaction (‡) for delta (p = 0.0268) and theta (p < 0.0001), a genotype*treatment interaction (*) for gamma (p < 0.0001) and high gamma (p = 0.0454), and a main effect of alcohol (#) for alpha (p = 0.0008) and beta (p < 0.0001).
Effects of alcohol and ASIC1A on fear memory
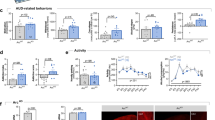
To explore potential impacts of alcohol and ASICs on behavior, we next tested Pavlovian fear conditioning. This learning and memory task depends on the amygdala [72], and has been suggested to be sensitive to alcohol [36,37,38,39, 73] as well as ASIC1A disruption [26, 35]. Asic1a+/+ and Asic1a−/− mice were injected with a range of alcohol doses (0.25, 0.75, or 1.5 g/kg) or saline (0 g/kg) on day 1, and trained to associate a previously neutral context and auditory cue with footshocks (Fig. 3A). Behavioral responses (freezing) during training and on subsequent test days were quantified (Fig. 3B–D, Supplementary Fig. 2). As previously reported, during training Asic1a−/− mice exhibited a marked deficit in freezing acquisition in the absence of alcohol [26, 33], which was further evident following alcohol injections (Fig. 3B) (linear regression, genotype effect, p < 0.0001). There was also an alcohol effect, with higher alcohol doses increasing freezing during acquisition (dose effect, p < 0.0001), which might be due to locomotor and/or sedating effects of alcohol, although prior to footshocks alcohol evoked little or no freezing by itself in either genotype (Supplementary Fig. 2A). The genotype by dose interaction during training was not significant (p = 0.103).
A Fear conditioning paradigm. Day 1, mice were injected with alcohol (EtOH) or saline and trained to associate context and auditory cue with aversive footshocks. Memory was subsequently tested in the absence of alcohol. Day 2, context-evoked freezing was tested. Day 3, auditory cue-evoked freezing was tested in a novel context. B During training (Day 1), there was a significant effect of both genotype (p < 0.0001, n = 14–23 per alcohol group, n = 47–58 per saline group) and alcohol dose (p < 0.0001) but no interaction (p = 0.1031). C During context testing (Day 2), there was a significant dose*genotype interaction (p < 0.0001). D During auditory cue-evoked testing (Day 3), there was a significant dose*genotype interaction (p = 0.0370).
Context and auditory cue evoked memory were tested after injected alcohol had cleared, on days 2 and 3 (Fig. 3A). Asic1a−/− mice displayed significant deficits in both context and cue-evoked memory, as described previously [26, 33] (Fig. 3C, D, Supplementary Fig. 2C, D) (linear regression, genotype effect, p < 0.0001 for both tests). Interestingly, although alcohol exposure increased freezing during training, this exposure led to substantial memory impairment (less freezing) during testing to both context and auditory cues, with higher doses during training causing greater impairment during testing (dose effect p < 0.0001, both context and cue). Moreover, there were significant genotype by alcohol dose interactions (context testing p < 0.0001; cued testing p = 0.0370). These results are consistent with the amnesia-inducing effects of alcohol [62,63,64,65,66], and suggest alcohol differentially impaired memory in mice lacking ASIC1A (Fig. 3C, D). However, the large baseline effects of ASIC1A disruption make it challenging to discern the nature of these differential effects. Therefore, we next assessed the effects of alcohol on tasks in which ASIC1A disruption does not cause baseline differences.
Stimulating and sedating effects of alcohol and ASIC1A
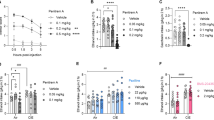
ASIC1A disruption by itself does not alter locomotor activity [33], whereas alcohol induces prominent effects in a dose-dependent manner [74]. Thus, locomotor responses provide a practical advantage for testing behavioral interactions between alcohol and ASIC1A. We assessed locomotor activity for 15 min following injection with a range of eight different alcohol doses (0.0–2.5 g/kg, i.p.). The resulting dose-response data were fitted to a model (see Methods), shown as dashed curves in Fig. 4A (also see time courses, Supplementary Fig. 3). The two genotypes displayed similar levels of activity following saline injections. As alcohol dose increased from 0.25 to 1.5 g/kg, locomotor activity in both genotypes increased above saline levels, indicating that alcohol elicited locomotor stimulation. Higher alcohol doses (2.0–2.5 g/kg for wild-types and 2.5 g/kg for Asic1a−/− mice) suppressed activity below saline levels, suggesting sedation. Importantly, there was a significant dose by genotype interaction (p = 0.0006) driven by an upward and rightward shift in the Asic1a−/− mice, suggesting more stimulation and less sedation. Despite these differences in behavior, blood alcohol levels between genotypes were similar after alcohol injection in a separate cohort of mice (1.5 and 3.0 g/kg, i.p.) (Fig. 4B), suggesting the different locomotor responses to alcohol were unlikely due to different absorption or metabolism.
A Biphasic, dose-dependent locomotor response to alcohol in the open field. A best-fit regression model revealed a dose*genotype interaction (p = 0.0006, n = 6–15 per group). B Blood alcohol level did not differ between genotypes at any dose tested, and there was no interaction with dose (2-way ANOVA, p-values > 0.05 for genotype effect and genotype*dose interaction, n = 2–7 per group). C Loss of righting reflex (LORR) duration following 3.5 g/kg EtOH i.p. was decreased in Asic1a−/− mice (t-test, p = 0.0051, n = 17–19 per group). D Total time at each level of intoxication (Table 1) across a range of alcohol doses. Asic1a–/– mice spent more time at a lower level of intoxication (level 1) (2-way repeated measures ANOVA, genotype effect, p = 0.0403, n = 17–19 per group), and less time at a higher able level of intoxication (level 3) (genotype effect, p = 0.0012). Significant planned comparisons are indicated with asterisks. E Latency to reach each level of intoxication across a range of alcohol doses. Asic1a–/– mice took longer to reach level 2 overall (genotype effect, p = 0.0024) and took longer to reach level 3 at the lowest dose (genotype*dose interaction, p = 0.0480). Significant planned comparisons are indicated with asterisks.
Because of the ASIC1A-dependent effects of alcohol dose in the open field, we wondered whether ASIC1A would affect other assessments of acute intoxication. To test the contribution of ASIC1A to alcohol-induced sedation, we assessed the loss of righting reflex, which is tested with higher alcohol doses than those used in the open field. Asic1a−/− mice recovered their righting reflex significantly faster than wild-type mice after an i.p. injection of 3.5 g/kg of alcohol (Fig. 4C) (t-test, p = 0.0051), suggesting that mice lacking ASIC1A were less severely obtunded by a high alcohol dose.
Because the sedating and intoxicating effects of alcohol tend to progress in severity from minor ataxia to loss of consciousness, we also classified behaviors along this progression using an alcohol intoxication scale spanning 30 min post-injection (Table 1). This scale allowed us to assess multiple alcohol doses with greater sensitivity than loss of righting reflex. We found that latency to reach each intoxication level and total time spent at each level depended highly on alcohol dose (Fig. 4D, E, Supplementary Fig. 4B). The lowest dose (2.5 g/kg, i.p.) caused mice to spend the majority of time in intoxication levels 1 and 2, characterized largely by ataxia, while the highest dose (3.5 g/kg, i.p.) caused mice to shift to intoxication levels 3 and 4, characterized by immobility and loss of consciousness. Overall, compared to wild-type mice, the Asic1a–/– mice spent more time at a lower level of intoxication (level 1), and less time at a higher level of intoxication (level 3) (repeated measures 2-way ANOVAs, genotype effects, p = 0.0403 and p = 0.0012 respectively). ASIC1A disruption also affected latency taking longer to reach intoxication levels 2 and 3, especially at the lowest alcohol dose (repeated measures 2-way ANOVAs, genotype effect for level 2, p = 0.0024; genotype by dose interaction for level 3, p = 0.0480). Taken together, the loss of righting reflex test and intoxication levels suggest that ASIC1A disruption shifts intoxication-related behavior towards the lower end of the spectrum, i.e., less severe intoxication.
Discussion
Using multiple independent assays, these studies revealed diverse neural and behavioral effects of alcohol across a range of doses in wild-type mice that differed significantly in mice lacking ASIC1A. These results thus identify ASIC1A as a novel contributor to the complex molecular and behavioral actions of acute alcohol intoxication.
There are numerous ways by which ASIC1A function could affect alcohol intoxication. One previous study reported no direct effects of alcohol on ASICs, although that study used a different methodology that did not distinguish between ASIC1A homomers, ASIC1A/2 A heteromers, or other ASIC subunits [75]. In contrast, our results in CHO cells and BLA neurons suggest alcohol may enhance acid-induced activation of ASIC1A homomeric channels, which might contribute to alcohol intoxication. We speculate this effect of alcohol on channel function likely involves alcohol’s ability to form hydrogen bonds [10]. Our results further suggest bonding may be specific to the ASIC1A subunit, and possibly a site formed between multiple ASIC1A subunits, given that ASIC1A/2 A heteromers were unaffected. Further studies will be required to pinpoint potential sites for such interactions.
Other possibilities for how ASICs affect alcohol intoxication are also conceivable. For example, alcohol might influence ASICs through its ability to induce metabolic acidosis [76,77,78]. Alternatively, alcohol increases neurotransmitter release from presynaptic vesicles, which are highly acidic and can transiently lower synaptic pH [21], and might thus facilitate ASIC activation at synapses [79, 80]. Additionally, ASIC1A may modulate previously established actions of alcohol on neurotransmitter systems, such as glutamatergic signaling. For example, alcohol preferentially inhibits NMDA receptors over AMPA receptors [81] and disrupting ASIC1A increases the AMPA/NMDA receptor ratio at glutamatergic synapses in multiple brain areas [20, 82]. Thus, molecular interactions between alcohol and ASIC1A could be multifold. Additional studies will be necessary to discern which potential mechanisms may be most important.
Consistent with such mechanistic possibilities, ASIC1A disruption substantially impacted alcohol-evoked changes in local field potentials in the amygdala in vivo. ASIC1A disruption altered alcohol responses in the gamma, delta, and theta frequency ranges. Gamma suppression was greater, delta suppression was reduced, and theta was exclusively potentiated in Asic1a−/− mice. These observations are largely consistent with previous studies linking these specific frequency bands with learning and memory and/or responses to emotional stimuli [83,84,85,86,87,88,89,90,91,92,93], and seem likely to contribute to the behavioral interactions observed here, particularly the fear conditioning effects. Although the relationship between local field potentials and neuronal activity is complex, these data suggest that alcohol and ASICs interact to influence brain activity.
Consistent with the above-described interactions between alcohol and ASICs on neural responses in vitro and in vivo, multiple behavioral outcomes here supported a critical role for ASIC1A in alcohol intoxication. The fear conditioning results suggest ASIC1A contributes to the amnestic effects of alcohol intoxication. However, the nature of this interaction is difficult to fully interpret because ASIC1A disruption by itself produced such dramatic effects on conditioned fear memory. Although, previous studies suggested that fear conditioning deficits in Asic1a–/– mice were not due to impaired shock sensitivity, an inability to freeze, impaired hearing, or altered locomotor activity [25, 26, 33].
The other interactions between alcohol and ASIC1A disruption were more straightforward to interpret, including effects on locomotion, loss of righting reflex, and intoxication severity scores. In each of these assays, Asic1a−/− mice displayed less sedation and more activation. Differences were due to more than just a shift in dose-response, because in the open field assay the alcohol-evoked hyperactivity displayed by Asic1a−/− mice was never reached by Asic1a+/+ mice. Increased locomotor activity evoked by low-dose alcohol in mice likely parallels the excitement and disinhibition evoked in humans. Similarly, the hypoactivity, loss of coordination, stupor, and loss of consciousness in mice at high doses closely resemble features of human intoxication. Because ASIC1A is widely expressed in brain, its effects on alcohol intoxication may result from channel action in a wide variety of brain sites. Pinpointing sites of ASIC1A action on specific behavioral effects of alcohol will require additional, and likely extensive, studies. Because blood alcohol levels did not differ between Asic1a−/− and Asic1a+/+ mice, ASIC1A likely mediates these effects of alcohol rather than its absorption or clearance. In humans, genetic factors have been suggested to play a major role in acute responses to alcohol with an estimated heritability of 60% [94]. Importantly, people who report less sedation and/or more stimulation from alcohol are more likely to have a family history of AUD [5], a greater preference for alcohol [95], higher levels of alcohol consumption [96], as well as a higher risk of developing AUD [6,7,8]. This profile is remarkably similar to what we observed in Asic1a−/− mice, suggesting ASICs might contribute to AUD in humans.
In summary, this work identifies ASIC1A as a novel molecular contributor to the acute actions of alcohol. The results further suggest that disrupting ASIC1A in mice leads to phenotypes resembling human characteristics previously linked to AUD risk. Together these observations could have important clinical implications for people with genetic variations in ASIC1A, and may open new avenues for research into the mechanisms underlying AUD.
References
Glantz MD, Bharat C, Degenhardt L, Sampson NA, Scott KM, Lim CCW, et al. The epidemiology of alcohol use disorders cross-nationally: findings from the World Mental Health Surveys. Addict Behav. 2020;102:106128. https://doi.org/10.1016/j.addbeh.2019.106128.
Sacks JJ, Gonzales KR, Bouchery EE, Tomedi LE, Brewer RD. 2010 National and state costs of excessive alcohol consumption. Am J Prev Med. 2015;49:e73–9. https://doi.org/10.1016/j.amepre.2015.05.031.
Rehm J, Gmel GE Sr, Gmel G, Hasan OSM, Imtiaz S, Popova S, et al. The relationship between different dimensions of alcohol use and the burden of disease—an update. Addiction. 2017;112:968–1001. https://doi.org/10.1111/add.13757.
Kendler KS, Ohlsson H, Karriker-Jaffe KJ, Sundquist J, Sundquist K. Social and economic consequences of alcohol use disorder: a longitudinal cohort and co-relative analysis. Psychol Med. 2017;47:925–35. https://doi.org/10.1017/S0033291716003032.
Schuckit MA. Self-rating of alcohol intoxication by young men with and without family histories of alcoholism. J Stud Alcohol. 1980;41:242–9.
Schuckit MA, Smith TL. An 8-year follow-up of 450 sons of alcoholic and control subjects. Arch Gen Psychiatry. 1996;53:202–10.
King AC, de Wit H, McNamara PJ, Cao D. Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Arch Gen Psychiatry. 2011;68:389–99. https://doi.org/10.1001/archgenpsychiatry.2011.26.
King AC, McNamara PJ, Hasin DS, Cao D. Alcohol challenge responses predict future alcohol use disorder symptoms: a 6-year prospective study. Biol Psychiatry. 2014;75:798–806. https://doi.org/10.1016/j.biopsych.2013.08.001.
Koob GF, Arends MA, Le Moal M. Chapter 6 - Alcohol. Drugs, addiction, and the brain. San Diego: Academic Press; 2014. p. 173–219.
Dwyer DS, Bradley RJ. Chemical properties of alcohols and their protein binding sites. Cell Mol Life Sci Cmls. 2000;57:265–75. https://doi.org/10.1007/PL00000689.
Abrahao KP, Salinas AG, Lovinger DM. Alcohol and the brain: neuronal molecular targets, synapses, and circuits. Neuron. 2017;96:1223–38. https://doi.org/10.1016/j.neuron.2017.10.032.
Wemmie JA, Taugher RJ, Kreple CJ. Acid-sensing ion channels in pain and disease. Nat Rev Neurosci. 2013;14:461–71. https://doi.org/10.1038/nrn3529.
Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature. 1997;386:173–7. https://doi.org/10.1038/386173a0.
Hesselager M, Timmermann DB, Ahring PK. pH dependency and desensitization kinetics of heterologously expressed combinations of acid-sensing Ion channel subunits. J Biol Chem. 2004;279:11006–15. https://doi.org/10.1074/jbc.M313507200.
Wu J, Xu Y, Jiang YQ, Xu J, Hu Y, Zha XM. ASIC subunit ratio and differential surface trafficking in the brain. Mol Brain. 2016;9:4. https://doi.org/10.1186/s13041-016-0185-7.
Joeres N, Augustinowski K, Neuhof A, Assmann M, Gründer S. Functional and pharmacological characterization of two different ASIC1a/2a heteromers reveals their sensitivity to the spider toxin PcTx1. Sci Rep. 2016;6:27647. https://doi.org/10.1038/srep27647.
Sherwood TW, Lee KG, Gormley MG, Askwith CC. Heteromeric ASIC channels composed of ASIC2b and ASIC1a display novel channel properties and contribute to acidosis-induced neuronal death. J Neurosci: Off J Soc Neurosci. 2011;31:9723–34. https://doi.org/10.1523/JNEUROSCI.1665-11.2011.
Vullo S, Kellenberger S. A molecular view of the function and pharmacology of acid-sensing ion channels. Pharmacol Res. 2020;154:104166. https://doi.org/10.1016/j.phrs.2019.02.005.
Wemmie JA, Price MP, Welsh MJ. Acid-sensing ion channels: advances, questions and therapeutic opportunities. Trends Neurosci. 2006;29:578–86. https://doi.org/10.1016/j.tins.2006.06.014.
Kreple CJ, Lu Y, Taugher RJ, Schwager-Gutman AL, Du J, Stump M, et al. Acid-sensing ion channels contribute to synaptic transmission and inhibit cocaine-evoked plasticity. Nat Neurosci. 2014;17:1083–91. https://doi.org/10.1038/nn.3750.
Du J, Reznikov LR, Price MP, Zha X-M, Lu Y, Moninger TO, et al. Protons are a neurotransmitter that regulates synaptic plasticity in the lateral amygdala. Proc Natl Acad Sci. 2014;111:8961–6. https://doi.org/10.1073/pnas.1407018111.
Askwith CC, Wemmie JA, Price MP, Rokhlina T, Welsh MJ. Acid-sensing Ion channel 2 (ASIC2) modulates ASIC1 H+-activated currents in Hippocampal neurons. J Biol Chem. 2004;279:18296–305. https://doi.org/10.1074/jbc.M312145200.
Chiang P-H, Chien T-C, Chen C-C, Yanagawa Y, Lien C-C. ASIC-dependent LTP at multiple glutamatergic synapses in amygdala network is required for fear memory. Sci Rep. 2015;5:10143. https://doi.org/10.1038/srep10143.
González-Inchauspe C, Urbano FJ, Di Guilmi MN, Uchitel OD. Acid-sensing ion channels activated by evoked released protons modulate synaptic transmission at the mouse calyx of Held synapse. J Neurosci. 2017;37:2589–99. https://doi.org/10.1523/jneurosci.2566-16.2017.
Wemmie JA, Chen J, Askwith CC, Hruska-Hageman AM, Price MP, Nolan BC, et al. The acid-activated ion channel ASIC contributes to synaptic plasticity, learning, and memory. Neuron. 2002;34:463–77.
Wemmie JA, Askwith CC, Lamani E, Cassell MD, Freeman JH Jr., Welsh MJ. Acid-sensing ion channel 1 is localized in brain regions with high synaptic density and contributes to fear conditioning. J Neurosci. 2003;23:5496–502.
Li W-G, Liu M-G, Deng S, Liu Y-M, Shang L, Ding J, et al. ASIC1a regulates insular long-term depression and is required for the extinction of conditioned taste aversion. Nat Commun. 2016;7:13770. https://doi.org/10.1038/ncomms13770.
Price MP, Gong H, Parsons MG, Kundert JR, Reznikov LR, Bernardinelli L, et al. Localization and behaviors in null mice suggest that ASIC1 and ASIC2 modulate responses to aversive stimuli. Genes Brain Behav. 2014;13:179–94. https://doi.org/10.1111/gbb.12108.
Jiang Q, Wang CM, Fibuch EE, Wang JQ, Chu XP. Differential regulation of locomotor activity to acute and chronic cocaine administration by acid-sensing ion channel 1a and 2 in adult mice. Neuroscience. 2013;246:170–8. https://doi.org/10.1016/j.neuroscience.2013.04.059.
Mukhopadhyay M, Bera AK. Modulation of acid-sensing ion channels by hydrogen sulfide. Biochem Biophys Res Commun. 2020;527:71–5. https://doi.org/10.1016/j.bbrc.2020.04.092.
Mukhopadhyay M, Singh A, Sachchidanand S, Bera AK. Quercetin inhibits acid-sensing ion channels through a putative binding site in the central vestibular region. Neuroscience. 2017;348:264–72. https://doi.org/10.1016/j.neuroscience.2017.02.025.
Price MP, Lewin GR, McIlwrath SL, Cheng C, Xie J, Heppenstall PA, et al. The mammalian sodium channel BNC1 is required for normal touch sensation. Nature. 2000;407:1007–11. https://doi.org/10.1038/35039512.
Coryell MW, Ziemann AE, Westmoreland PJ, Haenfler JM, Kurjakovic Z, Zha XM, et al. Targeting ASIC1a reduces innate fear and alters neuronal activity in the fear circuit. Biol Psychiatry. 2007;62:1140–8. https://doi.org/10.1016/j.biopsych.2007.05.008.
Cohen MX. Analyzing neural time series data: theory and practice. Cambridge, MA: MIT press; 2014.
Taugher RJ, Lu Y, Fan R, Ghobbeh A, Kreple CJ, Faraci FM, et al. ASIC1A in neurons is critical for fear-related behaviors. Genes Brain Behav. 2017;16:745–55. https://doi.org/10.1111/gbb.12398.
Gould TJ. Ethanol disrupts fear conditioning in C57BL/6 mice. J Psychopharmacol (Oxf, Engl). 2003;17:77–81. https://doi.org/10.1177/0269881103017001702.
Gould TJ, Lommock JA. Nicotine enhances contextual fear conditioning and ameliorates ethanol-induced deficits in contextual fear conditioning. Behav Neurosci. 2003;117:1276–82. https://doi.org/10.1037/0735-7044.117.6.1276.
Gulick D, Gould TJ. Acute ethanol has biphasic effects on short- and long-term memory in both foreground and background contextual fear conditioning in C57BL/6 mice. Alcohol: Clin Exp Res. 2007;31:1528–37. https://doi.org/10.1111/j.1530-0277.2007.00458.x.
Seemiller LR, Gould TJ. Adult and adolescent C57BL/6J and DBA/2J mice are differentially susceptible to fear learning deficits after acute ethanol or MK-801 treatment. Behavioural Brain Res. 2021;410:113351. https://doi.org/10.1016/j.bbr.2021.113351.
Bocarsly ME, da Silva E Silva D, Kolb V, Luderman KD, Shashikiran S, Rubinstein M, et al. A mechanism linking two known vulnerability factors for alcohol abuse: heightened alcohol stimulation and low striatal dopamine D2 receptors. Cell Rep. 2019;29:1147–63.e5. https://doi.org/10.1016/j.celrep.2019.09.059.
Salamone JD, Cousins MS, Maio C, Champion M, Turski T, Kovach J. Different behavioral effects of haloperidol, clozapine and thioridazine in a concurrent lever pressing and feeding procedure. Psychopharmacology. 1996;125:105–12. https://doi.org/10.1007/BF02249408.
Chuck TL, McLaughlin PJ, Arizzi-LaFrance MN, Salamone JD, Correa M. Comparison between multiple behavioral effects of peripheral ethanol administration in rats: Sedation, ataxia, and bradykinesia. Life Sci. 2006;79:154–61. https://doi.org/10.1016/j.lfs.2005.12.045.
Blednov YA, Black M, Benavidez JM, Da Costa A, Mayfield J, Harris RA. Sedative and motor incoordination effects of ethanol in mice lacking CD14, TLR2, TLR4, or MyD88. Alcohol: Clin Exp Res. 2017;41:531–40. https://doi.org/10.1111/acer.13314.
R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2020.
RStudio Team. RStudio: Integrated Development Environment for R. Boston, MA: RStudio, PBC; 2020.
Wickham H, Averick M, Bryan J, Chang W, McGowan L, François R, et al. Welcome to the Tidyverse. J Open Source Softw. 2019;4:1686.
Fox J, Weisberg S. An {R} Companion to Applied Regression. Thousand Oaks, CA: Sage; 2019. p. car citation.
Venables WN, Ripley BD. Modern Applied Statistics with S. 4th ed. New York: Springer; 2002.
Hothorn T, Hornik K, van de Wiel MA, Zeileis A. Implementing a class of permutation tests: the coin package. J Stat Softw. 2008;28:23. https://doi.org/10.18637/jss.v028.i08.
Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:48. https://doi.org/10.18637/jss.v067.i01.
Nash JC, Varadhan R. Unifying optimization algorithms to aid software system users: optimx for R. J Stat Softw. 2011;43:14. https://doi.org/10.18637/jss.v043.i09.
Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest package: tests in linear mixed effects models. J Stat Softw. 2017;82:26. https://doi.org/10.18637/jss.v082.i13.
Chang W. extrafont: Tools for using fonts. R package version 0.17. 2014.
Smith ESJ, Zhang X, Cadiou H, McNaughton PA. Proton binding sites involved in the activation of acid-sensing ion channel ASIC2a. Neurosci Lett. 2007;426:12–7. https://doi.org/10.1016/j.neulet.2007.07.047.
García-Añoveros J, Samad TA, Zuvela-Jelaska L, Woolf CJ, Corey DP. Transport and localization of the DEG/ENaC ion channel BNaC1alpha to peripheral mechanosensory terminals of dorsal root ganglia neurons. J Neurosci: Off J Soc Neurosci. 2001;21:2678–86. https://doi.org/10.1523/JNEUROSCI.21-08-02678.2001.
Cadiou H, Studer M, Jones NG, Smith ESJ, Ballard A, McMahon SB, et al. Modulation of acid-sensing ion channel activity by nitric oxide. J Neurosci. 2007;27:13251–60. https://doi.org/10.1523/jneurosci.2135-07.2007.
Perra S, Pillolla G, Luchicchi A, Pistis M. Alcohol inhibits spontaneous activity of basolateral amygdala projection neurons in the rat: involvement of the endocannabinoid system. Alcohol: Clin Exp Res. 2008;32:443–9. https://doi.org/10.1111/j.1530-0277.2007.00588.x.
Zhu PJ, Lovinger DM. Ethanol potentiates GABAergic synaptic transmission in a postsynaptic neuron/synaptic bouton preparation from basolateral amygdala. J Neurophysiol. 2006;96:433–41. https://doi.org/10.1152/jn.01380.2005.
Silberman Y, Shi L, Brunso-Bechtold JK, Weiner JL. Distinct mechanisms of ethanol potentiation of local and paracapsular GABAergic synapses in the rat basolateral amygdala. J Pharmacol Exp Ther. 2008;324:251–60. https://doi.org/10.1124/jpet.107.128728.
Läck AK, Ariwodola OJ, Chappell AM, Weiner JL, McCool BA. Ethanol inhibition of kainate receptor-mediated excitatory neurotransmission in the rat basolateral nucleus of the amygdala. Neuropharmacology. 2008;55:661–8. https://doi.org/10.1016/j.neuropharm.2008.05.026.
Läck AK, Diaz MR, Chappell A, DuBois DW, McCool BA. Chronic ethanol and withdrawal differentially modulate pre- and postsynaptic function at glutamatergic synapses in rat basolateral amygdala. J Neurophysiol. 2007;98:3185–96. https://doi.org/10.1152/jn.00189.2007.
Robinson SL, Alexander NJ, Bluett RJ, Patel S, McCool BA. Acute and chronic ethanol exposure differentially regulate CB1 receptor function at glutamatergic synapses in the rat basolateral amygdala. Neuropharmacology. 2016;108:474–84. https://doi.org/10.1016/j.neuropharm.2015.12.005.
Lindemeyer AK, Liang J, Marty VN, Meyer EM, Suryanarayanan A, Olsen RW, et al. Ethanol-induced plasticity of GABAA receptors in the basolateral amygdala. Neurochem Res. 2014;39:1162–70. https://doi.org/10.1007/s11064-014-1297-z.
Gremel CM, Cunningham CL. Involvement of amygdala dopamine and nucleus accumbens NMDA receptors in ethanol-seeking behavior in mice. Neuropsychopharmacology. 2009;34:1443–53. https://doi.org/10.1038/npp.2008.179.
Gremel CM, Cunningham CL. Roles of the nucleus accumbens and amygdala in the acquisition and expression of ethanol-conditioned behavior in mice. J Neurosci. 2008;28:1076–84. https://doi.org/10.1523/jneurosci.4520-07.2008.
Sciascia JM, Reese RM, Janak PH, Chaudhri N. Alcohol-seeking triggered by discrete Pavlovian cues is invigorated by alcohol contexts and mediated by glutamate signaling in the basolateral amygdala. Neuropsychopharmacology. 2015;40:2801–12. https://doi.org/10.1038/npp.2015.130.
Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiological Rev. 2003;83:803–34. https://doi.org/10.1152/physrev.00002.2003.
Ziemann AE, Allen JE, Dahdaleh NS, Drebot II, Coryell MW, Wunsch AM, et al. The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell. 2009;139:1012–21. https://doi.org/10.1016/j.cell.2009.10.029.
Slawecki CJ. Altered EEG responses to ethanol in adult rats exposed to ethanol during adolescence. Alcohol: Clin Exp Res. 2002;26:246–54. https://doi.org/10.1111/j.1530-0277.2002.tb02531.x.
Pian JP, Criado JR, Walker BM, Ehlers CL. Differential effects of acute alcohol on EEG and sedative responses in adolescent and adult Wistar rats. Brain Res. 2008;1194:28–36. https://doi.org/10.1016/j.brainres.2007.11.057.
Ehlers CL, Desikan A, Wills DN. Developmental differences in EEG and sleep responses to acute ethanol administration and its withdrawal (hangover) in adolescent and adult Wistar rats. Alcohol. 2013;47:601–10. https://doi.org/10.1016/j.alcohol.2013.09.040.
Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–85. https://doi.org/10.1037//0735-7044.106.2.274.
Gulick D, Gould TJ. Interactive effects of ethanol and nicotine on learning in C57BL/6J mice depend on both dose and duration of treatment. Psychopharmacology. 2008;196:483–95. https://doi.org/10.1007/s00213-007-0982-x.
Pohorecky LA. Biphasic action of ethanol. Biobehav Rev. 1977;1:231–40. https://doi.org/10.1016/0147-7552(77)90025-0.
Zhou R-P, Leng T-D, Yang T, Chen F-H, Xiong Z-G. Acute ethanol exposure promotes autophagy-lysosome pathway-dependent ASIC1a protein degradation and protects against acidosis-induced neurotoxicity. Mol Neurobiol. 2019;56:3326–40. https://doi.org/10.1007/s12035-018-1289-0.
Halperin ML, Hammeke M, Josse RG, Jungas RL. Metabolic acidosis in the alcoholic: a pathophysiologic approach. Metabolism. 1983;32:308–15. https://doi.org/10.1016/0026-0495(83)90197-x.
Goldman H, Sapirstein L, Murphy S, Moore J. Alcohol and regional blood flow in brains of rats. Proc Soc Exp Biol Med. 1973;144:983–8.
Mitchell MA, Belknap JK. The effects of alcohol withdrawal and acute doses of alcohol on the acid-base balance in mice and rats. Drug Alcohol Depend. 1982;10:283–94. https://doi.org/10.1016/0376-8716(82)90031-X.
Roberto M, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Increased GABA release in the central amygdala of ethanol-dependent rats. J Neurosci. 2004;24:10159–66. https://doi.org/10.1523/jneurosci.3004-04.2004.
Imperato A, Di Chiara G. Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. J Pharmacol Exp Ther. 1986;239:219–28.
Nie Z, Madamba SG, Siggins GR. Ethanol inhibits glutamatergic neurotransmission in nucleus accumbens neurons by multiple mechanisms. J Pharmacol Exp Ther. 1994;271:1566–73.
Yu Z, Wu Y-J, Wang Y-Z, Liu D-S, Song X-L, Jiang Q, et al. The acid-sensing ion channel ASIC1a mediates striatal synapse remodeling and procedural motor learning. Sci Signal. 2018;11. https://doi.org/10.1126/scisignal.aar4481
Seidenbecher T, Laxmi TR, Stork O, Pape H-C. Amygdalar and hippocampal theta rhythm synchronization during fear memory retrieval. Science. 2003;301:846–50.
Pape H-C, Narayanan RT, Smid J, Stork O, Seidenbecher T. Theta activity in neurons and networks of the amygdala related to long-term fear memory. Hippocampus. 2005;15:874–80. https://doi.org/10.1002/hipo.20120.
Likhtik E, Stujenske JM, Topiwala MA, Harris AZ, Gordon JA. Prefrontal entrainment of amygdala activity signals safety in learned fear and innate anxiety. Nat Neurosci. 2014;17:106–13. https://doi.org/10.1038/nn.3582.
Karalis N, Dejean C, Chaudun F, Khoder S, Rozeske RR, Wurtz H, et al. 4-Hz oscillations synchronize prefrontal–amygdala circuits during fear behavior. Nat Neurosci. 2016;19:605–12. https://doi.org/10.1038/nn.4251.
Kling AS, Lloyd RL, Perryman KM. Slow wave changes in amygdala to visual, auditory, and social stimuli following lesions of the inferior temporal cortex in squirrel monkey (Saimiri sciureus). Behav Neural Biol. 1987;47:54–72. https://doi.org/10.1016/S0163-1047(87)90156-7.
Lloyd RL, Kling AS. Delta activity from amygdala in squirrel monkeys (Saimiri sciureus): Influence of social and environmental context. Behav Neurosci. 1991;105:223–9. https://doi.org/10.1037/0735-7044.105.2.223.
Fedele T, Tzovara A, Steiger B, Hilfiker P, Grunwald T, Stieglitz L, et al. The relation between neuronal firing, local field potentials and hemodynamic activity in the human amygdala in response to aversive dynamic visual stimuli. Neuroimage. 2020;213:116705. https://doi.org/10.1016/j.neuroimage.2020.116705.
Bauer EP, Paz R, Paré D. Gamma oscillations coordinate amygdalo-rhinal interactions during learning. J Neurosci. 2007;27:9369–79. https://doi.org/10.1523/jneurosci.2153-07.2007.
Popescu AT, Popa D, Paré D. Coherent gamma oscillations couple the amygdala and striatum during learning. Nat Neurosci. 2009;12:801–7. https://doi.org/10.1038/nn.2305.
Kanta V, Pare D, Headley DB. Closed-loop control of gamma oscillations in the amygdala demonstrates their role in spatial memory consolidation. Nat Commun. 2019;10:3970. https://doi.org/10.1038/s41467-019-11938-8.
Courtin J, Karalis N, Gonzalez-Campo C, Wurtz H, Herry C. Persistence of amygdala gamma oscillations during extinction learning predicts spontaneous fear recovery. Neurobiol Learn Mem. 2014;113:82–9. https://doi.org/10.1016/j.nlm.2013.09.015.
Heath AC, Madden PA, Bucholz KK, Dinwiddie SH, Slutske WS, Bierut LJ, et al. Genetic differences in alcohol sensitivity and the inheritance of alcoholism risk. Psychol Med. 1999;29:1069–81.
Chutuape MA, de Wit H. Relationship between subjective effects and drug preferences: ethanol and diazepam. Drug Alcohol Depend. 1994;34:243–51.
King AC, Houle T, Wit H, Holdstock L, Schuster A. Biphasic alcohol response differs in heavy versus light drinkers. Alcohol: Clin Exp Res. 2002;26:827–35.
Acknowledgements
We would like to thank Dr. Youngcho Kim for assistance in in vivo electrophysiology techniques.
Funding
JAW was supported by NIH National Institute of Mental Health grant R01MH113325, NIH National Institute of Drug Abuse grant R01DA052953, the Roy J. Carver Charitable Trust, the Roy J. Carver Chair, a U.S. Department of Veterans Affairs Merit Review Award, and the U.S. Department of Veterans Affairs. GISH was supported by NIH National Institute of Neurological Disorders and Stroke training grant T32NS007421, NIH National Institute of General Medical Sciences training grant T32GM067795, and the University of Iowa Ballard and Seashore Dissertation Fellowship. ACC was supported by NIH National Institute of Mental Health training grant T32MH019113. MJM was supported by the Iowa Neuroscience Institute Summer Scholar Award. BJD was supported by NS-112573. AKH was supported by a fellowship from UGC, Govt. of India. NSN was supported by NIH National Institute of Mental Health grant R01MH116043-01A1. Tools for CHO cell research (rASIC1a-IRES2-DsRed and pcDNA3.1-rASIC2A) were given to AKB by Francois Rugiero, University College London, UK and Peter McNaughton, University of Cambridge, UK, respectively.
Author information
Authors and Affiliations
Contributions
GISH conceptualized experiments, acquired data, analyzed data, interpreted data, and wrote the manuscript. ACC conceptualized experiments, acquired data, analyzed data, and interpreted data. MJM conceptualized experiments and acquired data. RJT acquired data, analyzed data, interpreted data, and wrote the manuscript. AKH acquired and analyzed data. JBH acquired and analyzed data. RF acquired data. JDL was an essential contributor to advanced data analysis. GZW acquired data. BJD conceptualized experiments, provided funding, and interpreted data. AKB conceptualized experiments. NSN conceptualized experiments and advised on data analysis. JAW conceptualized experiments, interpreted data, provided funding, and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Harmata, G.I.S., Chan, A.C., Merfeld, M.J. et al. Intoxicating effects of alcohol depend on acid-sensing ion channels. Neuropsychopharmacol. 48, 806–815 (2023). https://doi.org/10.1038/s41386-022-01473-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-022-01473-4