Abstract
Alcohol Use Disorder (AUD) is characterized by loss of control over drinking. Behavioral control is mediated, in part, by cortical dopamine signaling. Inhibition of catechol-O-methyltransferase (COMT), the enzyme primarily responsible for cortical dopamine inactivation, may increase cortical dopamine, especially among individuals with genetically mediated lower dopaminergic tone, such as COMT rs4680 (val158met) val-allele homozygotes. This study was a randomized, placebo-controlled, pharmacogenetic trial of the COMT inhibitor tolcapone. Ninety non-treatment-seeking AUD individuals were prospectively genotyped for rs4680 and randomized to tolcapone (200 mg t.i.d.) or placebo for 8 days. At baseline and on day 7, peripheral COMT activity was assayed, and participants completed an fMRI alcohol cue-reactivity task; on day 8, they completed a bar-lab paradigm. Primary outcomes were: (1) natural drinking during the medication period; (2) alcohol self-administration in the bar lab; and (3) alcohol cue-elicited cortical (right inferior frontal gyrus [rIFG]) and ventral striatal activation. At baseline, the rs4680 val-allele had an additive effect on COMT activity. Tolcapone, relative to placebo, reduced COMT activity in all genotype groups. COMT genotype moderated tolcapone’s effect on drinking during the medication period and in the bar lab, such that tolcapone, relative to placebo, reduced drinking only among val-allele homozygotes. Tolcapone did not affect cue-elicited ventral striatal activation but reduced rIFG activation; less rIFG activation on day 7 was associated with less drinking during the medication period. Taken together, these data suggest that COMT inhibition may reduce drinking specifically among individuals genetically predisposed to excessive COMT activity and potentially low cortical dopamine tone.
ClinicalTrials.gov identifier: NCT02949934 https://clinicaltrials.gov/ct2/show/NCT02949934
Similar content being viewed by others
Introduction
Alcohol Use Disorder (AUD) is characterized by dysregulated motivation for alcohol and loss of control over consumption. In the brain, motivated behavior and inhibitory control are mediated, in part, by cortical dopamine (DA) signaling [1]. In particular, interactions between the right inferior frontal cortex (rIFC) and the ventral striatum (VS) are believed to underlie inhibitory control [2] and are dopaminergically modulated [3]. The relationship between cortical DA tone and control has been hypothesized to be inverted-U-shaped, with both high and low tone associated with poor control [4,5,6]. Dysregulation of DA function in AUD has been studied for many years. Early positron emission tomography (PET) studies found lower striatal D2 receptor availability [7] and DA transmission [8] among AUD individuals, relative to controls. Concomitant PET and functional magnetic resonance imaging (fMRI) studies reported that lower striatal D2 availability was correlated with greater alcohol cue-elicited cortical activation [9], and greater rIFC D2 availability with greater striatal activation during inhibition [10], suggesting corticostriatal DA circuits underlie both alcohol cue reactivity and inhibition. Critically, a PET study with the novel cortical DA ligand [11C]FLB-457 reported that cortical DA transmission was substantially down-regulated among recently abstinent AUD individuals [11], suggesting that these individuals might lie on the left side of the inverted-U-shaped DA-control function.
In the striatum, DA is inactivated primarily through active reuptake at the presynaptic dopamine transporter (DAT). In the prefrontal cortex (PFC), however, the DAT is not highly expressed [12], and the principal method of DA inactivation is degradation by catechol-O-methyltransferase (COMT) [13]. Medications that inhibit COMT could thus increase cortical DA concentrations, potentially remediating AUD-associated cortical DA deficits. One such medication is tolcapone, a brain-penetrant [14], selective, reversible COMT inhibitor [15] that is FDA-approved to treat Parkinson’s disease and is normally co-administered with levodopa to reduce its peripheral metabolism. When administered independently of levodopa, tolcapone has limited effects on striatal DA or motor function [16, 17], likely because greater striatal expression of DAT, relative to COMT, offsets its acute effects. However, in the PFC, tolcapone potentiates extracellular DA release elicited by exogenous factors, including behaviorally salient stimuli (e.g., food cues) [18]. Tasks that require inhibitory control also elicit cortical DA release [19], and drugs that increase extracellular DA concentrations enhance performance on such tasks [20]. Thus, while tolcapone’s idiosyncratic hepatotoxicity [21] may limit its clinical utility in AUD, its potential to increase control-associated cortical DA tone makes it an appealing proof-of-concept medication to evaluate COMT inhibition as a target to improve control over drinking.
Animal and human imaging and laboratory models suggest that tolcapone improves inhibitory control and reduces drinking. Tolcapone increases cortical DA release during executive functioning tasks [18, 22], and among both alcohol-preferring and high-drinking Wistar rats, it reduced alcohol consumption elicited by alcohol cues [23]. Tolcapone has previously been tested among individuals with other addictive disorders (Gambling Disorder and Tobacco Use Disorder), with mixed results [24,25,26]. Among non-treatment-seeking AUD individuals, tolcapone (300 mg), relative to placebo, reduced drinking under natural conditions, and individuals with the greatest tolcapone-induced reduction in impulsive decision-making had the greatest decrease in drinking [27].
A single nucleotide polymorphism (SNP) in COMT, the gene that encodes COMT, may moderate tolcapone’s effects. The val allele of the rs4680 SNP (val158met), which engenders a methionine to valine amino acid substitution, is associated with a three- to four-fold increase in COMT activity relative to the met allele [28, 29], and may thereby reduce cortical DA tone. Individuals with AUD, already left-shifted on the inverted-U-shaped DA-control function, might thus have particularly low cortical DA if they are also homozygous for the rs4680 val allele. Although associations between rs4680 genotype and alcohol-related phenotypes have been mixed [30,31,32,33], many studies suggest that rs4680 genotype moderates the effects of dopaminergic medications, including tolcapone [34]. In three studies of healthy controls, tolcapone improved cognitive performance and decreased risky decision-making only among val-allele homozygotes [35,36,37]. Similarly, in an open-label tolcapone study among individuals with Gambling Disorder, clinical improvement was significantly greater among val-allele homozygotes [25]. Thus, among AUD individuals, tolcapone might be selectively effective in reducing drinking among rs4680 val-allele homozygotes.
Given these findings, the current study tested the interacting effects of tolcapone and COMT rs4680 genotype on drinking and alcohol cue-elicited cortical and striatal activation among non-treatment-seeking AUD individuals in a well-validated human laboratory and imaging paradigm [38, 39]. Primary outcomes were: (1) natural drinking during the first 6 days of medication ingestion; (2) alcohol self-administration in a bar-lab paradigm; and (3) alcohol cue-elicited rIFC and VS activation. Tolcapone, relative to placebo, was hypothesized to reduce drinking, alcohol self-administration, and rIFC, but not VS, cue-elicited activation specifically among rs4680 val-allele homozygotes, relative to heterozygotes or met-allele homozygotes.
Methods
Overview
The Medical University of South Carolina (MUSC) Institutional Review Board approved the study, which was an eight-day randomized controlled trial (ClinicalTrials.gov identifier: NCT02949934) conducted at MUSC between May 2016 and April 2021. All participants provided informed consent before participation, for which they were compensated. Participants were initially assessed by a centralized intake core (see Supplemental Methods) for AUD diagnostic criteria, daily drinking over the previous 30 days, and other inclusion/exclusion criteria and provided a blood sample that was used to measure liver transaminase (ALT and AST) levels and genotype rs4680. As a safeguard against potential tolcapone-induced hepatotoxicity, all participants were required to have ALT and AST values within the normal range at baseline. On Day 1, immediately before ingestion of the first dose of study medication, and again on Day 7 of medication ingestion, a blood sample was obtained to measure peripheral COMT activity and participants completed an fMRI alcohol cue-reactivity task [40]. On Day 7, drinking during the first 6 days of the medication period was assessed with the Timeline Follow-back (TLFB) [41]. On Day 8, participants completed an alcohol self-administration (bar-lab) paradigm [38]. As a safety precaution, only participants whose ALT and AST values, as reassessed on Day 7, remained within the normal range were permitted to complete the bar-lab paradigm. Participants were discontinued from the study if, at the beginning of the Day 1, Day 7, or Day 8 visits, they had any measurable breath alcohol concentration (BrAC) or a urine drug screen (UDS) positive for amphetamines, barbiturates, benzodiazepines, cannabis, cocaine, opioids, or phencyclidine. On Day 9, ALT and AST values were reassessed, and participants were debriefed and given brief motivational counseling to encourage them to reduce their heavy drinking.
Participants
Ninety participants were randomized to medication; this sample size was determined a priori, using G*Power 3.1 [42], to yield 95% power to detect a large effect size (f > 0.4/d > 0.8) for the interaction between rs4680 genotype and medication group. Participants, recruited via media advertisements, were required to be ages 21–40, reported drinking ≥20 standard drinks per week in the 30 days before assessment, and met DSM-5 (Diagnostic and Statistical Manual of Mental Disorders, 5th edition) diagnostic criteria for AUD of at least moderate severity, as assessed by the Structured Clinical Interview for DSM-5. Exclusion criteria were: current DSM-5 Substance Use Disorder for any other substance except nicotine; current use of illicit substances or any psychoactive medication, as evidenced by UDS and self-report; current DSM-5 Axis I diagnosis or suicidal/homicidal ideation; history of significant medical illness; and ALT or AST values greater than the upper limit of normal. Female participants could not be pregnant or nursing. At baseline, the Alcohol Use Disorders Identification Test (AUDIT) [43], TLFB, and Wechsler Abbreviated Scale of Intelligence, Second Edition [44] were used to assess AUD severity, past-30-day drinking, and IQ, respectively. Table 1 lists demographic and severity data for the 87 participants who returned on Day 7 and reported drinking data for the medication period (see further detail on participant flow through the study in Supplemental Fig. 1).
Genotyping
COMT rs4680 genotyping is further described in the Supplemental Methods. Since ~50% of the population carries the rs4680 heterozygous genotype, eligible heterozygotes were randomly selected for participation, while val- and met-allele homozygotes were over-selected so that each group ultimately represented approximately one-third of randomized participants.
Randomization and medication
Participants were randomized, based on their rs4680 genotype, to receive tolcapone (Day 1: 100 mg b.i.d.; Days 2–3: 100 mg t.i.d.; Days 4–7: 200 mg t.i.d.; Day 8: 200 mg b.i.d.) or placebo (1:1 allocation ratio) for 8 days. They were instructed to take the medication each morning, afternoon, and evening, and were observed to ingest doses immediately after the Day 1 scan, before the Day 7 scan, and before the Day 8 bar-lab paradigm. Tolcapone’s elimination half-life is 2 h [45]; thus, to ensure it was at Cmax during the Day 7 and Day 8 procedures, doses were administered 60 min before each procedure. Because rs4680 allele frequencies vary by race [46], randomization was stratified by both rs4680 genotype (val/val, val/met, or met/met) and participants’ self-identified race (European-American vs. all other races), yielding six strata. Sex was used as an urn randomization variable [47] and was balanced across medication groups within each stratum. To maintain genotype blinding, one investigator (K.E.V.) blinded rs4680 genotypes prior to another investigator (J.P.S. or M.H.) performing randomization. Participants and all investigators except K.E.V. (who did not subsequently interact with participants) were blind to genotype and both participants and all investigators were blind to medication assignment. Study medications were identically over-encapsulated with 25 mg riboflavin per dose and distributed in labeled blister packs. Urinary riboflavin was measured twice at baseline (participants were instructed to discontinue use of multivitamins and supplements containing riboflavin before participation) and on Day 7 with a fluorometric assay based on standard curves of weighed-in riboflavin [48], and samples were considered adherent if the Day 7 riboflavin value was ≥1000 ng/mL or had doubled relative to baseline.
Peripheral COMT activity
Before medication ingestion on Days 1 and 7, whole blood samples were collected by venipuncture. COMT activity was measured with an assay adapted from [49] and described further in the Supplemental Methods.
Neuroimaging
On Day 1 and Day 7, participants completed a neuroimaging session, prior to which they were assessed for alcohol withdrawal with the Clinical Institute Withdrawal Assessment of Alcohol-Revised (CIWA-Ar) [50] and breathalyzed. All participants had CIWA-Ar scores <4 before both scans, but, as noted in Supplemental Fig. 1, three participants with a BrAC >0 were excluded from scanning on Day 7. Participants completed a previously published alcohol cue-reactivity task [40] described further in the Supplemental Methods.
Regions of interest (ROIs) were the right inferior frontal gyrus (rIFG) pars triangularis, defined from the Harvard-Oxford cortical parcellation [51], and the right VS, defined, consistent with our previous work, as a 6 mm-radius sphere centered at the point [12 15 −6] in Montreal Neurological Institute (MNI) space. These ROIs were selected a priori to focus on a cortical region associated with inhibitory control [2] and a striatal region in which we have previously shown medication effects in AUD [39, 52]. For each participant, these ROIs were reverse-registered from the MNI-152 image to the participant’s anatomical image, and the average percentage change of the BOLD signal between alcohol image (ALC) and neutral beverage image (BEV) blocks (i.e., ALC vs. BEV percent signal change) was extracted using the FSL featquery tool.
Bar-lab paradigm
On Day 8, participants were breathalyzed, provided a gender- and weight-adjusted standard caloric lunch at 12:00 PM, and were observed to ingest the final medication dose at 1:00 PM. As noted in Supplemental Fig. 1, one participant with a BrAC>0 was excluded from participating in the bar-lab paradigm. At 2:00 PM, participants were administered a priming drink (1:3 ratio of their preferred 80-proof liquor and juice), adjusted for gender, age, and weight to produce a targeted BrAC of 30 mg% [53], and instructed to consume it within 5 min. Ten, twenty, and thirty min later, the seven-item Alcohol Urge Questionnaire (AUQ) [54], which has been validated for real-time assessment of alcohol craving in the laboratory [55], was administered. Forty min later, participants were presented a tray of four drinks, each with a targeted BrAC of 15 mg%, and told they could consume as many as they desired over the next hour. After an hour, this tray was removed and another tray of four drinks was made available over a second hour. To create a decisional balance between drinking and abstaining, participants were given a “bar credit” of $16 with which to “purchase” drinks, at the cost of $2/drink, and were told that any money they did not spend would be given to them the following day. After the procedure, participants were given dinner and remained until 10:00 PM. A BrAC measurement <20 mg% was required before departure, and a friend or taxi drove participants home.
Adverse effects (AEs)
A physical symptom checklist was used to assess the presence and severity (self-rated as none, mild, moderate, or severe) of 21 elicited AEs on Day 1 and Day 8, immediately before the bar-lab paradigm. An open-ended question also queried the presence of other AEs. For participants who participated during the COVID-19 pandemic, the checklist was administered on Day 7 instead of Day 8 but was inadvertently omitted for 12 participants.
Statistical analysis
Analyses were conducted with SPSS v25. For the COMT activity and imaging analyses, linear mixed models (SPSS MIXED) were tested in which time (Day 1 vs. 7), medication group, COMT genotype, and all interactions of these factors were tested as predictors of (1) hemoglobin-normalized normetanephrine concentrations and (2) ALC vs. BEV percent signal change in the rIFG and VS. All models used an unstructured variance-covariance matrix; the imaging models covaried for scanner. For the drinking analyses, general linear models (SPSS GLM) were tested in which medication group, COMT genotype, and their interaction were tested as predictors of drinks per day (DPD) and drinks per drinking day (DPDD) during the first 6 days of the medication period, covarying for baseline DPD/DPDD, age and AUDIT score (which were not included in the randomization scheme), and an indicator-coded variable indicating whether the participant completed the study before or during the COVID-19 pandemic. For the bar-lab analyses, the same GLMs were tested for peak AUQ score (highest measurement across the 10, 20, and 30 min post-priming-drink assessments; maximum possible range = 0–56) and the number of drinks consumed in the bar lab after the priming drink, again covarying for age and AUDIT score. For all models, if the interaction between genotype and medication group was not significant, it was removed from the model and only the main effect of medication was tested. Exploratory associations between COMT activity, drinking, and cue-elicited activation were tested with Pearson correlations. Finally, chi-square tests were used to compare medication adherence and AE rates between groups. For the three primary analyses (drinking during the medication period, bar-lab drinking, and cue-elicited brain activation), alpha was corrected to p = 0.0167. For the secondary analyses of COMT activity, AUQ scores, adherence and AE rates, and associations between outcomes, an uncorrected alpha of p = 0.05 was used.
Results
Peripheral COMT activity
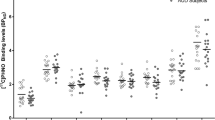
At baseline, there was an additive effect of the COMT val-allele on COMT activity (F(2, 84) = 35.77, p = 5.8 × 10−12), such that val-allele homozygotes had the greatest activity and met-allele homozygotes the least (val/val vs. met/met, d = 2.15; val/val vs. val/met, d = 1.09; val/met vs. met/met, d = 1.31) (Fig. 1a). Greater baseline COMT activity was associated at a trend level with more baseline drinking (DPD, r(87) = 0.18, p = 0.095; DPDD, r(87) = 0.19, p = 0.086). Between Days 1 and 7, tolcapone, relative to placebo, significantly reduced COMT activity in all genotype groups (F(1, 85) = 29.19, p = 5.9 × 10−9; mean inhibition: tolcapone = 56.4% (SD = 29.3%); placebo = 6.5% (SD = 22.1%)) (Fig. 1b). On day 7, greater COMT activity was significantly associated with more drinking during the previous 6 days in the placebo group (DPD, r(46) = 0.37, p = 0.011; DPDD, r(45) = 0.29, p = 0.051), but not the tolcapone group (DPD, r(41) = −0.16, p = 0.32; DPDD, r(41) = −0.15, p = 0.34), suggesting that tolcapone decoupled the baseline relationship between COMT activity and drinking. (One participant was abstinent during the medication period and hence did not have a value for DPDD during this period; thus, there is one less degree of freedom for statistics that test this parameter).
a Effect of COMT rs4680 genotype on COMT activity in red blood cells at baseline. b Effects of medication group and COMT rs4680 genotype on COMT activity on day 7 of medication ingestion. Figures are mean normetanephrine concentrations normalized to hemoglobin, ± standard errors from the linear mixed model. **p < 0.01.
Drinking during the medication period
Tolcapone, relative to placebo, did not significantly reduce DPD or DPDD during the medication period. However, the interaction between COMT genotype and medication group was significant, such that, as hypothesized, tolcapone, relative to placebo, significantly reduced DPD (F(2, 77) = 4.62, p = 0.013; Fig. 2a) and DPDD (F(2, 76) = 4.97, p = 0.009; Fig. 2b) among val-allele homozygotes (simple effect of medication in this group: DPD, F(1, 77) = 7.46, p = 0.008, d = 1.08; DPDD, F(1, 76) = 7.16, p = 0.009, d = 1.06), but not heterozygotes or met-allele homozygotes.
a Effects of medication group and COMT rs4680 genotype on drinks per day. b Effects of medication group and COMT rs4680 genotype on drinks per drinking day. Figures are means ± standard errors from the general linear model, controlling for age, AUDIT score, baseline drinking, and whether the participant completed the study before or during the COVID-19 pandemic. **p < 0.01.
Bar-lab paradigm
Mean BrAC from the priming drink was 22 mg% (SD = 8.4) and did not significantly differ between medication groups. Tolcapone, relative to placebo, did not significantly reduce peak AUQ score or the number of drinks consumed after the priming drink. However, the interaction between COMT genotype and medication group was significant for both of these outcomes (AUQ, F(2, 52) = 3.17, p = 0.050, Fig. 3a; bar-lab drinks, F(2, 51) = 4.68, p = 0.014, Fig. 3b). As hypothesized, tolcapone, relative to placebo, reduced both outcomes among val-allele homozygotes (simple effect of medication: AUQ, F(1, 52) = 4.61, p = 0.036, d = 1.02; bar-lab drinks, F(1, 51) = 3.10, p = 0.084, d = 0.80). Unexpectedly, tolcapone also significantly increased self-administration, but not craving, among met-allele homozygotes (simple effect of medication: F(1, 51) = 6.70, p = 0.013, d = 1.18).
Effects of medication group and COMT rs4680 genotype on (a) peak alcohol craving (Alcohol Urge Questionnaire [AUQ] score) in the bar lab following consumption of a priming drink; and (b) the number of drinks (out of 8 possible) that participants chose to self-administer in the bar lab. Figures are means ± standard errors from the general linear model, controlling for age and AUDIT score. *p < 0.05.
Neuroimaging
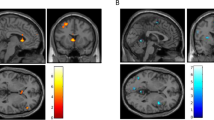
Between Days 1 and 7, tolcapone, relative to placebo, did not affect cue-elicited activation of the VS (F(1, 76) = 0.35, p = 0.56), but reduced activation of the rIFG (F(1, 76) = 5.13, p = 0.026), though this effect did not survive multiple comparisons correction. For rIFG activation, the medication groups did not significantly differ on Day 1 (F(1, 76) = 0.91, p = 0.34), but on Day 7, the simple effect of medication was significant F(1, 76) = 5.51, p = 0.022, d = 0.53; Fig. 4a). COMT genotype did not significantly moderate either effect. Across all participants, less rIFG activation on day 7 was associated with significantly fewer DPDD during the medication period (r(77) = 0.28, p = 0.012) (Fig. 4b), but not DPD (r(78) = 0.15, p = 0.21) or bar-lab drinks (r(55) = −0.16, p = 0.25).
a Inset: Right inferior frontal gyrus (rIFG) region of interest. Main figure: Effect of medication group on alcohol cue-elicited rIFG activation. Figures are means ± standard errors from the linear mixed model, controlling for scanner. b Association between cue-elicited rIFG activation and drinks per drinking day during the first 6 days of the medication period. *p < 0.05.
Adherence
Day 7 riboflavin samples were missing for two participants and were uninterpretable for one due to an elevated baseline value, all of whom were in the tolcapone group. Of the remaining 84 participants, Day 7 riboflavin values were adherent for 40/44 placebo participants and 40/40 tolcapone participants; this difference trended toward greater adherence in the tolcapone group (χ2(1, 84) = 3.82, p = 0.051).
AEs and liver function tests
Two participants were discontinued due to AEs prior to the Day 7 visit. One, randomized to tolcapone, had an unexpected pregnancy, and one, randomized to placebo, had a severe rash. At the end of the medication period, low energy was significantly more frequent among tolcapone participants (21/37 participants, 20 “mild” and 1 “severe”) than placebo participants (11/38 participants, 9 “mild” and 2 “moderate”) (χ2(1, 75) = 5.93, p = 0.015). Nausea was more frequent among tolcapone participants (5/37 participants, 3 “mild”, 1 “moderate”, 1 “severe”) than placebo participants (1/38 participants, “mild”) at a trend level (χ2(1, 75) = 3.02, p = 0.082). There were no significant differences between medication groups in other elicited or unelicited AEs. With respect to liver transaminase values (Supplementary Table 1), AST values did not significantly change between baseline, Day 7, or Day 9 in either medication group. ALT values increased between baseline and Day 7 among placebo participants and decreased among tolcapone participants; this effect (i.e., the interaction between medication group and time) was significant F(1, 85) = 4.54, p = 0.036), but had normalized by Day 9. All four participants who were discontinued due to AST or ALT elevation on Day 7 were in the placebo group.
Discussion
In this pharmacogenetic human laboratory study, COMT rs4680 genotype was associated with substantial differences in peripheral COMT activity at baseline, validating previous in vitro data on this SNP’s function [28]. Greater COMT activity at baseline was associated with more recent drinking, supporting previous findings of impaired cortical DA transmission among AUD individuals, relative to controls [11]. While tolcapone robustly inhibited COMT activity in all genotype groups, it reduced drinking and alcohol craving, both in the natural environment and in a bar-lab paradigm, selectively among rs4680 val-allele homozygotes. There was no main effect of tolcapone on drinking or craving, suggesting that genetic screening would be critical to identifying individuals likely to benefit from tolcapone treatment. There was, however, a main effect on alcohol cue-elicited brain activation, such that tolcapone reduced activation of the rIFG, a region long associated with inhibitory control. Lower rIFG activation was associated with less drinking during the medication period. Taken together, these data implicate down-regulated cortical DA transmission in AUD pathophysiology and suggest that COMT inhibition might be an effective pharmacological strategy for reducing drinking specifically among individuals genetically predisposed to low cortical DA tone.
The current findings support some aspects of a previous human laboratory study among AUD individuals and extend this work with our previously validated bar-lab and neuroimaging paradigm. Coker et al. [27] reported that tolcapone, relative to placebo, reduced self-reported drinking over 3 days, but did not affect consumption in a bar-lab paradigm; rs4680 genotype did not moderate either effect. However, they enrolled less heavily drinking participants (mean 21 drinks/week, vs. 42 drinks/week in our sample); tested a lower tolcapone dose (300 mg) for a shorter period (3 days); employed a group bar laboratory paradigm, in which multiple participants were tested simultaneously; and did not over-select rs4680 val- or met-allele homozygotes, limiting power to detect pharmacogenetic effects. Any or all of these factors might account for the differences between their results and ours. Importantly, Coker et al. found that tolcapone-mediated reduction in drinking was associated with reduction in impulsive choice, suggesting that tolcapone’s effect on drinking was mediated through inhibitory control.
In the current study, further clues to tolcapone’s potential mechanism of action in AUD can be derived from its effects on alcohol cue-elicited brain activation. Tolcapone did not affect cue-elicited VS activation, consistent with its putatively cortically specific effects, but unlike other medications we have tested known to affect striatal DA (e.g., naltrexone, aripiprazole) [39, 52]. Instead, tolcapone reduced cue-elicited rIFG activation, although rs4680 genotype did not moderate this effect. Within the cortex, rIFG has been reported to be one of three “hotspots” (along with the medial PFC and precentral gyrus) of cue-elicited activation across substances of abuse [56]. Among individuals with Gambling Disorder, tolcapone increased rIFG-striatal connectivity during delay discounting [26], and increases in frontoparietal (rIFG, frontopolar, and inferior parietal) activation during an executive function task were associated with symptom improvement during tolcapone treatment [25], suggesting that tolcapone might particularly modulate activation of this area. Given rIFG’s role in inhibitory control, others have speculated about what its activation by substance-related cues represents. As rIFG is activated by both natural reward and substance cues [57], it may simply reflect attentional salience and reward processing. Since cue-elicited activation of rIFG and broader dorsolateral PFC are enhanced when participants are instructed to regulate craving [58], another possibility is that individuals who display reduced rIFG activation are making less of an effort to resist craving—perhaps because pharmacological intervention (i.e., tolcapone) has reduced the need to do so.
The current study had several strengths, including the use of prospective genotyping, over-selection of rs4680 val- and met-allele homozygotes to increase power to detect pharmacogenetic effects, and measurement of peripheral COMT activity to validate genotype and pharmacological effects. However, several limitations require acknowledgement. First, participants were younger, non-treatment-seeking AUD individuals; further study is needed to determine whether these findings would extrapolate to older, treatment-seeking individuals with more severe AUD. Second, although sex was used as an urn randomization variable, sample size was too small to analyze moderation by sex. Third, although the study was powered to detect a large effect for the rs4680-tolcapone pharmacogenetic interaction, the cell sizes for this interaction were relatively small (n’s = 12–17). Given the absence of a tolcapone effect on drinking in rs4680 heterozygotes, future studies could increase cell sizes and power by focusing on only val- and met-allele homozygotes. Fourth, we measured peripheral, not central, COMT activity. The effect of rs4680 genotype on peripheral activity was consistent with its brain effect in vitro [29], but other factors might modulate brain COMT activity. Fifth, since COMT metabolizes other catecholamines, noradrenergic effects could account for some of these findings. However, the norepinephrine transporter inactivates 70–90% of synaptic norepinephrine [59], so these effects would be relatively small in comparison to dopaminergic effects. Finally, although tolcapone was well-tolerated and demonstrated no hepatoxicity in this short-term dosing study among individuals selected for normal liver function, its short half-life limits the ability to achieve steady-state dosing and makes daily use to control drinking somewhat impractical, and its potential hepatotoxicity over longer dosing intervals likely precludes extended use of this medication in the general AUD clinical population. This study tested tolcapone as a pharmacogenetic proof-of-concept probe; its efficacy in reducing drinking among rs4680 val-allele homozygotes suggests that COMT inhibition is a possible pharmacological target for AUD. Other brain-penetrant, non-hepatotoxic COMT inhibitors currently under development have demonstrated promise for modulating cortical DA and inhibitory control [60] and may ultimately achieve similar or enhanced therapeutic results in AUD.
References
Cools R, D’Esposito M. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry. 2011;69:e113–25.
Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn Sci. 2014;18:177–85.
Meder D, Herz DM, Rowe JB, Lehericy S, Siebner HR. The role of dopamine in the brain - lessons learned from Parkinson’s disease. Neuroimage 2019;190:79–93.
Goldman-Rakic PS, Muly EC 3rd, Williams GV. D(1) receptors in prefrontal cells and circuits. Brain Res Brain Res Rev. 2000;31:295–301.
Arnsten AF. Catecholamine regulation of the prefrontal cortex. J Psychopharmacol. 1997;11:151–62.
Zahrt J, Taylor JR, Mathew RG, Arnsten AF. Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci. 1997;17:8528–35.
Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann R, Ding YS, et al. Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol Clin Exp Res. 1996;20:1594–8.
Volkow ND, Wang G-J, Telang F, Fowler JS, Logan J, Jayne M, et al. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci. 2007;27:12700–6.
Heinz A, Siessmeier T, Wrase J, Hermann D, Klein S, Grüsser SM, et al. Correlation between dopamine D(2) receptors in the ventral striatum and central processing of alcohol cues and craving. Am J Psychiatry. 2004;161:1783–9.
Pfeifer P, Sebastian A, Buchholz HG, Kaller CP, Grunder G, Fehr C, et al. Prefrontal and striatal dopamine D2/D3 receptors correlate with fMRI BOLD activation during stopping. Brain Imaging Behav. 2022;16:186–98.
Narendran R, Mason NS, Paris J, Himes ML, Douaihy AB, Frankle WG. Decreased prefrontal cortical dopamine transmission in alcoholism. Am J Psychiatry. 2014;171:881–8.
Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI. Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J Neurosci. 1998;18:2697–708.
Matsumoto M, Weickert CS, Akil M, Lipska BK, Hyde TM, Herman MM, et al. Catechol O-methyltransferase mRNA expression in human and rat brain: evidence for a role in cortical neuronal function. Neuroscience 2003;116:127–37.
Marsala SZ, Gioulis M, Ceravolo R, Tinazzi M. A systematic review of catechol-0-methyltransferase inhibitors: efficacy and safety in clinical practice. Clin Neuropharmacol. 2012;35:185–90.
Maj J, Rogoz Z, Skuza G, Sowinska H, Superata J. Behavioural and neurochemical effects of Ro 40-7592, a new COMT inhibitor with a potential therapeutic activity in Parkinson’s disease. J Neural Transm Park Dis Dement Sect. 1990;2:101–12.
Budygin EA, Gainetdinov RR, Kilpatrick MR, Rayevsky KS, Mannisto PT, Wightman RM. Effect of tolcapone, a catechol-O-methyltransferase inhibitor, on striatal dopaminergic transmission during blockade of dopamine uptake. Eur J Pharm. 1999;370:125–31.
Hauser RA, Molho E, Shale H, Pedder S, Dorflinger EE. A pilot evaluation of the tolerability, safety, and efficacy of tolcapone alone and in combination with oral selegiline in untreated Parkinson’s disease patients. Tolcapone De Novo Study Group. Mov Disord. 1998;13:643–7.
Lapish CC, Ahn S, Evangelista LM, So K, Seamans JK, Phillips AG. Tolcapone enhances food-evoked dopamine efflux and executive memory processes mediated by the rat prefrontal cortex. Psychopharmacol (Berl). 2009;202:521–30.
van der Meulen JA, Joosten RN, de Bruin JP, Feenstra MG. Dopamine and noradrenaline efflux in the medial prefrontal cortex during serial reversals and extinction of instrumental goal-directed behavior. Cereb Cortex. 2007;17:1444–53.
Schmeichel BE, Zemlan FP, Berridge CW. A selective dopamine reuptake inhibitor improves prefrontal cortex-dependent cognitive function: potential relevance to attention deficit hyperactivity disorder. Neuropharmacology 2013;64:321–8.
Benabou R, Waters C. Hepatotoxic profile of catechol-O-methyltransferase inhibitors in Parkinson’s disease. Expert Opin Drug Saf. 2003;2:263–7.
Tunbridge EM, Bannerman DM, Sharp T, Harrison PJ. Catechol-o-methyltransferase inhibition improves set-shifting performance and elevates stimulated dopamine release in the rat prefrontal cortex. J Neurosci. 2004;24:5331–5.
McCane AM, Czachowski CL, Lapish CC. Tolcapone suppresses ethanol intake in alcohol-preferring rats performing a novel cued access protocol. Alcohol Clin Exp Res. 2014;38:2468–78.
Ashare RL, Wileyto EP, Ruparel K, Goelz PM, Hopson RD, Valdez JN, et al. Effects of tolcapone on working memory and brain activity in abstinent smokers: a proof-of-concept study. Drug Alcohol Depend. 2013;133:852–6.
Grant JE, Odlaug BL, Chamberlain SR, Hampshire A, Schreiber LR, Kim SW. A proof of concept study of tolcapone for pathological gambling: relationships with COMT genotype and brain activation. Eur Neuropsychopharmacol. 2013;23:1587–96.
Kayser AS, Vega T, Weinstein D, Peters J, Mitchell JM. Right inferior frontal cortex activity correlates with tolcapone responsivity in problem and pathological gamblers. Neuroimage Clin. 2017;13:339–48.
Coker AR, Weinstein DN, Vega TA, Miller CS, Kayser AS, Mitchell JM. The catechol-O-methyltransferase inhibitor tolcapone modulates alcohol consumption and impulsive choice in alcohol use disorder. Psychopharmacol (Berl). 2020;237:3139–48.
Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics 1996;6:243–50.
Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, et al. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75:807–21.
Schellekens AF, Franke B, Ellenbroek B, Cools A, de Jong CA, Buitelaar JK, et al. COMT Val158Met modulates the effect of childhood adverse experiences on the risk of alcohol dependence. Addict Biol. 2013;18:344–56.
Hendershot CS, Lindgren KP, Liang T, Hutchison KE. COMT and ALDH2 polymorphisms moderate associations of implicit drinking motives with alcohol use. Addict Biol. 2012;17:192–201.
Guillot CR, Fanning JR, Liang T, Berman ME. COMT Associations with Disordered Gambling and Drinking Measures. J Gambling Stud. 2015;31:513–24.
Olfson E, Bierut LJ. Convergence of genome-wide association and candidate gene studies for alcoholism. Alcohol Clin Exp Res. 2012;36:2086–94.
Schacht JP. COMT val158met moderation of dopaminergic drug effects on cognitive function: a critical review. Pharmacogenom J. 2016;16:430–8.
Apud JA, Mattay V, Chen J, Kolachana BS, Callicott JH, Rasetti R, et al. Tolcapone improves cognition and cortical information processing in normal human subjects. Neuropsychopharmacology 2007;32:1011–20.
Giakoumaki SG, Roussos P, Bitsios P. Improvement of prepulse inhibition and executive function by the COMT inhibitor tolcapone depends on COMT Val158Met polymorphism. Neuropsychopharmacology 2008;33:3058–68.
Farrell SM, Tunbridge EM, Braeutigam S, Harrison PJ. COMT Val(158)Met genotype determines the direction of cognitive effects produced by catechol-O-methyltransferase inhibition. Biol Psychiatry. 2012;71:538–44.
Anton RF, Schacht JP, Voronin KE, Randall PK. Aripiprazole Suppression of Drinking in a Clinical Laboratory Paradigm: Influence of Impulsivity and Self-Control. Alcohol Clin Exp Res. 2017;41:1370–80.
Schacht JP, Voronin KE, Randall PK, Anton RF. Dopaminergic Genetic Variation Influences Aripiprazole Effects on Alcohol Self-Administration and the Neural Response to Alcohol Cues in a Randomized Trial. Neuropsychopharmacology 2018;43:1247–56.
Schacht JP, Anton RF, Randall PK, Li X, Henderson S, Myrick H. Stability of fMRI striatal response to alcohol cues: a hierarchical linear modeling approach. Neuroimage 2011;56:61–8.
Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Allen JP, Litten RZ, editors. Measuring alcohol consumption: psychosocial and biochemical methods. Totowa, NJ: Humana Press; 1992. p. 41–72.
Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–91.
Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption-II. Addiction 1993;88:791–804.
Wechsler D. Wechsler Abbreviated Scale of Intelligence–Second Edition (WASI-II). San Antonio, TX: NCS Pearson; 2011.
Dingemanse J, Jorga KM, Schmitt M, Gieschke R, Fotteler B, Zurcher G, et al. Integrated pharmacokinetics and pharmacodynamics of the novel catechol-O-methyltransferase inhibitor tolcapone during first administration to humans. Clin Pharm Ther. 1995;57:508–17.
Palmatier MA, Kang AM, Kidd KK. Global variation in the frequencies of functionally different catechol-O-methyltransferase alleles. Biol Psychiatry. 1999;46:557–67.
Stout RL, Wirtz PW, Carbonari JP, Del Boca FK. Ensuring balanced distribution of prognostic factors in treatment outcome research. J Stud Alcohol Suppl. 1994;12:70–5.
Anton RF. New methodologies for pharmacological treatment trials for alcohol dependence. Alcohol Clin Exp Res. 1996;20:3A–9A. 7 Suppl
Lorenz M, Paul F, Moobed M, Baumann G, Zimmermann BF, Stangl K, et al. The activity of catechol-O-methyltransferase (COMT) is not impaired by high doses of epigallocatechin-3-gallate (EGCG) in vivo. Eur J Pharm. 2014;740:645–51.
Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict. 1989;84:1353–7.
Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006;31:968–80.
Schacht JP, Randall PK, Latham PK, Voronin KE, Book SW, Myrick H, et al. Predictors of Naltrexone Response in a Randomized Trial: Reward-Related Brain Activation, OPRM1 Genotype, and Smoking Status. Neuropsychopharmacology 2017;42:2640–53.
Watson PE. Total body water and blood alcohol levels: Updating the fundamentals. In: Crow KE, Batt RD, editors. Human Metabolism of Alcohol (Vol 1): Pharmacokinetics, medicolegal aspects, and general interest. Boca Raton: CRC Press; 1989.
Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin Exp Res. 1995;19:600–6.
MacKillop J. Factor structure of the alcohol urge questionnaire under neutral conditions and during a cue-elicited urge state. Alcohol Clin Exp Res. 2006;30:1315–21.
Hanlon CA, Dowdle LT, Gibson NB, Li X, Hamilton S, Canterberry M, et al. Cortical substrates of cue-reactivity in multiple substance dependent populations: transdiagnostic relevance of the medial prefrontal cortex. Transl Psychiatry. 2018;8:186.
Noori HR, Cosa Linan A, Spanagel R. Largely overlapping neuronal substrates of reactivity to drug, gambling, food and sexual cues: a comprehensive meta-analysis. Eur Neuropsychopharmacol. 2016;26:1419–30.
Jasinska AJ, Stein EA, Kaiser J, Naumer MJ, Yalachkov Y. Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies. Neurosci Biobehav Rev. 2014;38:1–16.
Zhou J. Norepinephrine transporter inhibitors and their therapeutic potential. Drugs Future. 2004;29:1235–44.
Byers S, Buchler IP, DePasquale M, Rowley HL, Kulkarni RS, Pinder L, et al. Novel, non-nitrocatechol catechol-O-methyltransferase inhibitors modulate dopamine neurotransmission in the frontal cortex and improve cognitive flexibility. Psychopharmacol (Berl). 2020;237:2695–707.
Acknowledgements
We gratefully acknowledge Katy Fuqua, Kelsey Gnade, Mark Ghent, and Lindsay Meredith for assisting with participant recruitment, assessment, and administration of study procedures; Jaclyn Condo, P.A., for assessing medical inclusion/exclusion criteria; and James Purl, James Coatsworth, and Scott Henderson for assistance with fMRI data acquisition.
Funding
This research was supported by National Institutes of Health (NIH) grants P50 AA010761 (Charleston Alcohol Research Center, PI: Howard Becker), R01 AA026859 (Schacht), and R00 AA021419 (Schacht). Tolcapone was provided by Bausch Health under an investigator-initiated, unrestricted grant. Neither NIH nor Bausch played any role in the decision to submit these data for publication.
Author information
Authors and Affiliations
Contributions
Dr Schacht conceived the study, acquired funding, oversaw the implementation of all study procedures, conducted the statistical analyses, and drafted the paper. Ms Im conducted genotyping and the RBC COMT activity and urinary riboflavin assays. Dr Hoffman created and oversaw the urn randomization program and assisted with statistical analyses. Dr Voronin oversaw participant recruitment and assessment. Dr Book provided medical oversight. Dr Anton conceived the study, acquired funding, and provided medical oversight. All authors critically reviewed the paper and approved it for publication.
Corresponding author
Ethics declarations
Competing interests
In the past 3 years, Dr Anton has been a consultant for Alkermes, Denovo, Dicerna, Foxo Bioscience, Imbrium, Otsuka, and Sophrosyne Pharma, and has received grant funding from Laboratorio Farmaceutico C.T. He is chair of and a participant in the Alcohol Clinical Trials Initiative (ACTIVE), which is sponsored by the American Society of Clinical Psychopharmacology and has been supported (in the past or currently) by Abbvie, Alkermes, Amygdala, Arbor, Dicerna, Ethypharm, Glaxo Smith Kline, Indivior, Janssen, Eli Lilly, Lundbeck, Mitsubishi, Otsuka, Pfizer, and Schering. In the past 3 years, Dr Schacht has received grant funding from Laboratorio Farmaceutico C.T. Drs Hoffman, Voronin, and Book and Ms Im report no biomedical competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Schacht, J.P., Yeongbin Im, Hoffman, M. et al. Effects of pharmacological and genetic regulation of COMT activity in alcohol use disorder: a randomized, placebo-controlled trial of tolcapone. Neuropsychopharmacol. 47, 1953–1960 (2022). https://doi.org/10.1038/s41386-022-01335-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-022-01335-z
This article is cited by
-
Adaptor protein complex 2 in the orbitofrontal cortex predicts alcohol use disorder
Molecular Psychiatry (2023)