Abstract
Individuals diagnosed with post-traumatic stress disorder (PTSD) are often comorbid for substance use disorders. Cannabis is widely used by PSTD patients, and the literature is mixed on whether cannabis use ameliorates or exacerbates patient responses to stress-associated conditioned stimuli (stress-CS). We determined if cannabis use affects responsivity to stress-CS in rats receiving 2 h stress in the presence of an odor stress-CS. Three weeks after acute stress, rats self-administered cannabinoids (delta9-tetrahydrocannabinol + cannabidiol; THC + CBD) for 15 days, and the stressed males consumed more THC + CBD than sham males. We then used the stress-CS or a novel odor (stress-NS) to reinstate THC + CBD seeking. Surprisingly, the stress-NS reinstated THC + CBD seeking, an effect blocked by N-acetylcysteine. Moreover, the stress-CS inhibited THC + CBD-CS induced reinstatement. To determine if the unexpected effects of stress-NS and -CS resulted from THC + CBD altering conditioned stress, the effect of THC + CBD use on stress-NS/CS-induced coping behaviors and spine morphology was quantified. In THC + CBD-treated rats, stress-NS increased active coping (burying). Conversely, stress-CS reduced active coping and increased passive coping (immobility) and other behavioral parameters associated with stress responses, including self-grooming and defecation. Transient spine head expansion in nucleus accumbens core is necessary for cue-induced drug seeking, and THC + CBD self-administration prevented the increase in head diameter by stress-CS in control rats. These data show THC + CBD self-administration altered the salience of environmental cues, causing neutral cues to promote active behavior (drug seeking and burying) and stress-CS to switch from active to passive behavior (inhibiting drug seeking and immobilization). We hypothesize that cannabis may exacerbate conditioned stress responses.
Similar content being viewed by others
Introduction
Experiencing a single traumatic event induces post-traumatic stress disorder (PTSD) in a subpopulation of people [1]. A person with PTSD is 2–4 times more likely to have a substance use disorder (SUD) than a person without PTSD [2, 3], and among the addictive drugs consumed by PTSD patients, cannabis use is second only to alcohol [2]. Many clinical studies suggest that patients use cannabis as self-medication to cope with PTSD symptoms [4,5,6]. However, some studies offer contrary data, that cannabis use exacerbates PTSD symptoms and treatment outcomes [7, 8].
In parallel with these clinical studies, preclinical research shows that prior exposure to stressful events increases behavioral responses to subsequent drug administration and facilitates the acquisition of cocaine, alcohol and heroin self-administration [9,10,11]. These studies suggest that stress and drug use share common neural substrates [12, 13]. In support, both addictive drug use and acute stress exposure induce long-lasting neuroadaptations in glutamatergic transmission in the nucleus accumbens core (NAcore), including increases in AMPA glutamate receptor signaling and dendritic spine density, and decreased function and expression of astroglial glutamate transporters (GLT-1) [9]. Moreover, clinical studies using N-acetylcysteine (NAC) as treatment proved effective at reducing symptoms of PTSD and/or drug craving by restoring the levels of GLT-1 [14,15,16]. Also, when acute stress is paired with a conditioned stimulus (CS), the stress-CS reinstates drug seeking for cocaine, alcohol, and heroin, and NAC pretreatment prevents this reinstatement [10, 11]. While there is substantial preclinical work on neurobiological interactions between stress and many classes of addictive drug, there are no studies on interactions between stress and cannabis use.
The aim of this study was to use a rodent model of acute stress and cannabis self-administration to determine whether cannabis and stress produce adaptations in NAcore that alter drug seeking and conditioned stress-induced coping behaviors. Accordingly, a distinct odor was paired with a single restraint stress session followed 3 weeks later by self-administration of a mixture of cannabinoids (delta9-tetrahydrocanabinol+cannabidiol; THC + CBD) [17]. Employing this model, we investigated the impact of acute stress-CS on cannabis seeking using extinction training followed by cannabis cue-induced reinstatement and whether NAC treatment prevented reinstatement. We also examined the effect of cannabis use on behavioral coping strategies using a defensive burying task (DBT). Finally, cue-induced drug seeking is associated with transient expansion of dendritic spine heads in NAcore [18], and we quantified the effect of cannabis use and stress-CS on NAcore dendritic spine morphology.
Methods
Animals
Male and female Sprague–Dawley rats (~250 g; Charles River Laboratories) were paired housed with a 12:12 h dark/light cycle with food and water available ad libitum. The animals were 2 months old and all experiments occurred in the light cycle. Rats were allowed to acclimate to the vivarium for one week. All procedures were approved by the Animal Care and Use Committee of the Medical University of South Carolina and performed in accordance with National Institutes of Health guidelines.
Acute restraint stress and scent exposure
The acute stress group was restrained for 2 h in a restraining device paired with an odor cue (lemon or sandalwood; Sun essential, Phoenix, AZ, USA) using a counter balanced design, that became the stress-CS. Sham rats were exposed to the same odors in the home-cage for 2 h, this odor is termed sham-CS. The neutral odor stimulus (NS) was an odor different from the one associated with stress (stress group) or added to the home-cage (sham group). Two weeks after the acute stress or sham, rats were implanted with indwelling catheters as previously described [17], and the following week animals started self-administration.
THC + CBD self-administration and extinction
Following surgery, rats were exposed to 5 days of THC + CBD vapor in 10:1 ratio as previously described [17]. This ratio of cannabinoids was found to be more reinforcing than THC alone using self-administration or conditioned place preference protocol [17, 19]. THC + CBD self-administration depends on CB1-receptor stimulation [17]. Before THC + CBD self-administration, food was restricted to 25 g standard chow 24 h before two sessions of food training (2 h/session). Following food training, rats had food ad libitum. Rats then underwent daily 90 min THC + CBD self-administration sessions on a fixed ratio 1 schedule. Active lever presses delivered 0.4 + 0.04 mg/kg (day 1–5) and 0.2 + 0.02 mg/kg (day 6–15) THC + CBD and were paired with cues (light + tone) followed by a 20-s timeout. A discrimination index was calculated: (active lever presses − inactive lever presses)/(active lever presses + inactive lever presses) where 0 equals no discrimination and 1 equals complete discrimination]. After 15 days of self-administration, rats underwent 10 days of extinction where active lever presses no longer resulted in delivery of drug or cues. Following self-administration and extinction, male rats were assigned to a reinstatement experiment (Fig. 2) or a defensive burying experiment (Fig. 3). Female rats were only tested in the reinstatement experiment after stress and THC + CBD self-administration.
N-acetylcysteine treatment and reinstatement
Male rats assigned to the reinstatement experiment received daily injections of saline (Veh) or NAC (100 mg/kg, ip; Sigma‐Aldrich, St. Louis, Missouri) four days before the first reinstatement test. NAC was dissolved in saline and the pH was adjusted to 7.2. Veh and NAC were administered 2 h prior to the extinction or reinstatement session.
No drug cue reinstatement
Rats were first exposed to the CS and NS in a within-subject crossover design, without drug-associated cues, and with two days of extinction between each test. The odors were placed in operant chambers from 5 min before the beginning of the test until its completion. Lever presses were monitored under extinction condition for 90 min.
With drug cue
Rats were then exposed to the CS and NS in a within-subject crossover design with drug-associated cues present. Two days of extinction between testin was performed. The odors were placed in operant chambers from 5 min before the beginning of the test until its completion. Lever presses were monitored during cued-reinstatement for 90 min.
Defensive burying
The DBT was used to evaluate the coping strategies in response to the stress-CS or a NS as previously described in [20]. Briefly, a standard home-cage (18.5 × 10 × 8.5) was divided in two zones of equal area: the bedding and the odor cue zones. Bedding was placed in one half of the cage opposite to a dish containing the odor. Each rat was placed facing away from the odor on the bedding side of the cage. Behavior was digitally recorded for 15 min and several stress-related parameters were quantified using Ethovision XT software (Leesburg, VA, USA). These parameters include: the burying index (the percentage of the odor cue zone covered by the bedding), immobility-burying ratio (time spent immobile/time spent burying), grooming and defecation.
Dendritic spine quantification
NAcore tissue slices and dendritic spine labeling procedure were performed as previously described [20](SI Methods). DiI-labeled dendrites were imaged on a laser scanning confocal microscope, Carl Zeiss LSM 880 with Airscan (Oberkochen, Germany) at ×63 (520 × 1532 frame; 0.23 μm/Z step). Following deconvolution, a 3-D perspective was rendered by the Filament module of Imaris software package version 8 (Bitplane; Saint Paul, MN). Only spines on dendrites beginning >75 μm and ending <200 μm distal to the soma and after the first branch point were quantified on cells localized to the NAcore.
Statistical analyses
All data were analyzed using GraphPad Prism, Version 9 (GraphPad Software, La Jolla, CA). Three outliers identified with ROUT (Q = 1%) were omitted from all dependent variables in Figs. 1 and 3, and eight rats were not able to be processed for dendritic spine morphology due to procedural problems (Fig. 4). Self-administration and reinstatement data were analyzed using two-way or three-way repeated measures ANOVA followed by Sidak’s or Bonferroni post hoc analysis. Defensive burying data were evaluated using two-way ANOVAs with Sidak’s post hoc comparison. Spine morphology was analyzed using two-way ANOVA, and cumulative frequency distribution were analyzed by Kolmogorov–Smirnov test. See Supplementary Methods and Tables S1 and S2 for complete statistical and methodological information.
Results
Acute restraint stress increased THC + CBD self-administration
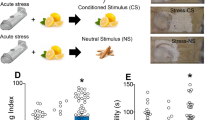
Male Sprague–Dawley rats were exposed to restraint stress (n = 39) or sham (n = 21) for 2 h and simultaneously exposed to an odor (sandalwood or lemon oil scent, crossover design). Three weeks after the stress, rats were trained to self-administer THC + CBD for 15 days followed by 10 days of extinction training (Fig. 1A). After the extinction period, some rats were examined for reinstatement of drug seeking and others for DBT (Fig. 1A). Stressed rats pressed more on the active lever over the 15 days of self-administration than sham rats (group: F1,58 = 4.83, p = 0.031; days: F8.023,465.3 = 5.63, p < 0.001) (Fig. 1B). Stressed rats also consumed more THC + CBD than sham (group: F1,58 = 10.88, p = 0.0017; days: F8.892,515.7 = 2.01, p = 0.037) (Fig. 1C). Animals in the sham and stress groups showed equivalent discrimination between the active and inactive levers (Fig. 1D). No differences were measured between groups on the active lever presses during extinction training (Fig. 1E).
A Experimental timeline outlining THC + CBD self-administration and extinction. B Acute restraint stress increased the active lever pressing during THC + CBD self-administration in males (see Fig. S3 for females). C Stressed male rats took more THC + CBD compared with sham tween the active (THC + CBD infusion) and inactive (no consequence) levers during the last 5 days animals. D There were no differences between the sham and stress groups in discriminating be of self-administration (SA). The dotted line represents a 2:1 ratio of active to inactive. E Time course of active lever presses during extinction. Data are shown as mean ± SEM. Number of animals is expressed in brackets. *p < 0.05, comparing stress to sham.
To determine if females showed the same effects as males on cannabis consumption we examined a cohort of female rats treated as in Fig. 1A. Female rats had higher intake of THC + CBD than males, and unlike males the females did not show augmented intake in stress versus sham rats (Fig. S1).
Cannabinoid withdrawal promoted drug seeking to a neutral stimulus
During THC + CBD self-administration, drug infusions were paired with a light/tone Pavlovian cue, and after extinction training some rats (21 stressed and 13 sham rats) underwent 90 min of odor-induced reinstatement with or without the drug-associated cues (Fig. 2A). Each group was split into vehicle and NAC treatment group so that there was no difference in THC + CBD consumption between NAC and vehicle (Fig. S2). NAC was examined because of its ability to increase glutamate transport and thereby inhibit reinstated drug seeking for all addictive drugs examined, including THC + CBD [17, 21]. Also, NAC prevents stress-induced increases in cocaine and alcohol reinstatement [10]. Four days before the first reinstatement test and during extinction training, rats began daily injections of NAC (100 mg/kg, ip) or vehicle (saline) (Fig. 2A). The first two reinstatement tests were in the absence of drug cues. Rats were exposed to either the stress-CS odor previously associated with the stressful or sham experience, or a novel odor stimulus (NS), using a randomized crossover design. For sham rats, the NS was a novel odor and the CS was an odor added to the home-cage during sham conditioning. For stress rats the NS was a novel different odor from the CS associated with stress. Stress-NS rats reinstated active lever pressing. In contrast, neither the sham-NS nor stress-CS groups reinstated compared to extinction baseline responding (Fig. 2B). Next, the same rats were examined for drug cue-induced reinstatement during which the light/tone cue associated with THC + CBD infusion was restored to the active lever. While the drug cue reinstated THC + CBD seeking in the sham-NS and stress-NS groups, presenting the stress odor blocked drug cue-induced reinstatement (Fig. 2B) (three-way ANOVA for Fig. 2B overall interaction F3,51 = 3.65, p = 0.018; see Table S1 for main effect and other interaction F values). Inactive lever presses were not different between any treatment group in Figs. 2B and S3A.
A Experimental timeline outlining the reinstatement protocol. B The neutral stimulus induced cannabis seeking in stressed rats in the absence of drug cues, and the stress-CS prevented drug cue-induced reinstatement. C NAC treatment blocked reinstated behavior in all groups. Data are shown as mean ± SEM. Each dot in bar represents a rat and the number of rats is expressed in brackets (B, sham N = 6, stress N = 13. C, sham N = 7, stress N = 8). *p < 0.05, compared to extinction within each group using a Bonferroni post hoc analysis.
Reinstatement induced by stress-NS in the absence of drug cue and by sham-NS and stress-NS groups in the presence of drug cue was absent in rats pretreated with NAC (Fig. 2C) (three-way ANOVA for Fig. 2C overall interaction F3,39 = 0.91, p = 0.45; see Table S1 for main effect and other interaction F values). Inactive lever presses were not different between any treatment group in Figs. 2C and S3B.
Akin to males, the stress-NS induced reinstatement in females in the absence of drug cue, both the sham-NS and stress-NS groups reinstated to drug cue, and stress-CS inhibiting drug cue-induced reinstatement (Fig. S4A). Inactive lever presses were not different between any treatment group in Fig. S4A, B.
Cannabinoid withdrawal altered stress-CS induced behavioral coping strategies
The effect of THC + CBD self-administration on stress-NS and stress-CS promoting and reducing drug seeking, respectively, is in marked contrast to other drugs, including alcohol, heroin and cocaine, where a stress-NS has no effect on drug seeking and a stress-CS precipitates drug seeking [10, 11]. We hypothesized that cannabis use promoted the salience of the stress-CS such that it immobilized rats thereby inhibiting drug seeking and increasing the salience of the stress-NS to induce active coping behavior, including reinstating THC + CBD seeking. To examine the hypothesis that THC + CBD self-administration had altered stress responding, we employed a modified DBT to estimate changes in active (burying) and passive (immobility) coping strategies, and other behavioral parameter as grooming and defecation that have been associated with stress responses [22,23,24]. Because male and female rats showed equivalent responses to stress-NS and -CS, to conserve animals only male rats were exposed to stress or sham plus odor, trained to self-administer THC + CBD and extinguished for this series of experiments (Fig. 1A). To establish baseline responses in the DBT, some rats went through a parallel yoked vehicle self-administration/extinction protocol (Fig. S5; stress n = 16 and sham n = 8). After extinction training, stressed rats were divided into stress-CS and stress-NS groups such that the groups did not differ in THC + CBD consumption (Fig. S6). Rats were then exposed to either stress-CS or -NS in a 15 min DBT session and sacrificed immediately afterwards for quantifying dendritic spine morphology in NAcore (Fig. 3A). Compared to vehicle, THC + CBD self-administration increased the burying index in both the sham-CS and stress-NS groups and reduced the burying index in the stress-CS group (the percentage of the odor cue zone covered by the bedding, see “Methods” for details) (interaction: F2,44 = 9.80, p < 0.001, see Table S1 for main effect and other interaction F values) (Fig. 3B). Similarly, the percent of total time spent burying was elevated after THC + CBD in the stress-NS group and reduced in the stress-CS group compared to vehicle rats (interaction: F2,44 = 6.34, p = 0.004) (Fig. 3C). Conversely, the percent time spent immobile was elevated after THC + CBD in the stress-CS group (interaction: F2,44 = 7.86, p = 0.001) (Fig. 3D). The overall effect of THC + CBD on burying and immobilization is revealed in the ratio of time spent immobilized:burying (Fig. 3E). The stress-NS group had a reduced ratio and the stress-CS group had an elevated ratio after THC + CBD use compared to vehicle (interaction: F2,44 = 4.16, p = 0.022). Two other measures associated with stress responses are grooming and defecation [23, 24]. Stress-CS increased grooming after THC + CBD (interaction: F2,44 = 8.68, p = 0.001) (Fig. 3F) and there was a drug effect of THC + CBD to increase the number of fecal pellets (drug: F1,44 = 10.29, p = 0.002) (Fig. 3G).
A Experimental timeline outlining the defensive burying task after rats had received stress/sham followed by vehicle or THC + CBD self-administration (see timeline in Fig. 1A for this portion of the experiment). B The stress-CS reduced and the NS increased the burying index in rats that self-administered THC + CBD. C The stress-CS reduced and stress-NS increased the percent time spent burying in rats that self-administered THC + CBD. D The stress-CS increased the time spent immobile in rats that self-administered THC + CBD. E The stress-CS increased and stress-NS reduced the immobility-burying ratio in rats that self-administered THC + CBD. F The stress-CS increased the grooming in rats that self-administered THC + CBD. G Rats trained to self-administer THC + CBD defecated more than vehicle rats. Data are shown as mean ± SEM. Each dot in bar represents an analyzed animal. Number of rats is expressed in D. *p < 0.05 compared to Sham-CS within each drug group using a Sidak post hoc; #p < 0.05 comparing THC + CBD to vehicle within each stress/sham group.
Cannabinoid withdrawal prevented the structural plasticity induced by the stress-CS
The NAcore is embedded in brain circuitry underpinning the translation of salient environmental stimuli into adaptive behavioral responses [16]. Acute restraint stress induces enduring increases in dendritic spine density in NAcore neurons and stress-CS increases spine head diameter [20]. Moreover, the increase in spine density may contribute to resilience in behavioral responding to stress-CS since the increase in spine density is positively correlated with stress coping behavior (burying) [25]. To determine if THC + CBD use altered the effects of acute restraint stress and subsequent stress-CS exposure, rats were sacrificed immediately after the 15 min DBT session in Fig. 3 and brain slices containing NAcore were diolistically labeled with a membrane targeted dye (DiI) to quantify dendritic spine morphology in NAcore neurons (Fig. 4A, B). THC + CBD self-administration decreased spine density compared to vehicle rats, and acute restraint stress produced an enduring elevation in spine density compared to sham (Fig. 4C) (vehicle vs THC + CBD: F1,167 = 25.21, p < 0.001; group: F2,167 = 6.53, p = 0.001). The cumulative frequency plots show that the reduction in spine density by THC + CBD self-administration occurred in all three stress groups (Fig. 4D).
A Example of a labeled neuron and a dendritic segment that was quantified. B Representative dendritic segments from each treatment group, pseudocolored to match the data in C and D. C THC + CBD decreased spine density in NAcore. D Cumulative frequency distribution of spine density comparing THC + CBD to vehicle rats for each group. E THC + CBD abolished the stress-CS induced increase in spine head diameter. F Cumulative frequency distribution of spine head diameter comparing THC + CBD to vehicle rats for each group. Data are shown as mean ± SEM. Each dot in bar represents an analyzed segment. N is shown as number of neurons quantified over number of animals in each condition. *p < 0.05 compared to Sham-CS within each drug group using a Sidak post hoc; #p < 0.05 comparing THC + CBD to vehicle within each stress/sham group.
Exposure to the stress-CS increased the spine head diameter in the vehicle group and this effect of stress-CS was abolished after THC + CBD self-administration (interaction: F2,167 = 8.14, p < 0.001). Accordingly, the cumulative frequency plot reveals that THC + CBD reduced the spine head diameter after sham-CS and stress-CS compared with vehicle (Fig. 4E, F).
Potential relationships between coping behaviors in the DBT and spine morphology were examined using Pearson’s correlation analysis (Fig. 5 and Table S2). As with our previous publication [25], in vehicle-control rats active coping (burying index) was positively correlated with both spine density and head diameter (Fig. 5A, B). This correlation was lost in THC + CBD rats (Table S2). Also, spine head diameter was negatively correlated to the immobility-burying ratio in THC + CBD rats (Fig. 5C). Together, these data indicate that the decrease in spine density and lack of stress-CS induced increase in head diameter in THC + CBD rats was associated with increased passive relative active stress coping behaviors.
A Positive correlation between spine per μm and burying index during 15 min of defensive burying task in vehicle-treated animals. B Positive correlation between spine head diameter (μm) and burying index during 15 min of defensive burying task in vehicle-treated animals. C Negative correlation between spine head diameter and immobility:burying ratio. Spine morphology data is represented as the mean of segments by animal. Each dot represents an animal.
Discussion
Using a combination of a conditioned stress and cannabis self-administration, we showed that acute restraint stress potentiated THC + CBD consumption in males, but not females. Moreover, in stressed rats, THC + CBD withdrawal promoted drug seeking and active coping (defensive burying) in response to a neutral odor s(stress-NS). In contrast, exposure to a stress conditioned odor (stress-CS) inhibited both drug cue-induced THC + CBD seeking and active burying behavior while simultaneously increasing passive coping behaviors such as the immobility, self-grooming and defecation [23, 24]. THC + CBD use reduced dendritic spine density and prevented the capacity of stress-CS to increase dendritic spine head diameter in NAcore. Surprisingly, THC + CBD also promoted generalization of stress responding to neutral stimuli not associated with acute stress exposure, resulting in the stress-NS reinstating THC + CBD seeking and inducing active coping responses in the DBT. Taken together, these data indicate that the use of cannabis alters responding to stress conditioned stimuli by promoting passive over active coping mechanisms, and causes generalization of active coping responses to neutral stimuli. As discussed below, we hypothesize that the THC + CBD alterations in overall stress responding may be related to enduring drug effects on dendritic spine morphology and plasticity.
THC + CBD promotes passive coping to stressful stimuli
Stress exposure reinstates drug seeking in rodents and drug craving in humans [26, 27]. However, most animal studies use exposure to a primary stressor to reinstate drug seeking [26]. Given that PTSD patients avoid re-exposure to the primary stressor and that the symptoms of stress are triggered by environmental stimuli [28, 29], we chose to evaluate a stress-CS previously paired with a single restraint stress or a neutral odor (stress-NS). Previously we found that stress-CS, but not stress-NS reinstated drug seeking for cocaine and alcohol in the absence of drug-associated cues [10, 11]. However, here we found that stress-CS did not reinstate cannabinoid seeking and surprisingly prevented THC + CBD cue-induced reinstatement. Moreover, THC + CBD enabled a stress-NS to initiate drug seeking in the absence of THC + CBD-conditioned cues. Importantly, these contrary effects of the stress-NS and stress-CS after THC + CBD use were observed in both male and female rats.
From these experiments, we hypothesized that cannabis use promoted the salience of the stress-CS such that it immobilized rats and inhibited drug cue-induced seeking and promoted the salience of the stress-NS sufficiently to initiate THC + CBD seeking in the absence of drug cues. We used a DBT [22] to more directly monitor stress coping strategies where an active coping strategy consists of burying the stress-CS source and a passive coping strategy involves a profile of behaviors that contribute to avoiding direct confrontation with the stressor (immobilization, grooming and defecation) [22,23,24]. We found that rats trained to self-administer THC + CBD showed greater active coping than vehicle trained rats when presented with an NS. This stress coping profile was reversed in the presence of the stress-CS where THC + CBD rats showed reduced active burying and an increase in passive coping behaviors including immobility-burying ratio, grooming and defecation. In animals, passive coping in the DBT is associated with higher activation of the hypothalamic-pituitary-adrenal axis (increased plasma levels of corticosterone and ACTH) than active coping strategies [30, 31] and we previously showed that exposure to the CS during DBT increases corticosterone to a level consistent with the original stressor [20]. Moreover, in humans, passive coping strategies can exacerbate PTSD symptomology [32, 33], suggesting that cannabis use may be exacerbating reactivity to stressful stimuli.
THC + CBD promotes generalizing stress responses to neutral stimuli
A characteristic symptom of PTSD is the progressive generalization of stress responses to previously neutral stimuli unrelated to stress cues [34]. While exposure to the stress-NS did not affect reinstated drug seeking in rats trained to self-administer cocaine or alcohol, after THC + CBD self-administration the stress-NS reinstated drug seeking and promoted active coping strategies by increasing burying and reducing the immobility-burying ratio. These data indicate that cannabis use 49 be aggravating the cardinal symptom of PTSD to generalize stress responding to stimuli unassociated with stress [1, 35] and that cannabis use is distinct from other addictive drugs in this regard [10, 11].
THC + CBD disrupts compensatory plasticity in accumbens to stress exposure
Stress is known to alter the structural plasticity in brain regions involved in addictive behaviors [36]. For example, restraint stress produces spine loss in medial prefrontal cortex [37, 38], and increases spine density in amygdala and NAcore [25, 39, 40]. In contrast, THC exposure reduces spine density in prefrontal cortex, hippocampus and NAcore [17, 41, 42]. Here, we found a similar reduction in NAcore spine density after self-administered THC + CBD regardless of whether animals were sham or restraint stressed. This effect of THC is in contrast with other addictive drugs that either increase (cocaine, alcohol) or produce no change (heroin) in NAcore dendritic spine density [18]. Moreover, in response to cues that promote drug seeking, NAcore spine head diameter is increased in animals trained to administer heroin, cocaine or alcohol [18]. We observed a similar stress-CS increased spine head diameter in vehicle rats. However, after THC + CBD self-administration spine head diameter decreased in response to the stress-CS.
It is tempting to link the lack of increase in NAcore spine head diameter by stress-CS in THC + CBD trained rats with the lack of stress-CS induced reinstatement and the increase in passive relative to active coping responses. Supporting this possibility, increased spine head diameter in NAcore is positively correlated with cue-induced reinstatement of drug seeking [43], and the stress-CS failed to increase drug seeking after THC + CBD self-administration. Moreover, the positive correlation between head diameter or density and the burying index in vehicle rats was absent in THC + CBD trained rats suggesting that increases in dendritic head diameter and density promotes active coping to the stress-CS in vehicle-treated animals. Also, spine head diameter was negatively correlated with the immobility-burying ratio indicating that the reduction in spine head diameter by the stress-CS in THC + CBD rats may have contributed to the stress-CS increases immobility relative to burying behavior. A similar relationship was shown between decreased mature dendrites in the amygdala after THC and a fear extinction deficit in adolescent rats [44].
Potential clinical relevance
Cannabis has been suggested as a treatment for PTSD [45,46,47]. However, recent longitudinal studies showed that cannabis use is associated with higher PTSD symptoms severity, including greater traumatic thought intrusions [7, 8]. We demonstrated that the enduring plasticity induced by a stressful event potentiated cannabinoid consumption in male rats, consistent with clinical studies that veterans diagnosed with PTSD and reporting higher combat exposure are more likely to use cannabis [48, 49]. Moreover, we found that after THC + CBD rats generalized active stress coping responses to a neutral odor, consistent with a cardinal symptom of PTSD to generalize stress responses to previously neutral stimuli [1, 35]. Finally, our findings that daily NAC treatment during cannabinoid withdrawal prevented the generalization of stress responses to a neutral stimulus and inhibited a neutral stimulus from inducing THC + CBD seeking complements a double-blind clinical trial showing that chronic NAC treatment reduced both PTSD symptoms and drug craving in veterans comorbid for PTSD and SUDs [14]. The potential ameliorative action of NAC on PTSD symptoms may be related to its capacity to elevate astroglial glutamate transporters (GLT-1) since acute restraint stress reduces GLT-1 in the NAcore and restoring GLT-1 prevented stress-induced increases in the acquisition of cocaine self-administration [9]. However, NAC effects on endocannabinoids may contribute to its ability to block reinstatement since NAC increases anandamide concentrations in striatum [50] and THC + CBD self-administration lowers presynaptic CB1-receptor efficacy [51].
Conclusions
Given increasing use of cannabis to self-medicate PTSD, it is urgent to understand the long-term impact of this drug on brain circuitry and stress responding. We showed that a single stressful event increased future cannabinoid consumption in male rodents, and that cannabinoid use changed conditioned stress responses and caused neutral stimuli to initiate active stress coping strategies. Moreover, these cannabinoid-induced changes in stress responses were associated with changes in the morphology of neurons in the NAcore. Our data seem most consistent with the clinical literature showing that cannabis use may be associated with greater PTSD symptom severity.
References
American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. 5th ed. Washington, DC: American Psychiatric Publishing; 2013. p. 947.
Bedard-Gilligan M, Bahorik AL, Satre DD, Kline-Simon AH, Weisner CM, Campbell CI. Alcohol, cannabis, and other drug use: engagement and outcome in PTSD treatment. Psychol Addict Behav. 2018;32:277–88.
Pietrzak RH, Goldstein RB, Southwick SM, Grant BF. Medical comorbidity of full and partial posttraumatic stress disorder in US adults: results from Wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. Psychosom Med. 2011;73:697–707.
Bonn-Miller MO, Babson KA, Vandrey R. Using cannabis to help you sleep: heightened frequency of medical cannabis use among those with PTSD. Drug Alcohol Depend. 2014;136:162–5.
Grant S, Pedersen ER, Neighbors C. Associations of posttraumatic stress disorder symptoms with marijuana and synthetic cannabis use among young adult U.S. veterans: a pilot investigation. J Stud Alcohol Drugs. 2016;77:509–14.
Metrik J, Bassett SS, Aston ER, Jackson KM, Borsari B. Medicinal versus recreational cannabis use among returning veterans. Transl Issues. Psychol Sci. 2018;4:6–20.
Mammen G, Rueda S, Roerecke M, Bonato S, Lev-Ran S, Reh, J. Association of cannabis with long-term clinical symptoms in anxiety and mood disorders: a systematic review of prospective studies. J Clin Psychiatry. 2018;79:17r11839.
Metrik J, Stevens AK, Gunn RL, Borsari B, Jackson KM. Cannabis use and posttraumatic stress disorder: prospective evidence from a longitudinal study of veterans. Psychol Med. 2020:1–11.
Garcia-Keller C, Kupchik YM, Gipson CD, Brown RM, Spencer S, Bollati F, et al. Glutamatergic mechanisms of comorbidity between acute stress and cocaine self-administration. Mol Psychiatry. 2016;21:1063–9.
Garcia-Keller C, Cora Smiley C, Monforton C, Melton S, Kalivas PW, Gass J. N-Acetylcysteine treatment during acute stress prevents stress-induced augmentation of addictive drug use and relapse. Addict Biol. 2020;25:e12798.
Carter JS, Kearns AM, Vollmer KM, Garcia-Keller C, Weber RA, Baker NL, et al. Long-term impact of acute restraint stress on heroin self-administration, reinstatement, and stress reactivity. Psychopharmacology. 2020;237:1709–21.
Aupperle RL, Melrose AJ, Stein MB, Paulus MP. Executive function and PTSD: disengaging from trauma. Neuropharmacology. 2012;62:686–94.
Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–38.
Back SE, McCauley JL, Korte KJ, Gros DF, Leavitt V, et al. A double-blind, randomized, controlled pilot trial of N-acetylcysteine in veterans with posttraumatic stress disorder and substance use disorders. J Clin Psychiatry. 2016;77:e1439–46.
Amen SL, Piacentine LB, Ahmad ME, Shi-Jiang LI, Mantsch JR, et al. Repeated N-acetyl cysteine reduces cocaine seeking in rodents and craving in cocaine-dependent humans. Neuropsychopharmacology. 2011;36:871–8.
Scofield MD, Heinsbroek JA, Gipson CD, Kupchik YM, Spencer S, et al. The nucleus accumbens: mechanisms of addiction across drug classes reflect the importance of glutamate homeostasis. Pharm Rev. 2016;68:816–71.
Spencer S, Neuhofer D, Chioma VC, Garcia-Keller C, Schwartz DJ, et al. A model of delta(9)-tetrahydrocannabinol self-administration and reinstatement that alters synaptic plasticity in nucleus accumbens. Biol Psychiatry. 2018;84:601–10.
Mulholland PJ, Chandler LJ, Kalivas PW. Signals from the fourth dimension regulate drug relapse. Trends Neurosci. 2016;39:472–85.
Vann RE, Gamage TF, Warner JA, Marshall EM, Taylor NL, et al. Divergent effects of cannabidiol on the discriminative stimulus and place conditioning effects of Delta(9)-tetrahydrocannabinol. Drug Alcohol Depend. 2008;94:191–8.
Garcia-Keller C, Carter JS, Kruyer A, Kearns AM, Hopkins JL, et al. Behavioral and accumbens synaptic plasticity induced by cues associated with restraint stress. Neuropsychopharmacology. 2021;46:1848–56.
Kalivas BC, Kalivas PW. Corticostriatal circuitry in regulating diseases characterized by intrusive thinking. Dialogues Clin Neurosci. 2016;18:65–76.
Fucich EA, DA Morilak. Shock-probe defensive burying test to measure active versus passive coping style in response to an aversive stimulus in rats. Bio Protoc. 2018;8:e2998.
Kalueff AV, Stewart AM, Song C, Berridge KC, Graybiel AM, et al. Neurobiology of rodent self-grooming and its value for translational neuroscience. Nat Rev Neurosci. 2016;17:45–59.
Nakade Y, Fukuda H, Iwa M, Tsukamoto K, Yanagi H, et al. Restraint stress stimulates colonic motility via central corticotropin-releasing factor and peripheral 5-HT3 receptors in conscious rats. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1037–44.
Garcia-Keller C, Carter JS, Kruyer A, Kearns AM, Hopkins JL, et al. Behavioral and accumbens synaptic plasticity induced by cues associated with restraint stress. Neuropsychopharmacology. 2021;46:1848–56.
Mantsch JR, Baker DA, Funk D, Lê AD, Shaham Y, et al. Stress-induced reinstatement of drug seeking: 20 years of progress. Neuropsychopharmacology. 2016;41:335–56.
Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann NY Acad Sci. 2008;1141:105–30.
Liberzon I, Abelson JL. Context processing and the neurobiology of post-traumatic stress disorder. Neuron. 2016;92:14–30.
Suh J, Ressler KJ. Common biological mechanisms of alcohol use disorder and post-traumatic stress disorder. Alcohol Res. 2018;39:131–45.
Koolhaas JM, Korte SM, De Boer SF, Van Der Vegt BJ, Van Reenen CG, et al. Coping styles in animals: current status in behavior and stress-physiology. Neurosci Biobehav Rev. 1999;23:925–35.
De Boer SF, Slangen JL, Van der Gugten J. Plasma catecholamine and corticosterone levels during active and passive shock-prod avoidance behavior in rats: effects of chlordiazepoxide. Physiol Behav. 1990;47:1089–98.
Olff M, Langeland W, Gersons BP. The psychobiology of PTSD: coping with trauma. Psychoneuroendocrinology. 2005;30:974–82.
Mattson E, James L, Engdahl B. Personality factors and their impact on PTSD and post-traumatic growth is mediated by coping style among OIF/OEF veterans. Mil Med. 2018;183:e475–80.
Deslauriers J, Toth M, Der-Avakian A, Risbrough VB, et al. Current status of animal models of posttraumatic stress disorder: behavioral and biological phenotypes, and future challenges in improving translation. Biol Psychiatry. 2018;83:895–907.
Paredes D, Morilak DA. A rodent model of exposure therapy: the use of fear extinction as a therapeutic intervention for PTSD. Front Behav Neurosci. 2019;13:46.
Christoffel DJ, Golden SA, Russo SJ. Structural and synaptic plasticity in stress-related disorders. Rev Neurosci. 2011;22:535–49.
Goldwater DS, Pavlides C, Hunter RG, Bloss EB, Hof PR, et al. Structural and functional alterations to rat medial prefrontal cortex following chronic restraint stress and recovery. Neuroscience. 2009;164:798–808.
Radley JJ, Morrison JH. Repeated stress and structural plasticity in the brain. Ageing Res Rev. 2005;4:271–87.
Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S, et al. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc Natl Acad Sci USA. 2005;102:9371–6.
Vyas A, Jadhav S, Chattarji S. Prolonged behavioral stress enhances synaptic connectivity in the basolateral amygdala. Neuroscience. 2006;143:387–93.
Rubino T, Prini P, Piscitelli F, Zamberletti E, Trusel M, et al. Adolescent exposure to THC in female rats disrupts developmental changes in the prefrontal cortex. Neurobiol Dis. 2015;73:60–9.
Rubino T, Realini N, Braida D, Guidi S, Capurro V, et al. Changes in hippocampal morphology and neuroplasticity induced by adolescent THC treatment are associated with cognitive impairment in adulthood. Hippocampus. 2009;19:763–72.
Gipson CD, Kupchik YM, Shen H, Reissner KJ, Thomas CA, et al. Relapse induced by cues predicting cocaine depends on rapid, transient synaptic potentiation. Neuron. 2013;77:867–72.
Saravia R, Ten-Blanco M, Julià-Hernández M, Gagliano H, Andero R, et al. Concomitant THC and stress adolescent exposure induces impaired fear extinction and related neurobiological changes in adulthood. Neuropharmacology. 2019;144:345–57.
Elms L, Shannon S, Hughes S, Lewis N, et al. Cannabidiol in the treatment of post-traumatic stress disorder: a case series. J Alter Complement Med. 2019;25:392–7.
Hill MN, Campolongo P, Yehuda R, Patel S, et al. Integrating endocannabinoid signaling and cannabinoids into the biology and treatment of posttraumatic stress disorder. Neuropsychopharmacology. 2018;43:80–102.
Jetly R, Heber A, Fraser G, Boisvert D. The efficacy of nabilone, a synthetic cannabinoid, in the treatment of PTSD-associated nightmares: a preliminary randomized, double-blind, placebo-controlled cross-over design study. Psychoneuroendocrinology. 2015;51:585–8.
Boden MT, Babson KA, Vujanovic AA, Short NA, Bonn-Milleret MO, et al. Posttraumatic stress disorder and cannabis use characteristics among military veterans with cannabis dependence. Am J Addictions. 2013;22:277–84.
Loflin M, Earleywine M, Bonn-Miller M. Medicinal versus recreational cannabis use: Patterns of cannabis use, alcohol use, and cued-arousal among veterans who screen positive for PTSD. Addict Behav. 2017;68:18–23.
Smaga I, Bystrowska B, Gawliński D, Pomierny B, Stankowicz P, et al. Antidepressants and changes in concentration of endocannabinoids and N-acylethanolamines in rat brain structures. Neurotox Res. 2014;26:190–206.
Neuhofer D, Spencer S, Chioma VC, Beloate LN, Schwartz D, et al. The loss of NMDAR-dependent LTD following cannabinoid self-administration is restored by positive allosteric modulation of CB1 receptors. Addict Biol. 2020;25:e12843.
Acknowledgements
We thank Eric Dereschewitz and Lucio Vaccaro for advice and technical assistance. Also, we thank the National Institute of Drug Abuse (NIDA) Drug Supply Program for providing (-)-trans-Delta9-tetrahydrocannabinol (THC) in ethyl alcohol (95%) and Cannabidiol (CBD) (ADL-19496-11) (Synthetic).
Funding
This work was supported by the National Institute of Health NIH, DA12513, DA046373 and VA BX004727 (PK), DA016511 (PK and CR), NIH K99DA047426-01A1 (CGK), U.S. Department of Defense Grant W81XWH-13-2-0075 (PK), National Science Foundation Grant OIA-1539034 (PK) and U.S. Veterans Affairs VA BX004727 (PK). The Carl Zeiss LSM 880 confocal microscope was acquired through an NIH/OD grant (1S10OD021532).
Author information
Authors and Affiliations
Contributions
RH, PWK, and CGK designed the research; RH, CGK, ADC, and MEM performed research; RH, CMR, CGK, and PWK analyzed data; RH, CGK, and PWK wrote the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Hodebourg, R., Meyerink, M.E., Crow, A.D. et al. Cannabinoid use is enhanced by stress and changes conditioned stress responses. Neuropsychopharmacol. 47, 1037–1045 (2022). https://doi.org/10.1038/s41386-022-01287-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-022-01287-4