Abstract
Individuals with schizophrenia show impairments in associative learning. One well-studied, quantifiable form of associative learning is Pavlovian fear conditioning. However, to date, studies of fear conditioning in schizophrenia have been inconclusive, possibly because they lacked sufficient power. To address this issue, we pooled data from four independent fear conditioning studies that included a total of 77 individuals with schizophrenia and 74 control subjects. Skin conductance responses (SCRs) to stimuli that were paired (the CS + ) or not paired (CS−) with an aversive, unconditioned stimulus were measured, and the success of acquisition of differential conditioning (the magnitude of CS + vs. CS− SCRs) and responses to CS + and CS− separately were assessed. We found that acquisition of differential conditioned fear responses was significantly lower in individuals with schizophrenia than in healthy controls (Cohen’s d = 0.53). This effect was primarily related to a significantly higher response to the CS− stimulus in the schizophrenia compared to the control group. Moreover, the magnitude of this response to the CS− in the schizophrenia group was correlated with the severity of delusional ideation (p = 0.006). Other symptoms or antipsychotic dose were not associated with fear conditioning measures. In conclusion, individuals with schizophrenia who endorse delusional beliefs may be over-responsive to neutral stimuli during fear conditioning. This finding is consistent with prior models of abnormal associative learning in psychosis.
Similar content being viewed by others
Introduction
An impairment in associative learning may represent a critical step on the path linking genetic and environmental risk factors for schizophrenia to the formation both positive and negative symptoms [1]. For instance, delusions have been suggested to arise from incorrected inferences from evidence [2], possibly related to a bias toward assigning meaning to neutral or irrelevant events and internal representations [3]. On the other hand, schizophrenia has also been associated with diminished responses to and learning from salient stimuli, such as rewards [4]. This impairment could in turn explain persistent deficits in motivation and social functioning in schizophrenia. Thus, both inappropriate and deficient assignments of salience have been associated with schizophrenia and may potentially underlie positive and negative symptoms, respectively. However, direct empirical support for these models remains limited.
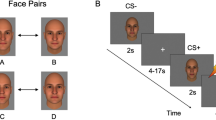
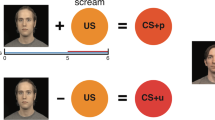
Pavlovian fear conditioning is one simple experimental paradigm that can be used to quantitatively measure associative learning [5] and test such models directly in humans. In a typical fear conditioning experiment, two distinct stimuli are presented: (1) a neutral stimulus that is repeatedly paired with an aversive unconditioned stimulus (US), such as an electrical shock, unpleasant picture or loud noise, the CS + , and (2) another neutral stimulus that is never paired with the US, the CS−. Over the course of the experiment, subjects learn to associate the presentation of the CS + with the US. As a result, subjects show similar responses to the CS + that were initially elicited by the US, for example a galvanic skin conductance response. Thus, the main outcome measure, reflecting the success of acquisition of conditioned fear responses and the ability to form associations, is the difference between the responses to the CS + and the CS− stimuli. A hypothesis based on models of abnormal associative learning in schizophrenia [2, 3] posits that individuals with schizophrenia exhibit increased responses to the neutral stimulus (i.e., the CS−) during fear conditioning [6,7,8]. Conversely, a decrease in relevant cue-induced salience detection would result in a decreased response to the CS + stimulus in schizophrenia [7, 9]. Together, the expected pattern, increased responses to neutral stimuli and decreased responses to the conditioned stimuli, would result in impaired acquisition of conditioned fear responses in schizophrenia.
Although schizophrenia has been studied extensively using Pavlovian conditioning paradigms, no consistent picture has yet emerged [6, 8, 10,11,12,13,14,15,16,17]. Some studies have found evidence for diminished differences between responses to CS + and CS− stimuli in schizophrenia and a higher response to the CS− [6, 8, 11], whereas other studies have not found statistically significant differences [10, 13]. These discrepant findings may be in part due to the small sample sizes of these previously published studies. Also, clinical features associated with the putative deficits in acquisition of conditioned fear in schizophrenia have not been identified.
Thus, here we aimed to address some of these unresolved questions by pooling data from four previously published studies of fear conditioning in schizophrenia. We limited this analysis to research published in the past 20 years in order to maximize the similarity in methods and definitions of schizophrenia, as well as the availability of the data, across the studies. All four studies included in these analyses collected skin conductance responses (SCRs) to CS + and CS− stimuli during a Pavlovian fear conditioning paradigm in individuals diagnosed with schizophrenia and healthy control subjects and measured symptom severity in the schizophrenia group using standard instruments [8, 10, 11, 13]. In this pooled sample of 77 individuals with schizophrenia and 74 healthy control subjects, we tested whether impaired acquisition of differential fear conditioning was evident in schizophrenia. We then tested the following specific predictions, that compared to the controls, the participants with schizophrenia would show: (1) higher responses to the CS− stimulus, which would be associated with higher levels of delusional ideation, and (2) lower responses to the CS + stimulus, which would associated with more severe negative symptoms.
Materials and methods
The sample
Data from four independent studies were included in the analyses of this study, comprising a total sample of 77 individuals with schizophrenia and 74 healthy control subjects (Table 1). One study [6] with relevant data was not included in the current analyses because the original data were no longer available. Following quality control procedures, one individual with schizophrenia from Holt et al. 2009 [11] was excluded from all analyses due to that subject’s outlier status (see below); therefore 76 subjects were included in the final combined schizophrenia group. All four studies defined schizophrenia using DSM-IV criteria assessed using a standardized, structured clinical interview [18]. In all studies, fear conditioning responses were measured using skin conductance. Each individual study had obtained ethics committee approval separately (from the Partners Healthcare Institutional Review Board or Lothian National Health Service Research Ethics Committee), and each participant had signed an ethics committee approved informed consent document before enrollment. The four studies differed in some aspects of their design and methods, including whether the data was collected inside an MRI scanner or not, whether the US was a mild electrical shock or an unpleasant picture, the reinforcement rate, number of trials, duration of stimulus presentation, and duration of the overall experiment. These specific methodological characteristics of the studies are described in Table 2.
Data quality control
In one participant of the schizophrenia group of the Holt et al., 2009 study, the CS + vs. CS− contrast was more than four times higher than the interquartile interval from the mean. This subject was therefore excluded from all subsequent statistical analyses.
Clinical measures
In three out of four studies, the severity of delusional symptoms was measured using the Peter’s Delusion Inventory (PDI) [19, 20], which is a self-report questionnaire measuring delusional ideation validated for use in both non-clinical and schizophrenia samples [21, 22]. Two studies used a 40-item version of the PDI [19] and one study use the 21-item PDI [20]. These data were normalized across studies by dividing the total score by the total number of items. In addition, positive, negative, and general symptoms of schizophrenia, as well as the total burden of symptoms, were assessed in all studies using the Positive and Negative Syndrome Scale (PANSS) [23]. Also, information about treatment with antipsychotic medication (current daily dose) was obtained for each subject with schizophrenia and converted to chlorpromazine equivalents.
Statistical tests
Primary analyses
Our main hypothesis was that the difference in SCRs between the CS + and CS− was significantly different between the schizophrenia and control groups (i.e., lower in the schizophrenia group). This hypothesis was tested using a linear mixed effects model: CS + vs. CS− contrast ~ schizophrenia diagnosis + sex + age. Study (i.e., of the four original cohorts) was entered as a random factor into the model. Separate linear mixed effects models were generated to test whether the two groups differed in response to the CS + or CS− stimuli separately, while controlling for age and sex, and again including study as a random factor.
In a subset of the schizophrenia group (n = 56) who had completed the PDI, we tested if the response to the CS− was correlated with the total PDI score using bi-square robust linear regression, with sex, age and study as covariates. Similarly, we tested in the full schizophrenia sample (n = 76) whether the response to the CS + was correlated with the PANSS negative symptom subscale score, using bi-square robust linear regression, with sex, age and study as covariates.
Secondary, exploratory analyses
We explored whether the severity of positive, negative, general, or total symptoms, or current antipsychotic medication dose, were associated with any of the fear conditioning measures in the schizophrenia group, while controlling for age, sex and study.
Confirmatory meta-analysis
We also conducted a meta-analysis in order to (a) confirm the results of the linear mixed effects model, (b) calculate a Cohen’s d value for the combined sample, and (c) to obtain an estimate of the heterogeneity of the studies.
We chose to use robust regression since this method provides more accurate estimates of the true effect, while appropriately controlling for false positives, with sufficient power [24]. Linear mixed effects analyses were carried out using the lme4 package in R [25] and robust regression was conducted using the robustbase package in R [26]. The meta-analysis was conducted using the metafor package for R [27]. Note that the meta-analysis did not take sex or age into account, but relied on the fact that the samples were approximately matched on these demographic variables in each individual study. The code used for the analyses is available here: https://github.com/ltuominen/SCRSCZ.
Results
Group differences in Pavlovian fear conditioning
In the pooled sample, the difference between the magnitude of SCRs to the CS + and the CS− stimuli was significantly smaller in the schizophrenia, compared to the control, group (b = −0.134, t143 = −2.57, p = 0.011), (Fig. 1; also see Fig. 2 and Supplementary Table 1 for results of the individual studies). The meta-analysis confirmed the findings of the linear mixed effects model (Cohen’s d = 0.5299, standard error = 0.1666, z = 3.1807, p = 0.0015, 95% CI = 0.2034–0.8565; Supplementary Table 1). There was no evidence for heterogeneity across studies (I2 = 0%).
The left panel shows that the difference between skin conductance responses (SCR) to the CS + and CS− is lower in the schizophrenia (SCZ) than in the control (CTR) group (b = −0.134, t143 = −2.57, p = 0.011). The middle panel shows that SCR to the CS + is on average numerically lower in SCZ than in CTR but this difference was not significant (b = −0.047, t143 = −0.966, p = 0.336). Finally, the right panel shows that the SCR to the CS− is significantly higher in SCZ compared to CTR (b = 0.087, t143 = 1.99, p = 0.048). For illustration, the individual datapoints have been adjusted for differences in age, sex and study.
This figure shows that the mean difference between skin conductance responses to the CS + and CS− stimuli is numerically smaller in the schizophrenia (SCZ) compared to the control group (CTR) in each of the four studies included in the full analysis (but statistically significant only in Holt et al. 2009). Data presented in these graphs are not corrected for sex, age or study effects. As a preprocessing step, the Romaniuk et al. 2010 data were normalized to the maximum response and the scale is thus a magnitude smaller than in the other three studies.
Group differences in responses to the CS+ and CS−
The responses to the CS + were on average slightly lower numerically in the schizophrenia participants compared to the controls, but this difference was not statistically significant (b = −0.047, t143 = −0.966, p = 0.336), whereas the response to the CS− was significantly higher in the schizophrenia than in the control group (b = 0.087, t143 = 1.99, p = 0.048). Supplementary Table 2 provides effect sizes for the CS + and CS− for each study and the combined sample. The overall point estimate indicated that the increased response to the CS− (0.087) in the schizophrenia group accounted for 65% of the overall (0.134) decrease in the CS + vs. CS− contrast relative to the controls, while the non-significant decrease in response to the CS + accounted for the remainder of the between-group difference.
Correlations with clinical variables in the schizophrenia group
The PDI total score was positively correlated with the response to the CS− (b = 0.333, t50 = 2.860, p = 0.0062) (Fig. 3). However levels of negative symptoms were not correlated with lower response to the CS + , nor were any other PANSS subscale scores, or current antipsychotic dose, correlated with any of the fear conditioning measures (Supplementary Table 3).
This plot shows the association between skin conductance responses (SCRs) to the CS− stimulus and the Peters Delusion Inventory total score (PDI total score), adjusted for the number of items, in 56 participants with schizophrenia. The regression line (b = 0.333, t50 = 2.860, p = 0.0062) has been adjusted for age, sex and study.
Discussion
Summary of findings
Here we showed that acquisition of differential conditioned fear responses is impaired in schizophrenia, using a pooled sample derived from four prior independent studies. The estimated effect size of schizophrenia diagnosis on fear acquisition in this sample (Cohen’s d = 0.53) was moderate and comparable to the effect of schizophrenia on hippocampal (Cohen’s d = 0.46) [28] or lateral ventricle volumes (Cohen’s d = 0.37) [28]. Despite differences in the designs and experimental paradigms of each study, there was no evidence for heterogeneity of the effect. Our analyses also revealed that the smaller difference between the CS + and CS− was primarily due to higher responses to the CS− stimuli in the schizophrenia group, and that this response to the CS− was proportional to the severity of delusional ideation.
Abnormalities in Pavlovian fear conditioning in schizophrenia
Several hypothetical models have proposed that the salience of innocuous, neutral cues encountered in the environment is increased during the evolution of positive symptoms, and during this process greater attention is allocated to these benign stimuli [2, 3]. This attentional shift is in some cases followed by a misinterpretation (and in some instances, a delusional explanation) of the sensory percept, according to these models. The CS− stimulus presented during Pavlovian fear conditioning paradigms may represent such a neutral cue in an experimental context [6, 8]. Thus, our findings of an elevated response to the CS− in schizophrenia and a correlation between delusions and SCRs to the CS− are consistent with these models as well as several fMRI [29, 30] and behavioral [9, 31] studies which reported elevated responses to neutral stimuli in schizophrenia. However, additional studies are needed to confirm these findings and, if confirmed, identify the molecular mechanisms that may account for such aberrant responses to neural information.
Increased spontaneous dopamine transients has been hypothesized to underlie the increased salience of neutral cues in psychotic states [3, 6, 7]. However, in addition to dopamine, other neurotransmitters, such as glutamate [32, 33], acetylcholine [34, 35], and serotonin [36] also play a role in fear conditioning. Future studies which measure dopamine release or tone, or the role of other neurotransmitters in abnormalities in fear conditioning in schizophrenia, can potentially identify the molecular mechanisms that give rise to such changes in associative learning in psychosis.
Lack of group difference in response to the CS+
While the response to the CS + was on average numerically lower in the schizophrenia group in the current study, there were no statistically significant between-group differences in responses to the CS + . Yet, the point estimate of the increased response to the CS− did not entirely explain the group difference in the acquisition of conditioned fear; some residual deficit may be attributable to a decreased response to the CS + in the schizophrenia group. Studies using reinforcement learning paradigms have shown that while deficits in learning from rewards is impaired in schizophrenia, learning from aversive outcomes may be spared [37]. These prior results are consistent with our current findings of relatively intact responses to the CS + (a negative reinforcer).
Also, performance on some reinforcement learning paradigms that have been used in studies of schizophrenia are affected by deficits in higher cognitive functions such as working memory [38]. At least in healthy individuals, the acquisition of conditioned fear appears to be somewhat insensitive to cognitive load [39]. However, the relationship between fear conditioning deficits and cognitive impairments in schizophrenia has not been examined; future well-powered studies could investigate this question.
Implicated neural circuitry
Consistent with our findings, prior studies using fMRI have reported increased responses to neutral stimulus in the amygdala [29], ventral striatum [6], midbrain [8], and ventromedial prefrontal cortex [10, 40] in schizophrenia or clinical high-risk participants. In addition, an impaired ability to differentiate CS + events from CS− events in schizophrenia is consistent with studies showing that schizophrenia is associated with diminished volume of the amygdala [28] and the finding that the lateral amygdala, where the association between the CS + and US is encoded, may be specifically affected in schizophrenia [41, 42]. One potential explanation of the current study’s findings is that, because the ventromedial prefrontal cortex exerts top-down control over the amygdala [43], disruption of the connections between these two regions in schizophrenia could lead to diminished inhibition of fear and greater responses to the CS− [44,45,46].
Fear conditioning in schizophrenia vs. anxiety disorders
Individuals with anxiety disorders also tend to have slightly higher SCRs to CS− stimuli during fear acquisition [47]. Since responses to the CS + are also higher in anxiety disorders, this finding has been thought to reflect overgeneralization of conditioned fear responses to CS− events [47]. However, we have shown that conditioned fear responses are in fact undergeneralized in schizophrenia [13]. Overgeneralization is therefore unlikely to explain the elevated responses to the CS− in schizophrenia. In future work, identifying symptom-specific patterns in associative learning using standardized, relatively uniform experimental paradigms may facilitate the development of mechanism-based treatments for these specific symptom clusters.
Limitations
Several limitations of this pooled analysis include those of the original studies, such as the fact that the majority of the individuals with schizophrenia enrolled were receiving treatment with antipsychotic medication, which are typically dopamine D2 receptor antagonists; these agents may affect fear conditioning [48]. However, antipsychotic dose was not correlated with any of the skin conductance measures in the schizophrenia sample. Nevertheless, adequately powered future studies should investigate whether unmedicated individuals with schizophrenia display similar deficits in fear acquisition. Another limitation of this pooled analysis is that one recent Pavlovian fear conditioning study of 10 individuals with schizophrenia and 11 healthy controls was not included because the original data were no longer available [6]. However, the findings of this study were in line with the results reported here, i.e., the schizophrenia group showed a significantly smaller difference between SCRs to the CS + and CS−, due to both lower response to the CS + and higher response to the CS−.
In addition, the substantial overlap between the schizophrenia and control groups in the distribution of the key fear conditioning measures (i.e., there was heterogeneity within each sample) indicates that currently fear conditioning measures cannot be used for diagnostic purposes. This overlap is consistent with the interpretation that these findings are more closely linked to specific neurophysiological features and symptoms (i.e., delusions) associated with the disorder, rather than to schizophrenia itself. Finally, studies with null findings may not have been available for these analyses due to publication bias; thus the findings of this study require further replication by a single well-powered study.
Conclusions
In a pooled sample derived from four independent studies, we found that schizophrenia is associated with impaired acquisition of differential fear conditioning, due to an increased response to the neutral stimulus. Moreover, this elevated response to the neutral stimulus was proportional to the severity of delusional ideation. Fear conditioning is a simple experimental paradigm which could be used in future work to establish the mechanisms associated with psychotic symptoms and how they manifest during the early course of the illness.
References
Hall J, Romaniuk L, McIntosh AM, Steele JD, Johnstone EC, Lawrie SM. Associative learning and the genetics of schizophrenia. Trends Neurosci. 2009;32:359–65.
Maher BA. Delusional thinking and perceptual disorder. J Individ Psychol. 1974;30:98.
Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160:13–23.
Gold JM, Waltz JA, Prentice KJ, Morris SE, Heerey EA. Reward Processing in Schizophrenia: a Deficit in the Representation of Value. Schizophrenia Bull. 2008;34:835–47.
Lonsdorf TB, Menz MM, Andreatta M, Fullana MA, Golkar A, Haaker J, et al. Don’t fear ‘fear conditioning’: Methodological considerations for the design and analysis of studies on human fear acquisition, extinction, and return of fear. Neurosci Biobehav Rev. 2017;77:247–85.
Jensen J, Willeit M, Zipursky RB, Savina I, Smith AJ, Menon M, et al. The formation of abnormal associations in schizophrenia: neural and behavioral evidence. Neuropsychopharmacology. 2008;33:473–9.
Maia TV, Frank MJ. An integrative perspective on the role of dopamine in schizophrenia. Biol Psychiatry. 2017;81:52–66.
Romaniuk L, Honey GD, King JR, Whalley HC, McIntosh AM, Levita L, et al. Midbrain activation during Pavlovian conditioning and delusional symptoms in schizophrenia. Arch Gen Psychiatry. 2010;67:1246–54.
Roiser J, Stephan K, Den Ouden H, Barnes T, Friston K, Joyce E. Do patients with schizophrenia exhibit aberrant salience? Psychological Med. 2009;39:199–209.
Holt DJ, Coombs G, Zeidan MA, Goff DC, Milad MR. Failure of neural responses to safety cues in schizophrenia. Arch Gen Psychiatry. 2012;69:893–903.
Holt DJ, Lebron-Milad K, Milad MR, Rauch SL, Pitman RK, Orr SP, et al. Extinction memory is impaired in schizophrenia. Biol Psychiatry. 2009;65:455–63.
Hofer E, Doby D, Anderer P, Dantendorfer K. Impaired conditional discrimination learning in schizophrenia. Schizophrenia Res. 2001;51:127–36.
Tuominen L, DeCross SN, Boeke E, Cassidy CM, Freudenreich O, Shinn AK, et al. Neural abnormalities in fear generalization in schizophrenia and associations with negative symptoms. Biol Psychiatry: Cogn Neurosci Neuroimaging. 2021. https://doi.org/10.1016/j.bpsc.2021.01.006.
Peters H, Murphee O. The conditioned reflex in the chronic schizophrenic. J Clin Psychol. 1954;10:126–30.
Lynn R. Russian theory and research on schizophrenia. Psychological Bull. 1963;60:486.
Baer PE, Fuhrer MJ. Cognitive factors in differential conditioning of the GSR: use of a reaction time task as the UCS with normals and schizophrenics. J Abnorm Psychol. 1969;74:544.
O’Connor N, Rawnsley K. Two types of conditioning in psychotics and normals. J Abnorm Soc Psychol. 1959;58:157.
First MB, Gibbon M, Spitzer RL, Williams JBW. User’s guide for the structured clinical interview for DSM–IV Axis I disorders. New York: Biometrics Research (SCID-I, Version 2.0, October 1995 Final Version) 1995.
Peters ER, Joseph SA, Garety PA. Measurement of delusional ideation in the normal population: introducing the PDI (Peters et al. Delusions Inventory). Schizophr Bull. 1999;25:553–76.
Peters E, Joseph S, Day S, Garety P. Measuring Delusional Ideation: the 21-Item Peters et al. Delusions Inventory (PDI). Schizophrenia Bull. 2004;30:1005–22.
Preti A, Rocchi MB, Sisti D, Mura T, Manca S, Siddi S, et al. The psychometric discriminative properties of the Peters et al Delusions Inventory: a receiver operating characteristic curve analysis. Compr Psychiatry. 2007;48:62–9.
Kao Y-C, Wang T-S, Lu C-W, Cheng T-H, Liu Y-P. The psychometric properties of the Peters et al. Delusions Inventory (PDI) in Taiwan: reliability, validity, and utility. Soc Psychiatry Psychiatr Epidemiol. 2012;47:1221–34.
Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76.
Pernet CR, Wilcox RR, Rousselet GA. Robust correlation analyses: false positive and power validation using a new open source matlab toolbox. Front Psychol. 2013;3:606.
Pinheiro J, Bates D, DebRoy S, Sarkar D. R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. 2013. available at: https://ftp.uni-bayreuth.de/math/statlib/R/CRAN/doc/packages/nlme.pdf. Accessed 27 September 2021.
Maechler M, Rousseeuw P, Croux C, Todorov V, Ruckstuhl A, Salibian-Barrera M, et al. Package ‘robustbase’. Basic Robust Statistics. 2021. available at: http://robustbase.r-forge.r-project.org/. Accessed 27 September 2021.
Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48.
van Erp TG, Hibar DP, Rasmussen JM, Glahn DC, Pearlson GD, Andreassen OA, et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry. 2016;21:547–53.
Holt DJ, Kunkel L, Weiss AP, Goff DC, Wright CI, Shin LM, et al. Increased medial temporal lobe activation during the passive viewing of emotional and neutral facial expressions in schizophrenia. Schizophrenia Res. 2006;82:153–62.
Hall J, Whalley HC, McKirdy JW, Romaniuk L, McGonigle D, McIntosh AM, et al. Overactivation of fear systems to neutral faces in schizophrenia. Biol Psychiatry. 2008;64:70–3.
Holt DJ, Titone D, Long LS, Goff DC, Cather C, Rauch SL, et al. The misattribution of salience in delusional patients with schizophrenia. Schizophr Res. 2006;83:247–56.
Walker DL, Davis M. The role of amygdala glutamate receptors in fear learning, fear-potentiated startle, and extinction. Pharmacol Biochem Behav. 2002;71:379–92.
Batten SR, Pomerleau F, Quintero J, Gerhardt GA, Beckmann JS. The role of glutamate signaling in incentive salience: second‐by‐second glutamate recordings in awake Sprague‐Dawley rats. J Neurochemistry. 2018;145:276–86.
Acquas E, Wilson C, Fibiger HC. Conditioned and unconditioned stimuli increase frontal cortical and hippocampal acetylcholine release: effects of novelty, habituation, and fear. J Neurosci. 1996;16:3089–96.
Rajebhosale P, Ananth M, Crouse R, Jiang L, Hernández GL, Arty C, et al. Basal forebrain cholinergic neurons are part of the threat memory engram. BioRxiv. 2021:2021.05.02.442364.
Bauer EP. Serotonin in fear conditioning processes. Behavioural Brain Res. 2015;277:68–77.
Heinz A, Murray GK, Schlagenhauf F, Sterzer P, Grace AA, Waltz JA. Towards a unifying cognitive, neurophysiological, and computational neuroscience account of schizophrenia. Schizophrenia Bull. 2019;45:1092–100.
Collins AG, Brown JK, Gold JM, Waltz JA, Frank MJ. Working memory contributions to reinforcement learning impairments in schizophrenia. J Neurosci. 2014;34:13747–56.
Carter RM, Hofstötter C, Tsuchiya N, Koch C. Working memory and fear conditioning. Proc Natl Acad Sci. 2003;100:1399–404.
Quarmley M, Gur RC, Turetsky BI, Watters AJ, Bilker WB, Elliott MA, et al. Reduced safety processing during aversive social conditioning in psychosis and clinical risk. Neuropsychopharmacology. 2019;44:2247–53.
Armio R-L, Laurikainen H, Ilonen T, Walta M, Salokangas RKR, Koutsouleris N, et al. Amygdala subnucleus volumes in psychosis high-risk state and first-episode psychosis. Schizophrenia Res. 2020;215:284–92.
Barth C, Nerland S, de Lange A-MG, Wortinger LA, Hilland E, Andreassen O, et al. In vivo amygdala nuclei volumes in schizophrenia and bipolar disorders. Schizophrenia bulletin, 2021;47:1431–41.
Motzkin JC, Philippi CL, Wolf RC, Baskaya MK, Koenigs M. Ventromedial prefrontal cortex is critical for the regulation of amygdala activity in humans. Biol Psychiatry. 2015;77:276–84.
Anticevic A, Tang Y, Cho YT, Repovs G, Cole MW, Savic A, et al. Amygdala connectivity differs among chronic, early course, and individuals at risk for developing schizophrenia. Schizophrenia Bull. 2013;40:1105–16.
Tian L, Meng C, Yan H, Zhao Q, Liu Q, Yan J, et al. Convergent evidence from multimodal imaging reveals amygdala abnormalities in schizophrenic patients and their first-degree relatives. PloS ONE. 2011;6:e28794.
Hoptman MJ, D’Angelo D, Catalano D, Mauro CJ, Shehzad ZE, Kelly AC, et al. Amygdalofrontal functional disconnectivity and aggression in schizophrenia. Schizophrenia Bull. 2010;36:1020–8.
Duits P, Cath DC, Lissek S, Hox JJ, Hamm AO, Engelhard IM, et al. Updated meta‐analysis of classical fear conditioning in the anxiety disorders. Depression Anxiety. 2015;32:239–53.
Menon M, Jensen J, Vitcu I, Graff-Guerrero A, Crawley A, Smith MA, et al. Temporal difference modeling of the blood-oxygen level dependent response during aversive conditioning in humans: effects of dopaminergic modulation. Biol Psychiatry. 2007;62:765–72.
Funding
The collection of the original data was funded by the National Institute of Mental Health (K23 MH076054 (DJH), K24 MH002025 (DCG), R01 MH095904 (DJH)), EU_117 grant from the Translational Medicine Research Collaboration (JH), the National Alliance for Research on Depression and Schizophrenia with the Sidney R. Baer Jr Foundation (DJH). The funding agencies had no role in the design of the studies, in the data collection, analyses, interpretation, writing of the paper, nor in the decision to submit the paper for publication. All views expressed here are those of the authors.
Author information
Authors and Affiliations
Contributions
LR, MM, DG, JH, and DH designed, collected and analyzed the data for the original studies. LT analyzed the pooled data and wrote the first draft of the paper. LR, MM, DG, JH, and DH provided critically important scientific feedback during the analysis phase and on the first draft, all authors accepted the final paper and are in agreement to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Tuominen, L., Romaniuk, L., Milad, M.R. et al. Impairment in acquisition of conditioned fear in schizophrenia. Neuropsychopharmacol. 47, 681–686 (2022). https://doi.org/10.1038/s41386-021-01193-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-021-01193-1
This article is cited by
-
Changes in responses of the amygdala and hippocampus during fear conditioning are associated with persecutory beliefs
Scientific Reports (2024)
-
Data-driven, connectome-wide analysis identifies psychosis-specific brain correlates of fear and anxiety
Molecular Psychiatry (2024)
-
Threat Responses in Schizophrenia: A Negative Valence Systems Framework
Current Psychiatry Reports (2024)