Abstract
Anxious traits are elevated in eating disorders (EDs), are considered risk factors for ED development, and trait anxiety has been linked to ED psychopathology. How trait anxiety relates to ED neurobiology is not well understood. In this study 197 individuals across the ED spectrum (anorexia nervosa n = 91; other specified EDs n = 34; bulimia nervosa n = 56; binge ED n = 16), and 120 healthy controls were assessed for anxious traits and learned to expect and receive caloric or neutral taste stimuli during brain imaging. Amygdala sucrose expectation response differed across groups (Wilk’s lambda = 0.945, p = 0.023), and was higher on the left in anorexia nervosa compared to healthy controls (p = 0.002). Expected sucrose receipt response across taste reward regions was not different between groups. In the ED sample, trait anxiety negatively moderated the relationship between amygdala expectation and right dorsal (p = 0.0062) and ventral (p = 0.0046) anterior insula receipt response. A subgroup analysis showed similar results for anorexia nervosa, and partially in bulimia nervosa. Across EDs, appetitive motivation correlated positively with bilateral orbitofrontal cortex, caudate head, and ventral striatal sucrose receipt response (r = 0.215 to 0.179, p = 0.002 to 0.012). Across the study sample, trait anxiety showed an inverted-U-shaped relationship with right (r = 0.147, p = 0.034) and left (r = 0.162, p = 0.016) amygdala expectation response. Amygdala sucrose expectation response is elevated in anorexia nervosa, correlates with sucrose receipt response, and this relationship is negatively moderated by trait anxiety across EDs. Trait anxiety may have an important role in how expectation drives taste stimulus receipt brain response and perhaps food approach in individuals with EDs.
Similar content being viewed by others
Introduction
Eating disorders (EDs) are severe psychiatric disorders with complex bio-psycho-social etiology [1]. Individuals with anorexia nervosa (AN) are underweight and may intermittently binge-eat or purge, individuals with bulimia nervosa (BN) tend to be at normal to high weight and regularly binge-eat and purge, while individuals with binge-eating disorder (BED) regularly binge-eat without compensatory behaviors [2]. The Other Specified Feeding and Eating Disorders (OSFED) category encompasses EDs that do not meet full criteria for AN, BN or BED. While individuals with EDs present with a range of behaviors from food restriction to overeating, they typically share high body dissatisfaction and drive for thinness. Other transdiagnostic behaviors that are thought to contribute to the often-chronic course of EDs include difficulty tolerating strong emotional states, anxiety, sadness or anger [3, 4].
Various studies have suggested that anxious traits such as trait anxiety and harm avoidance are potential risk factors for EDs [5,6,7]. Individuals with those traits experience negative emotions including fears, worries, and anxiety across many situations and tend to perceive environmental stimuli as threatening [8,9,10]. Trait anxiety has been associated with ED psychopathology previously [11,12,13]. For instance, one study found that trait anxiety was related to low self-confidence and avoidance of social eating across EDs [14]. Furthermore, anxiety moderated the relationship between body dissatisfaction and disordered eating, suggesting that non-specific anxiety contributes to ED behaviors and severity [15]. Brain research that focused on trait anxiety irrespective of EDs found this temperament trait to be associated with amygdala activation, implicating the amygdala as potentially important in ED neurobiology [16,17,18]. In fact, a few studies have found elevated or reduced amygdala response in groups with EDs in response to body image, taste or emotional conflict tasks [19,20,21].
Neurobiological studies have repeatedly suggested that brain reward circuits are part of ED pathophysiology [22]. Recent results from our group across a transdiagnostic sample of individuals with EDs indicated that brain response in the motivational salience brain circuitry is related to body mass index (BMI) and striatal-hypothalamic food control pathways, reinforcing ED behaviors [23]. That study supported the hypothesis that extremes of food restriction or overeating alter dopamine related brain response, and anxious conditioning to food intake may recruit those circuits to engage in fearful avoidance as opposed to food approach [24, 25].
How trait anxiety and the neurobiology of reward circuits interact across EDs is not well understood but could have important implications on food intake behaviors [26, 27]. The majority of research that investigated fears and anxiety in individuals with EDs has used food pictures, but those studies had limited success in identifying underlying mechanisms of EDs [28]. Applying caloric and non-caloric taste stimuli during brain imaging, however, better relates to actual eating and thus fear of caloric food intake, engages well defined neural pathways and can be used to integrate fear and reward circuitry [23]. Neurotransmitter receptor studies repeatedly indicated relationships between receptor binding and measures for anxious traits in individuals with EDs [29]. Anticipatory anxiety and arousal have been associated with meal anxiety in a study that investigated interoception in AN using a sympathetic agonist, and that study highlighted anxious traits as important for altered interoception and anxious anticipation in the disorder [30]. That study suggested that non-specific anxious traits directly affect ED-related psychopathology.
Studies in the past showed that neural response to stimulus expectation and receipt are closely linked [31, 32]. This led us to hypothesize that brain response to expecting a caloric stimulus might drive activation in response to receipt of that stimulus. Furthermore, we hypothesized that trait anxiety would drive the interaction between expectation and caloric stimulus response. Such a finding would be relevant for the clinical care of individuals with EDs as it could indicate that treatments focusing on anxious traits could be useful for ameliorating food-related anxiety in individuals with EDs and normalizing eating behavior [33].
Here we investigated the above described transdiagnostic study sample across the ED spectrum [23] to test response to caloric stimulus (sucrose) expectation and expected receipt, both contrasted against non-caloric taste stimulus, and the effects of anxious traits. We hypothesized that elevated trait anxiety in individuals with EDs would be associated with brain response to caloric taste stimulus expectation and receipt. The amygdala is a brain region central to expectation, vigilance, anxiety and threat [34, 35]. We expected that the ED sample would show elevated amygdala response to expectation of a high caloric sucrose solution stimulus compared to the non-caloric stimulus and that expectation response would negatively bias and reduce response in brain reward regions during expected taste receipt, which could interfere with food intake [36].
Methods and materials
Participants
The Colorado Multiple Institutional Review Board approved the study. All participants provided written informed consent. Procedures including recruitment and sample size were conducted according to the approved and funded study (NIMH-R01MH103436). We recruited 197 women with an ED: 69 AN restricting subtype, 22 AN binge-eating/purging subtype, 17 OSFED atypical AN subtype, 17 OSFED purging disorder subtype, 56 BN, 3 OSFED binge-eating subtype, and 13 binge ED (BED). To increase power for comparison with HC, we combined restrictive and binge-eating/purging AN subgroups (AN, severe food restriction), OSFED Atypical AN and Purging Disorder subgroups (OSFEDr, intermediate restrictive eating, normal BMI), as well as OSFED binge-eating and BED groups (BED, loss of control eating, elevated BMI). ED participants were recruited from ED partial hospitalization specialty care (EDCare Denver or Children’s Hospital Colorado) within the first 2 weeks of treatment, to mitigate effects of acute starvation or dehydration [37]. Following NIMH’s Research Domain Criteria (RDoC) instructions, we recruited “any interested ED patient” who was admitted to treatment. In addition, we recruited 120 healthy control women (HC) without lifetime psychopathology through local advertisements.
Participants were right-handed without history of head trauma, neurological disease, major medical illness, bipolar disorder, psychosis, or current (past 3 months) substance use disorder. HC were studied during the first 10 days of the menstrual cycle to reduce hormonal effects. For EDs, treatment stage was the primary variable we controlled for, but we recorded days from last menstrual cycle as a proxy to test for effects of hormonal variation.
Assessments
Psychiatric diagnoses were assessed using the Structured Clinical Interview for DSM-5 (doctoral-level interviewer) [38]. Participants completed the State-Trait Anxiety Inventory [8], Temperament and Character Inventory for Harm Avoidance [39], Behavior Inhibition/Fear-Fight-Freeze/Behavior Approach System (BIS/FFFS/BAS) for Flight-Fight-Freeze System (FFFS), BIS Anxiety, BAS-Reward Responsiveness, BAS Drive and BAS-Fun Seeking [40], Eating Disorder Inventory–3 for Drive for Thinness (intense fear of weight gain), Bulimia (tendency to engage in binge eating), and Body Dissatisfaction (discontentment with size of body regions) [41], Beck Depression Inventory-II [42], and participants blindly rated sugar solutions for sweetness and pleasantness using a 9-point Likert scale.
Brain imaging methods
Functional magnetic resonance imaging (fMRI)
Between 0700 and 0900 h, ED participants ate their meal-plan breakfast and healthy controls ate a quality- and calorie-matched breakfast. FMRI of the brain was performed between 0800 and 0900 h on either a 3T GE Signa or Siemens Skyra 3T scanner (see Supplementary Material).
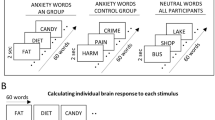
Taste reward task
The design (Supplementary Material) was adapted from O’Doherty et al. [43]. Participants learned to associate three unconditioned taste stimuli (US: 1 molar [M] sucrose solution, no solution, or artificial saliva) with paired conditioned visual stimuli (CS). Each CS was probabilistically associated with its US such that 80% of sucrose and no solution CS trials were followed by sucrose or no solution, respectively. CS and US for expectation and expected receipt of sucrose or artificial saliva were analyzed. For this study, only correct expectation–receipt trials were analyzed.
fMRI analysis
Image preprocessing and analysis were performed using SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/). Images were realigned to the first volume, normalized to the Montreal Neurological Institute template, smoothed at 6 mm full-width-at-half-maximum Gaussian kernel. Data were preprocessed with slice time correction and modeled with a hemodynamic response convolved function using the general linear model, including temporal and dispersion derivatives. A 128-s high-pass filter (removing low-frequency BOLD signal fluctuations), 6 motion parameters (as first-level analysis regressors), and SPM’s FAST (pre-whitening attenuation of autocorrelation effects) were applied [44].
Taste expectation and receipt analysis
We developed first-level models to predict the response in each voxel as a function of the following conditions: (1) sucrose expectation: trials with CS predicting sucrose receipt contrasted against trials with CS predicting artificial saliva (non-caloric taste stimulus); (2) expected sucrose receipt: trials with expected US caloric sucrose receipt contrasted against trials with expected US non-caloric artificial saliva receipt.
Region of interest (ROI) data extraction
We extracted beta values from predefined regions of interest bilaterally (http://marsbar.sourceforge.net/, automated anatomical labeling Atlas, AAL [45]): amygdala for sucrose expectation (anxiety, anticipation), and dorsal anterior insula, ventral anterior insula, middle, medial and inferior orbitofrontal cortex (OFC), head of caudate nucleus, ventral striatum [46] and nucleus accumbens [47] for expected sucrose and artificial saliva receipt (taste and reward circuitry) [23].
Statistical analysis
SPSS 28 software was used for statistical analyses (IBM, Armonk, N.Y.). Demographic and behavior data were analyzed using MANOVA. MANOVA and correlation analyses within HC or ED groups were used to test effects of potential confounding categorical or continuous variables such as comorbidity, medication use, BMI or age. Group-comparison studies were conducted with and without potential confounding covariates in the group-comparison (MANOVA, or MANCOVA for estimated marginal means). Brain imaging results are frequently non-normally distributed, and an additional group-comparison analysis was conducted using rank transformed values (Supplementary Material). Partial η2 was calculated for effect size in addition to power calculations. Post hoc group comparisons were furthermore Bonferroni corrected.
Regression analyses tested associations between behavior and brain activation, and results were multiple comparisons controlled using false discovery rate (FDR) [48].
Moderator analysis (PROCESS, SPSS) was used to test the effects of anxiety on the relationship between sucrose amygdala expectation response (X) and reward circuitry taste receipt response (Y). The primary hypothesis was that higher anxiety would moderate brain response to sucrose receipt. Those results were also FDR corrected for multiple comparisons.
Results
Demographic and behavioral variables
Demographic and behavioral variables are shown in Table 1. The overall age range was narrow with all mean values between 22 and 29 years of age, but AN and OSFED groups were younger than HC participants. AN was lower and BED higher in BMI compared to the HC group. Regular menses occurred in 16 AN (18%, 15 ± 7 days form last cycle), 17 OSFEDr (50%, 16 ± 8 days), all HC (6 ± 3 days), 33 BN (59%, 12 ± 8 days) and 6 BED (38%, 10 ± 6 days). Of the HC participants, 1 was American Indian/Alaska Native (0.8%), 13 were Asian (10.8%), 6 were Black or African American (5.0%), 3 were Asian/White (2.5%), 97 were White (80.8%); in the ED sample, 1 was American Indian/Alaska Native (0.5%), 2 were Asian (1.0%), 6 were Black or African American (3%), 2 were Asian/White (1.0%), 3 were Black, African American/White (1.5%), 1 did not identify race (0.5%), 1 was White/American Indian/Black, African American (0.5%), 181 were White (91.9%).
Harm avoidance, trait and state anxiety, drive for thinness, body dissatisfaction, bulimia, were higher across ED groups compared to HC; BIS FFFS and BIS Anxiety were higher in AN, OSFEDr and BN groups compared to HC; binge and purge frequency was higher in BN compared to the other study groups; calories consumed during breakfast were similar across groups.
Across EDs, 108 (55%) individuals were on an antidepressant, and 27 (14%) on an antipsychotic. Ninety-eight (50%) individuals had major depressive disorder (MDD), 28 (14%) obsessive compulsive disorder (OCD), 52 (26%) posttraumatic stress disorder (PTSD), and 78 (40%) had generalized anxiety disorder (GAD). Across ED groups, there were no significant differences for comorbidity or medication use, except for lower anxiety disorder rate in BED compared to BN.
Brain imaging results
In both HC and ED groups, scanner and age showed significant effects for sucrose expectation and receipt. In addition, within the ED group, PTSD had a significant effect on sucrose expectation, and comorbid anxiety disorder on sucrose receipt. Group-comparison analyses were conducted with and without covariates. Contrary to results in our previous study on prediction error response and unexpected stimulus receipt or omission, BMI was not significantly correlated with any regional response to stimulus expectation or expected stimulus receipt in either of the study groups.
Group by condition analysis, expectation, and receipt response
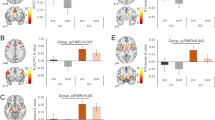
The 5-group by condition analysis for sucrose expectation (Table 2) showed an overall significant effect for group with Wilk’s lambda = 0.945, p = 0.023, a significant group effect (F = 4.08, p = 0.003) and post hoc analyses indicated higher left-sided amygdala response in the AN compared to the HC group (p = 0.002); the additional analysis with the covariates age, scanner, and PTSD showed similar results for left-sided group effect (F = 2.90, p = 0.022, Table 2, Fig. 1A), as did an analysis using rank transformed data (Supplementary Material).
An additional analysis contrasting HC against the combined ED sample (Supplementary Material) showed higher amygdala response in the ED group compared to HC in the analysis without covariates (Wilks lambda = 0.979, p = 0.035, right amygdala ED > HC p = 0.049; left amygdala ED > HC p = 0.010), as well as in the analysis with covariates age, scanner and PTSD (Wilks lambda = 0.974, p = 0.017, right amygdala ED > HC p = 0.037; left amygdala ED > HC p = 0.005).
The 5-group by condition analysis for expected sucrose receipt was not significant for either MANOVA or MANCOVA (including age, scanner and generalized anxiety disorder as covariates; Supplementary Material), nor was an additional analysis contrasting the combined ED group against HC participants.
Sucrose expectation brain response—demographic and behavior correlations
In the HC group, left amygdala response correlated positively with BIS-FFFS (r = 0.249, p = 0.006, CI 95% = 0.071 to CI 95% = 0.416).
In the ED group, amygdala response bilaterally correlated with age (R: r = −0.231, p = 0.001, CI 95% = −0.343 to CI 95% = −0.123; L: r = −0.205, p = 0.004, CI 95% = −0.314 to CI 95% = −0.091), body dissatisfaction (R: r = −0.193, p = 0.007, CI 95% = −0.324 to CI 95% = −0.068; L: r = −0.172, p = 0.017, CI 95% = −0.310 to CI 95% = −0.031), state anxiety (R: r = −0.162, p = 0.025, CI 95% = −0.286 to CI 95% = −0.042; L: r = −0.163, p = 0.025, CI 95% = −0.295 to CI 95% = −0.022), and right amygdala response with trait anxiety (r = −0.172, p = 0.017, CI 95% = −0.290 to CI 95% = −0.052), but not harm avoidance.
Across the entire study sample, there was a quadratic relationship between trait anxiety and bilateral amygdala expectation response (R: r = 0.147, F = 3.422, p = 0.034; L: r = 0.162, F = 4.221, p = 0.016) (Fig. 1B). Harm avoidance or BIS Anxiety showed no significant relationships with amygdala activation.
Sucrose receipt brain response—demographic and behavior correlations
In the HC group, there were no significant correlations after multiple comparison correction (FDR).
In the ED group, BAS-Drive was positively correlated with bilateral medial orbitofrontal cortex (R: r = 0.190, p = 0.008, CI 95% = 0.064 to CI 95% = 0.311; L: r = 0.215, p = 0.002, CI 95% = 0.084 to CI 95% = 0.345) (Fig. 2), caudate head (R: r = 0.181, p = 0.011, CI 95% = 0.038 to CI 95% = 0.302; L: r = 0.187, p = 0.009, CI 95% = 0.054 to CI 95% = 0.309), ventral striatum (R: r = 0.200, p = 0.005, CI 95% = 0.074 to CI 95% = 0.313; L: r = 0.179, p = 0.012, CI 95% = 0.054 to CI 95% = 0.290) and left nucleus accumbens (r = 0.192, p = 0.007, CI 95% = 0.059 to CI 95% = 0.307).
Correlations between sucrose expectation and expected sucrose receipt
Amygdala expectation response was significantly correlated with ipsilateral expected sucrose receipt response in ED groups across all (range r = 0.180 to 0.408, p = 0.008 to 0.000000003) and in HC across most regions (range r = 0.117 to 0.297, p = 0.202 to 0.0009) (Supplementary Material).
Moderator analysis of anxiety on sucrose expectation–receipt interaction
In the HC group (Supplementary Material) no regional moderator analysis showed significant interactions after multiple comparison correction.
In the ED group, trait anxiety negatively moderated the relationship between expectation and receipt responses that remained significant after multiple comparison correction (FDR) for right amygdala sucrose expectation response and response to sucrose receipt in right dorsal anterior insula (F = 7.663, p = 0.006) and right ventral anterior insula (F = 8.21, p = 0.005) (Table 3). A test for subgroup effects in those regions indicated that in AN, there were significant moderator effects in the right dorsal anterior insula (F = 5.54, p = 0.021) and right ventral anterior insula (F = 6.37, p = 0.014), and in the BN group in the right ventral anterior insula (F = 4.88, p = 0.032).
An exploratory analysis of harm avoidance, depression scores, body dissatisfaction or drive for thinness did not show significant moderator effects in the HC or ED groups.
Discussion
This study indicates that amygdala response is elevated in AN during expectation of caloric sweet taste stimuli compared to the HC group, while the other ED study groups only tended to have higher activation. Response to taste of the caloric stimulus was not different across groups. Amygdala caloric taste expectation and taste stimulus receipt response across taste reward regions were closely positively correlated, and across the ED sample, trait anxiety inversely moderated that relationship with the right insula. This result was confirmed in the smaller AN and partially in the BN subgroups. The study suggests that caloric sweet taste stimulus anticipation in individuals with EDs elicit a strong vigilance response, which drives reward circuit activation during taste stimulus receipt. However, trait anxiety diminishes that relationship, which could contribute to controlling food intake in individuals with EDs.
Caloric stimulus amygdala expectation versus stimulus receipt response
Amygdala response to stimulus expectation was higher in the multivariate analysis in AN compared to HC, suggesting that expecting high caloric sucrose contrasted against non-caloric artificial saliva resulted in higher arousal. The response in the other ED groups tended to be higher but that was not significant after multiple comparison corrections. On the contrary, the expected sucrose receipt did not differ across groups. Neurobiological studies in the past have repeatedly associated amygdala response with negative emotionality and trait anxiety [16,17,18]. Individuals with EDs share not only the ED-specific behaviors drive for thinness, body dissatisfaction, and fear of weight gain, but also personality traits such as negative emotionality, perfectionism, and negative urgency [49,50,51]. High negative emotionality is characterized by a tendency to react with anxiety, fear, anger or sadness, and is associated with high trait anxiety [8,9,10, 52]. How negative emotionality including trait anxiety and ED behaviors interact neurobiologically is not well understood. This study indicates that caloric stimulus expectation elicits elevated amygdala response, especially in AN, which could be a state marker for negative emotionality toward the stimulus. The lack of group differences for stimulus receipt indicates that neural response to stimulus anticipation is more indicative of altered neurobiology in this group than the response to expected receipt.
Caloric stimulus expectation predicts receipt response and is moderated by trait anxiety
Functional imaging in HCs had suggested that expectation biases neural response to stimulus receipt [36], while anticipatory anxiety has been shown to bias food intake in individuals with EDs [53]. The interaction of anxiety with the neural response to caloric stimulus expectation and receipt could provide a model for how anxiety affects ED-related neurobiology. Both the ED and HC groups showed very strong positive correlations between amygdala stimulus expectation activation and stimulus receipt response in taste reward-relevant regions. This supports previous studies linking expectation and receipt response and emphasizes that the amygdala is a key region for vigilance and anxiety processing that modulates cortical and subcortical regions that respond to taste receipt [31, 32]. Importantly, in the ED sample, trait anxiety moderated this relationship between the right amygdala and the right dorsal and ventral anterior insula. The insula is an important brain region for taste perception and body-related interoception and has strong connections with the striatal reward circuitry [54,55,56]. The right anterior insula has been specifically associated with self-recognition, the “abstract representation of oneself” and interoceptive awareness [57, 58]. It is, therefore, possible that high trait anxiety interferes with both normal taste and reward processing, as well as interoceptive awareness during food tasting. Trait anxiety could be an important link in ED pathophysiology by altering the normal taste expectation–receipt response.
Trait anxiety and its relationship with amygdala response
Trait anxiety in the ED sample was negatively correlated with amygdala expectation response, which was significant on the right side. Across the whole study sample, a quadratic regression was the best fit for the trait anxiety–amygdala relationship, with an inverted-U-shaped curve that was significant bilaterally. Trait anxiety has been associated with ED psychopathology previously. Studies suggested that trait anxiety is related to low self-confidence and avoidance of social eating [14], and is associated with altered biological stress response across EDs [59]. Anxious traits have been also found to be important for altered interoception and anxious anticipation in AN [30]. It is therefore possible that anxious traits recruit amygdala-related circuitry and interfere with food approach [60].
An inverted-U pattern had been demonstrated previously for anxiety and arousal-related behaviors and underlying biological mechanisms [61, 62]. Whether very high levels of trait anxiety led to a desensitization of amygdala response in the ED sample perhaps because very high arousal levels are not sustainable requires further exploration. Harm avoidance was not significantly related to brain response in either study group, supporting previous studies that the underlying neurobiology of trait anxiety and harm avoidance differ [63]. State anxiety was also negatively related to amygdala expectation response; however, trait anxiety is more stable than state anxiety and we focused on the trait measure and its relationship with ED neurobiology.
Behavioral approach system is related to caloric stimulus receipt response
Response to sucrose stimulus receipt in bilateral medial orbitofrontal cortex, caudate head, and ventral striatum in the ED group was significantly positively correlated with the BAS-Drive score. BAS-Drive reflects a person’s tendency to pursue rewards, a person’s “appetitive motivation”, and has been associated with cortical and subcortical response to food and non-food stimuli [40, 64, 65]. For instance, research that presented food pictures in the past found positive correlations with BAS-Drive score and orbitofrontal and ventral striatal activation [65]. The orbitofrontal cortex is an important region for reward valuation, and caudate and striatal regions process reward motivation. Individuals with EDs typically attempt to consciously control their food intake and it is possible that taste stimulation in this group is highly associated with the unconscious biological drive to pursue food reward, however, trait anxiety moderates that activation.
Limitations
The study investigated a large transdiagnostic sample according to NIMH’s RDoC guidelines and group contrasts were analyzed for ED subgroups and the combined sample; however, OSFED and BED groups were small, and the restrictive OSFED and BED categories included different subgroups. A control group without ED psychopathology but with higher depression or anxiety scores could have further helped separate ED-specific versus comorbidity-driven brain response. All ED subgroups had higher amygdala response to caloric stimulus expectation but that was only significant in AN, while all participants with EDs had significantly higher trait anxiety and no group differed in sucrose receipt response. Effect size and power in the analyses were modest and larger groups may have identified significantly higher amygdala response also in other ED subgroups. BMI was not related to brain activation contrary to prediction error response previously, supporting that unexpectancy and thus dopamine related brain response is modulated by the amount of food intake; however, neural response to expected food stimulus receipt is not, suggesting different underlying neurotransmitter mechanisms. We assessed and controlled for potentially confounding effects of comorbid conditions, age or scanner; however, residual effects or type II errors cannot be excluded. Anxiety and depression are typically correlated but neither depression nor body dissatisfaction or drive for thinness moderated the expectation–receipt relationships. Here we focused on trait anxiety as a relatively stable measure [66]. Whether anxiety can be manipulated in an experiment and directly change taste expectation or receipt brain response will be focus of future studies. Most individuals with EDs who present to treatment are White and the results may not be applicable across all racial or ethnic groups. The ROI-based approach of this study was decidedly narrow to be in line with our previous studies. An exploratory whole brain analysis indicated clusters of higher activation across somatosensory, parietal, occipital and temporal cortex in the ED compared to HC group but those were not significant at the voxel level (Supplementary Material).
In summary, elevated amygdala response in AN and the combined ED sample suggests elevated arousal to food stimuli. The relationship between amygdala expectation and right insular stimulus receipt response is moderated by trait anxiety in individuals with EDs. The influence of trait anxiety on brain taste response supports the notion that anxious traits may interfere with normal reward and interoception processing and thus have an important role in perpetuating ED pathophysiology and psychopathology. The study raises the question whether modifying the effects of trait anxiety and associated arousal via psychopharmacologic or psychotherapeutic interventions could have an important role in facilitating ED-specific treatment.
References
Crow SJ, Peterson CB, Swanson SA, Raymond NC, Specker S, Eckert ED, et al. Increased mortality in bulimia nervosa and other eating disorders. Am J Psychiatry. 2009;166:1342–6.
American Psychiatric Association. Desk reference to the diagnostic criteria from DSM-5. Washington, DC: American Psychiatric Publishing; 2013.
Cooper Z, Dalle Grave R. Eating disorders: transdiagnostic theory and treatment. In: Hofmann SG, Asmundson GJG, editors. The science of cognitive behavioral therapy. London: Elsevier Academic Press.; 2017. p. 337–57.
Sierra I, Senin-Calderon C, Roncero M, Perpina C. The role of negative affect in emotional processing of food-related images in eating disorders and obesity. Front Psychol. 2021;12:723732.
Jacobs MJ, Roesch S, Wonderlich SA, Crosby R, Thornton L, Wilfley DE, et al. Anorexia nervosa trios: behavioral profiles of individuals with anorexia nervosa and their parents. Psychol Med. 2009;39:451–61.
Lilenfeld LR. Personality and temperament. Curr Top Behav Neurosci. 2011;6:3–16.
Kaye W. Neurobiology of anorexia and bulimia nervosa. Physiol Behav. 2008;94:121–35.
Spielberger CD. Manual for the state-trate anxiety inventory. Palo Alto, CA: Consulting Psychologists Press, Inc.; 1983.
Gidron Y. Trait anxiety. In: Gellman MD, Turner JR, editors. Encyclopedia of behavioral medicine. New York, NY: Springer New York; 2013. p. 1989–89.
Cloninger CR, Bayon C, Svrakic DM. Measurement of temperament and character in mood disorders: a model of fundamental states as personality types. J Affect Disord. 1998;51:21–32.
Becker KR, Plessow F, Coniglio KA, Tabri N, Franko DL, Zayas LV, et al. Global/local processing style: Explaining the relationship between trait anxiety and binge eating. Int J Eat Disord. 2017;50:1264–72.
Byrne ME, Tanofsky-Kraff M, Kelly NM, Grammer AC, Jaramillo M, Mi SJ, et al. Pediatric loss-of-control eating and anxiety in relation to components of metabolic syndrome. J Pediatr Psychol. 2019;44:220–8.
Schaumberg K, Wonderlich S, Crosby R, Peterson C, Le Grange D, Mitchell JE, et al. Impulsivity and anxiety-related dimensions in adults with bulimic-spectrum disorders differentially relate to eating disordered behaviors. Eat Behav. 2020;37:101382.
Forrest LN, Sarfan LD, Ortiz SN, Brown TA, Smith AR. Bridging eating disorder symptoms and trait anxiety in patients with eating disorders: a network approach. Int J Eat Disord. 2019;52:701–11.
Juarascio AS, Perone J, Timko CA. Moderators of the relationship between body image dissatisfaction and disordered eating. Eat Disord. 2011;19:346–54.
Everaerd D, Klumpers F, van Wingen G, Tendolkar I, Fernandez G. Association between neuroticism and amygdala responsivity emerges under stressful conditions. Neuroimage. 2015;112:218–24.
Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry. 2007;164:318–27.
Haas BW, Omura K, Constable RT, Canli T. Emotional conflict and neuroticism: personality-dependent activation in the amygdala and subgenual anterior cingulate. Behav Neurosci. 2007;121:249–56.
Miyake Y, Okamoto Y, Onoda K, Kurosaki M, Shirao N, Okamoto Y, et al. Brain activation during the perception of distorted body images in eating disorders. Psychiatry Res. 2010;181:183–92.
Vocks S, Herpertz S, Rosenberger C, Senf W, Gizewski ER. Effects of gustatory stimulation on brain activity during hunger and satiety in females with restricting-type anorexia nervosa: an fMRI study. J Psychiatr Res. 2011;45:395–403.
Bang L, Ro O, Endestad T. Amygdala alterations during an emotional conflict task in women recovered from anorexia nervosa. Psychiatry Res Neuroimaging. 2016;248:126–33.
Steinglass JE, Berner LA, Attia E. Cognitive neuroscience of eating disorders. Psychiatr Clin North Am. 2019;42:75–91.
Frank GKW, Shott ME, Stoddard J, Swindle S, Pryor TL. Association of brain reward response with body mass index and ventral striatal-hypothalamic circuitry among young women with eating disorders. JAMA Psychiatry. 2021;78:1123–33.
Frank GKW. From desire to dread—a neurocircuitry based model for food avoidance in anorexia nervosa. J Clin Med. 2021;10:2228.
Castro DC, Cole SL, Berridge KC. Lateral hypothalamus, nucleus accumbens, and ventral pallidum roles in eating and hunger: interactions between homeostatic and reward circuitry. Front Syst Neurosci. 2015;9:90.
Guarda AS, Schreyer CC, Boersma GJ, Tamashiro KL, Moran TH. Anorexia nervosa as a motivated behavior: Relevance of anxiety, stress, fear and learning. Physiol Behav. 2015;152:466–72.
Schaumberg K, Reilly EE, Gorrell S, Levinson CA, Farrell NR, Brown TA, et al. Conceptualizing eating disorder psychopathology using an anxiety disorders framework: evidence and implications for exposure-based clinical research. Clin Psychol Rev. 2021;83:101952.
Lloyd EC, Steinglass JE. What can food-image tasks teach us about anorexia nervosa? A systematic review. J Eat Disord. 2018;6:31.
Frank GKW, Shott ME, DeGuzman MC. The neurobiology of eating disorders. Child Adolesc Psychiatr Clin N Am. 2019;28:629–40.
Khalsa SS, Hassanpour MS, Strober M, Craske MG, Arevian AC, Feusner JD. Interoceptive anxiety and body representation in anorexia nervosa. Front Psychiatry. 2018;9:444.
Nitschke JB, Sarinopoulos I, Oathes DJ, Johnstone T, Whalen PJ, Davidson RJ, et al. Anticipatory activation in the amygdala and anterior cingulate in generalized anxiety disorder and prediction of treatment response. Am J Psychiatry. 2009;166:302–10.
Nitschke JB, Dixon GE, Sarinopoulos I, Short SJ, Cohen JD, Smith EE, et al. Altering expectancy dampens neural response to aversive taste in primary taste cortex. Nat Neurosci. 2006;9:435–42.
Knatz Peck S, Towne T, Wierenga CE, Hill L, Eisler I, Brown T, et al. Temperament-based treatment for young adults with eating disorders: acceptability and initial efficacy of an intensive, multi-family, parent-involved treatment. J Eat Disord. 2021;9:110.
Belova MA, Paton JJ, Morrison SE, Salzman CD. Expectation modulates neural responses to pleasant and aversive stimuli in primate amygdala. Neuron. 2007;55:970–84.
Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34.
Nitschke JB, Sarinopoulos I, Mackiewicz KL, Schaefer HS, Davidson RJ. Functional neuroanatomy of aversion and its anticipation. Neuroimage 2006;29:106–16.
Frank GKW, Favaro A, Marsh R, Ehrlich S, Lawson EA. Toward valid and reliable brain imaging results in eating disorders. Int J Eat Disord. 2018;51:250–61.
First MB, Williams JBW, Karg RS, Spitzer RL. User’s guide for the structured clinical interview for DSM-5 disorders, research version (SCID-5-RV). Arlington, VA.: American Psychiatric Association.; 2015.
Cloninger CR, Przybeck TR, Svrakic DM, Wetzel RD. Center for psychobiology of personality. St. Louis, MO: Washington University; 1994.
Gray JA, McNaughton N. The neuropsychology of anxiety: an enquiry into the functions of the septo-hippocampal system. 2nd ed. Oxford; New York: Oxford University Press; 2000.
Garner D. Eating Disorder Inventory™-3 (EDI™-3). Lutz, FL: Psychological Assessment Resources, Inc.; 2004.
Beck AT, Steer RA, Ball R, Ranieri W. Comparison of beck depression inventories—IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–97.
O’Doherty JP, Dayan P, Friston K, Critchley H, Dolan RJ. Temporal difference models and reward-related learning in the human brain. Neuron. 2003;38:329–37.
Olszowy W, Aston J, Rua C, Williams GB. Accurate autocorrelation modeling substantially improves fMRI reliability. Nat Commun. 2019;10:1220.
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002;15:273–89.
O’Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science 2004;304:452–4.
Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, et al. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611.
Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B. 1995;57:289–300.
Culbert KM, Racine SE, Klump KL. Research review: What we have learned about the causes of eating disorders—a synthesis of sociocultural, psychological, and biological research. J Child Psychol Psychiatry. 2015;56:1141–64.
Dufresne L, Bussieres EL, Bedard A, Gingras N, Blanchette-Sarrasin A. Begin Ph DC. Personality traits in adolescents with eating disorder: a meta-analytic review. Int J Eat Disord. 2020;53:157–73.
Cassin SE, von Ranson KM. Personality and eating disorders: a decade in review. Clin Psychol Rev. 2005;25:895–916.
Costa PT Jr, McCrae RR. The Five-Factor Model of personality and its relevance to personality disorders. J Personal Disord. 1992;6:407–23.
Lloyd EC, Powell C, Schebendach J, Walsh BT, Posner J, Steinglass JE. Associations between mealtime anxiety and food intake in anorexia nervosa. Int J Eat Disord. 2021;54:1711–6.
Deen B, Pitskel NB, Pelphrey KA. Three systems of insular functional connectivity identified with cluster analysis. Cereb Cortex. 2011;21:1498–506.
Craig AD. How do you feel-now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70.
Rolls ET. Functions of the anterior insula in taste, autonomic, and related functions. Brain Cogn. 2016;110:4–19.
Devue C, Collette F, Balteau E, Degueldre C, Luxen A, Maquet P, et al. Here I am: the cortical correlates of visual self-recognition. Brain Res. 2007;1143:169–82.
Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–95.
Monteleone AM, Cascino G, Ruzzi V, Pellegrino F, Carfagno M, Raia M, et al. Multiple levels assessment of the RDoC “system for social process” in eating disorders: biological, emotional and cognitive responses to the trier social stress test. J Psychiatr Res. 2020;130:160–6.
Janak PH, Tye KM. From circuits to behaviour in the amygdala. Nature. 2015;517:284–92.
Cools R, D’Esposito M. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry. 2011;69:e113–25.
Arent SM, Landers DM. Arousal, anxiety, and performance: a reexamination of the inverted-U hypothesis. Res Q Exerc Sport. 2003;74:436–44.
Huggins AA, Belleau EL, Miskovich TA, Pedersen WS, Larson CL. Moderating effects of harm avoidance on resting-state functional connectivity of the anterior insula. Front Hum Neurosci. 2018;12:447.
Beaver JD, Lawrence AD, Passamonti L, Calder AJ. Appetitive motivation predicts the neural response to facial signals of aggression. J Neurosci. 2008;28:2719–25.
Beaver JD, Lawrence AD, van Ditzhuijzen J, Davis MH, Woods A, Calder AJ. Individual differences in reward drive predict neural responses to images of food. J Neurosci. 2006;26:5160–6.
Usala PD, Hertzog C. Evidence of differential stability of state and trait anxiety in adults. J Pers Soc Psychol. 1991;60:471–9.
Acknowledgements
We would like to thank all the individuals who have participated in this study.
Funding
The study was supported by NIMH grants MH096777 and MH103436.
Author information
Authors and Affiliations
Contributions
GKWF made substantial contributions to the conception and design of the work, acquisition of data, and data analysis and interpretation of data. MES, TP, SS, and TN all made substantial contributions to the data acquisition and interpretation of data. JS made substantial contributions to the data analysis and interpretation of data. All authors contributed to the preparation and review of this paper and have approved this paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Frank, G.K.W., Shott, M.E., Pryor, T. et al. Trait anxiety is associated with amygdala expectation and caloric taste receipt response across eating disorders. Neuropsychopharmacol. 48, 380–390 (2023). https://doi.org/10.1038/s41386-022-01440-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-022-01440-z
This article is cited by
-
Anticipatory and consummatory pleasure in eating disorders
Journal of Eating Disorders (2022)