Abstract
The persecutory delusion is the most common symptom of psychosis, yet its underlying neurobiological mechanisms are poorly understood. Prior studies have suggested that abnormalities in medial temporal lobe-dependent associative learning may contribute to this symptom. In the current study, this hypothesis was tested in a non-clinical sample of young adults without histories of psychiatric treatment (n = 64), who underwent classical Pavlovian fear conditioning while fMRI data were collected. During the fear conditioning procedure, participants viewed images of faces which were paired (the CS+) or not paired (the CS−) with an aversive stimulus (a mild electrical shock). Fear conditioning-related neural responses were measured in two medial temporal lobe regions, the amygdala and hippocampus, and in other closely connected brain regions of the salience and default networks. The participants without persecutory beliefs (n = 43) showed greater responses to the CS− compared to the CS+ in the right amygdala and hippocampus, while the participants with persecutory beliefs (n = 21) failed to exhibit this response. These between-group differences were not accounted for by symptoms of depression, anxiety or a psychosis risk syndrome. However, the severity of subclinical psychotic symptoms overall was correlated with the level of this aberrant response in the amygdala (p = .013) and hippocampus (p = .033). Thus, these findings provide evidence for a disruption of medial temporal lobe-dependent associative learning in young people with subclinical psychotic symptoms, specifically persecutory thinking.
Similar content being viewed by others
Prior neuroimaging studies of schizophrenia and psychosis risk syndromes have consistently detected anatomical and functional abnormalities of medial temporal lobe (MTL) areas such as the hippocampus and the amygdala1,2,3,4,5,6,7,8,9. Some studies have specifically linked MTL abnormalities in schizophrenia to the presence or severity of psychotic symptoms10,11,12,13,14,15, including persecutory delusions12,16,17. However, although findings of MTL abnormalities linked with psychosis have been well-replicated, it is unknown whether there are specific changes in cognitive or affective processes mediated by the MTL that contribute to the development of psychotic symptoms. It has been hypothesized that changes in the assignment of salience to incoming sensory stimuli by an MTL-striatal-midbrain circuit lead to abnormal perceptions and misinterpretations of those perceptions18,19,20, which then give rise to psychotic symptoms. The known central role of the MTL in associative learning and memory processes21 is consistent with this model, and prior studies have identified abnormalities in associative learning in individuals with schizophrenia22,23,24. However, it is unknown whether disruptions of associative learning processes mediated by the MTL are specifically linked to psychotic symptoms.
One form of associative learning that has been well-studied in animal models of psychiatric illness and in humans is Pavlovian aversive or threat conditioning, or what is commonly known as “fear” conditioning. This basic form of implicit learning is frequently measured in the laboratory using classical Pavlovian fear conditioning paradigms25,26,27,28. A recent meta-analysis that included 77 individuals with schizophrenia and 74 demographically-matched control subjects showed physiologic evidence for impaired fear conditioning in schizophrenia, particularly in those with active delusions29. Also, a prior study found some evidence for a role of the MTL in this abnormality, demonstrating hippocampal dysfunction during fear conditioning in schizophrenia30. However, the potentially confounding effect of treatment with antipsychotic medication in these prior studies limits their interpretability, particularly in light of evidence that D2 dopamine receptor blockade interferes with the acquisition or expression of conditioned fear responses in rodents31.
Therefore, to investigate the hypothesis that abnormalities in MTL-dependent associative learning are linked to psychotic symptoms, in the current study we employed a previously validated Pavlovian fear conditioning paradigm32,33 to measure MTL function during fear conditioning using fMRI. The participants of the study were young adults without a serious mental illness who endorsed varying levels of subclinical psychotic symptoms. Prior studies of fear conditioning conducted in healthy humans have shown that the human amygdala and hippocampus show conditioned fear responses26,34,35. Although the predicted pattern (based on rodent studies) of significantly higher responses to the CS+ compared to the CS− have been observed in the human amygdala34,35, more recent fMRI studies (including a meta-analysis) have also observed significantly larger responses to the CS− than to the CS+ in the amygdala and hippocampus of healthy human subjects36,37. This pattern of responses is consistent with the evidence that the amygdala and hippocampus are involved in both “threat” and “safety” -related learning38,39,40. Recent work has suggested that in a non-threatening context, safety signaling (i.e., CS− > CS+ responses) may dominate39, and a subset of safety-selective neurons in the amygdala may respond preferentially to the CS− during fear conditioning40,41. Given the prior evidence for abnormalities in fear and safety learning and memory processes in schizophrenia29,30,42, we hypothesized in the current study that such learning mediated by the MTL may be disrupted in individuals with subclinical psychotic symptoms.
Subclinical psychotic symptoms, often referred to as “psychotic experiences,” are common in the general population and are typically non-distressing and transient. Although the majority of individuals with psychotic experiences do not go on to develop frank psychosis, there is converging evidence, based on studies of epidemiologic and environmental risk factors for clinical psychosis43, as well as neuroimaging data44,45,46,47, for some degree of a continuum in the clinical and neurobiological expression of psychosis across different levels of severity in the general population, and for some biological mechanisms that are shared across psychotic experiences and clinical psychosis. One specific psychotic symptom that has been studied extensively across this continuum is persecutory beliefs, which can vary in severity from mild paranoia or persecutory thinking to full-blown persecutory delusions48. Since prior work has linked MTL dysfunction to the presence of persecutory beliefs or paranoia16,44, in the current study, we measured MTL responses during fear conditioning in non-help-seeking, young adults with persecutory beliefs. Based on this prior work, we predicted that we would observe evidence of abnormal MTL function during Pavlovian fear conditioning in those with persecutory beliefs, when compared to demographically-matched individuals without such beliefs.
Methods
Recruitment of participants
This study recruited and enrolled subjects via an ongoing study that focused on assessing various aspects of mental health in college students49,50 and the effectiveness of a behavioral intervention in this population51,52,53. In this parent study, we conducted in-person mental health screenings at three local universities over one or two days, in a high traffic area of the university. During the screening, study staff were available to consent participants and answer questions. Students who chose to participate in the overall study signed a consent form and completed a battery of self-report questionnaires.
For the current fMRI study, 72 non-help-seeking adults (65.6% female, age 18–25, mean age = 19.60) were identified as potentially eligible via these college campus screenings44,54 and then contacted and enrolled in the study. To recruit a mildly at-risk sample with a wide range of severity of psychotic experiences, as well as other types of psychopathology (e.g., depression), three groups of participants were enrolled: (1) those with an elevated score on the Peters Delusions Inventory (PDI,55; total PDI score > 7); (2) those with an elevated score on the Beck Depression Inventory (BDI,56; total BDI score > 5) but not an elevated PDI score (total PDI score < 8); and (3) those with low PDI and BDI scores (PDI < 8 and BDI < 6). These cut-offs of the PDI and BDI were chosen based on prior studies of these scales which indicated that these criteria identified approximately the top half of the distribution for depressive symptoms57, and subclinical psychosis58. Consistent with this, in our prior studies using these screening methods, these criteria typically identified approximately 20% (with elevated PDI scores) and 48% (with elevated BDI scores) of the distribution of the college students screened49,50. The goal of the study was to enroll young people with psychotic experiences and compare them to a control sample that also had a range of psychopathology, since psychotic experiences typically co-occur with other forms of psychopathology such as depression59,60. To test our specific hypothesis about persecutory beliefs, the enrolled cohort was divided into those who endorsed persecutory beliefs (one or both of the two persecutory items of the PDI) and those who did not.
Following quality control procedures, the data of 8 participants were excluded from the fMRI analyses due to excessive head motion during scanning (see criteria below). Thus, the data of 64 participants were included in the final analyses of this study (69% female, mean age = 19.7). All participants were proficient in English and had normal or corrected-to-normal vision based on Snellen test-based acuity61. At the time of enrollment, all subjects provided written informed consent. The study, including the experimental protocol, was approved by and conducted in accordance with the guidelines of the Partners Healthcare Institutional Review Board. The dataset analyzed is available from the authors by request.
Clinical measures
Self-report measures of psychotic experiences including persecutory thinking, depression, and anxiety were collected, using the 21-item version of the PDI55, the BDI56, and the Spielberger State and Trait Anxiety Inventory State scale—state subscale (STAI-S62), respectively. These questionnaires were administered on the same day that the fear conditioning procedure (with simultaneous fMRI data collection) was administered. In addition, the Structured Interview for Psychosis-Risk Syndromes (SIPS) interview was administered to all participants, to determine whether participants met criteria for a psychosis risk syndrome63.
The 21-item PDI is a widely used self-report questionnaire designed to assess delusional ideation, including persecutory beliefs, and other unusual experiences in the general population. The PDI has been validated for use in clinical and non-clinical groups64,65,66,67. A total score is calculated by summing the number of endorsed items. We measured persecutory beliefs using the previously identified “persecutory factor” of the PDI55,64,66, which includes two items: “Do you ever feel as if you are being persecuted in some way?” (item #4) and “Do you ever feel as if there is a conspiracy against you?” (item #5). The cohort of 64 subjects with usable fMRI data was divided into those who endorsed at least one of these two items (n = 21, the “Pers” group) and those who did not endorse either of these items (n = 43, the “NoPers” group). Within the Pers group, 4 endorsed both items; 8 endorsed only item #4, and 9 endorsed only item #5.
The BDI is a well-established 21-item self-report measure of depressive symptom severity over the past two weeks. Participants rate the degree to which they have experienced each symptom on a four-point scale from 0 to 3. The STAI-S contains 20 questions that measure the severity of anxiety symptoms that individuals are experiencing in the present on a Likert scale from 1 to 4.
The Structured Interview for Psychosis-Risk Syndromes (SIPS) and the Scale of Psychosis-Risk Symptoms (SOPS,63), administered by trained post-baccalaureate interviewers with direct supervision by a licensed psychiatrist, were used to rate the severity of symptoms of schizophrenia experienced by the participants. The SOPS consists of 19 items in 4 symptom domains: positive, negative, general, and disorganized. The five positive symptom items are: unusual thought content, suspiciousness, grandiosity, perceptual abnormalities, and disorganized communication, each rated on a score of 0 (none to minimal) to 6 (present and psychotic in intensity), with ratings of 3 to 5 representing attenuated psychosis. In this sample, 11 of the Pers group and 10 of the NoPers group met Attenuated Positive Symptom Syndrome (APSS) criteria based on the SIPS/SOPS. The Pers and NoPers groups showed no differences in gender frequencies and mean age (Table 1). There were expected differences between the two groups in levels of symptoms of depression and overall psychotic experiences, with significantly higher levels (all ps < 0.05) in the Pers group.
Fear conditioning paradigm
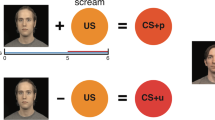
All subjects underwent a Pavlovian fear conditioning procedure while fMRI data and skin conductance responses (SCRs) were collected simultaneously. For each subject, one face pair of two possible face pairs was selected (counterbalanced across subjects). One face of the face pair was assigned to be the conditioned stimulus (CS+) and the other was assigned to be the neutral (CS−) stimulus. The selection of face pairs and assignment of the CS+ and CS− to the two faces of each pair were pseudo-randomized across the subjects.
During the fear conditioning paradigm, 13 CS+ trials and 13 CS− trials, each 2 s long, were presented in a pseudorandom order. The inter-trial intervals (ITI) were between 4 and 17 s in duration. The CS+ was immediately followed by a 500 ms long electrical shock (the unconditioned stimulus; US) that was administered to the shin of the left leg following 8 of the 13 CS+ trials. The intensity of the US ranged from 1.1 to 4 mA and was set individually to a level that was “highly annoying but not painful” to the participants prior to the procedure, as in previous studies33,42,68. To ensure that the participants were attending to the stimuli, a button-press task was performed during the fear conditioning procedure. During the procedure, 30% of the CS+ and CS− face stimuli appeared to briefly nod (for 500 ms) during the presentation. The participants were asked to press a button whenever they saw a nod. There were no significant differences between the Pers and NoPers groups in rates of detection of the nods (p = 0.86). See Fig. 1 for a schematic diagram of the paradigm and example stimuli. The fear conditioning phase was then followed by a fear generalization phase (findings for the fear generalization phase of this study will be reported separately).
Schematic diagram of the fear conditioning paradigm. (A) For each subject, one of two face-pairs was selected (pseudorandomly, counterbalanced across subjects). One face per pair was assigned to be the conditioned stimulus (CS+) and the other was assigned to be the neutral (CS−) stimulus. (B) During the fear conditioning paradigm, 13 CS+ trials and 13 CS− trials, each 2 s long, were presented in a pseudorandom order. The inter-trial intervals (ITI) were between 4 and 17 s long. Following 8 of the 13 CS+ trials, the unconditioned stimulus (US), a 500 ms electrical shock, was delivered to the shin of the left leg.
Skin conductance measurements
Skin conductance responses were measured using two MRI-compatible electrodes placed on the palm of each subject’s left hand at the beginning of the scan session. These data were recorded using a BIOPAC MP150 acquisition system (BIOPAC Systems, Inc, Goleta, CA) and an EDA100C MRI amplifier. Skin conductance was collected at a gain of 5 µS/V. During acquisition, data were low pass filtered at 1 Hz and digitized at 200 Hz.
MRI data acquisition
All MRI data were collected on a 3T Siemens Prisma scanner using a 64-channel head coil (Erlangen, Germany) at the Athinoula A. Martinos Center for Biomedical Imaging. T2*-weighted echo-planar images were collected during the fear conditioning and generalization paradigm (2 mm isotropic, matrix = 64 × 64, 45 slices, TR = 2000 ms, TE = 30 ms, flip angle = 90°). In addition, a T1-weighted 3D scan was collected using an MPRAGE sequence (spatial resolution 1 mm isotropic, matrix = 256 × 256, 176 slices, TR = 2530 ms, TE = 1.64, 3.5, 5.36, and 7.22 ms, flip angle = 7°).
Data analysis
Skin conductance analysis
Skin conductance data were analyzed using the ledalab V3.4.9 toolbox running on MATLAB R2016b (Mathworks; MA, USA). Data were downsampled into 10 Hz and manually cleaned for artifacts. A decomposition of the data into continuous signals of phasic and tonic activity was performed. A minimum amplitude threshold of 0.01 S, and maximum phasic values within response windows of 1–4 s after the events were extracted. To test for a fear conditioning response, the mean SCRs to the CS+ and CS− stimuli were compared using paired t-tests.
MRI data analyses
Preprocessing
Functional MRI data were preprocessed using the standard FSFAST processing pipeline in FreeSurfer version 6.0 (http://surfer.nmr.mgh.harvard.edu). Briefly, functional images were corrected for motion and slice timing and then spatially transformed into a common space (i.e., fsaverage on the cortical surface and MNI152 in the subcortical volume) and then spatially smoothed using a 3D Gaussian kernel (5 mm FWHM). A canonical hemodynamic response function was fitted to the CS+ and CS− events in each vertex and voxel. Temporal drift was accounted for by first- and second-degree polynomials in the first-level model. Motion parameters and timepoints with excess motion were used as regressors in the first level model.
Region-of-interest (ROI) analysis
The two primary ROIs, the amygdala and hippocampus, were defined based on anatomical criteria using each subject’s T1 anatomical scan, using the FreeSurfer segmentation algorithm69. In addition, six secondary ROIs were used in exploratory analyses of additional brain networks outside of the MTL known to play a role in fear conditioning. Five of these ROIs were defined using maps of fMRI responses to the CS+ v. CS− contrast generated using fMRI data collected in healthy control subjects in an independent fear conditioning study33. Three ROIs of the salience network (i.e., the anterior insula, dorsomedial prefrontal cortex (dmPFC), and thalamus) and two of the default mode network (i.e., precuneus and angular gyrus) were defined in this independent dataset. One additional ROI, the caudate nucleus, a key node of the salience network, was anatomically defined in each subject using the FreeSurfer automated segmentation procedure.
The ROIs defined in the previously collected fear conditioning data were constructed by identifying the border of each significant cluster of activation in that ROI (as identified by the FreeSurfer parcellation), at a threshold of p = 0.001, corrected for multiple comparisons using Monte-Carlo simulations with a voxel-wise p value threshold of 0.001, in the group template (fsaverage) space. Each of these template labels was then mapped onto each of the individual subjects of the current study (n = 64).
For the ROI analyses, average blood oxygenation level-dependent (BOLD) responses were extracted from each individual for the 8 ROIs (amygdala, hippocampus, anterior insula, dmPFC, thalamus, caudate, precuneus, and angular gyrus). For the primary analyses focused on the two MTL regions, the amygdala and hippocampus, repeated-measures ANOVAs were conducted for the left and right amygdala and hippocampus respectively, with condition (CS+ vs. CS−) as the within-group factor and group (Pers vs. NoPers) as the between-group factor. The ANOVAs were then repeated with depression, state anxiety scores, and APSS status added respectively as covariates. Similar analyses were conducted with the 6 additional, secondary ROIs.
Correlations
To determine whether there was a dimensional relationship between the severity of psychotic experiences and MTL responses during fear conditioning across the full cohort, correlations between PDI total score and the fear conditioning responses in the bilateral hippocampus and amygdala were explored using bisquare robust regression models.
Results
Skin conductance responses during fear conditioning
As expected, the full cohort, as well as both the Pers and NoPers groups separately, exhibited physiological evidence for differential fear conditioning, i.e., significantly greater skin conductance responses (SCRs) to the CS+ compared to the CS− (all ps < 0.05; Supplementary Fig. 1), with no significant difference in fear conditioning magnitude between the Pers and NoPers groups (t = − 1.422, p = 0.177).
Responses of the MTL during fear conditioning
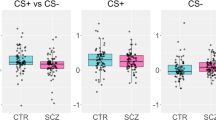
A repeated-measures ANOVA revealed a significant interaction between condition and group for the right amygdala (F[1, 60] = 12.890, p < 0.001), left hippocampus (F[1, 60] = 4.258, p = 0.043) and right hippocampus (F[1, 60] = 4.251, p = 0.043) but not the left amygdala (F[1, 60] = 2.332, p = 0.132; Fig. 2). The interaction between condition and group for the right amygdala and right hippocampus remained significant (p < 0.05) after controlling for potential confounding factors, such as anxiety, depression and APSS status; whereas the group by condition interaction for the left hippocampus was no longer significant after controlling for these potential confounds (Table 2).
Amygdala and hippocampal responses of the Pers and NoPers groups during fear conditioning. The average responses of the amygdala and hippocampus to the CS+ and CS− of the Pers and NoPers groups, relative to a baseline condition (responses to a uniformly gray screen displaying a central fixation cross), are plotted. The Pers and NoPers groups exhibited distinct response patterns of the right amygdala and hippocampus during fear conditioning, as reflected by significant group by condition interactions (right amygdala (B): p = .001; right hippocampus (D): p = .012, indicated by an asterisk *). These effects were not observed in the left hemisphere (left amygdala (A); left hippocampus (C); ps > .05 after controlling for psychiatric symptomatology). Error bars represent one standard error above and below the mean.
Follow-up t-tests revealed that the NoPers group (Fig. 2) exhibited the expected pattern of responses in the right amygdala (t = 4.416, p < 0.001, d = 0.535) and right hippocampus (t = 3.672, p = 0.001, d = 0.349), with significantly larger responses to the CS− compared to the CS+. In contrast, the Pers group failed to show this response in either the right amygdala or right hippocampus (Fig. 2; ps > 0.05). A between-group comparison of the responses of these two regions further confirmed this finding, showing a significantly greater CS− vs. CS+ response of the right amygdala (t = 3.566, p = 0.001, d = 0.328) and right hippocampus (t = 2.595, p = 0.012, d = 0.370) in the NoPers compared to the Pers group.
Secondary analyses
The analysis of the six additional ROIs revealed that the Pers group and the NoPers group (Supplementary Figs. 2 and 3) each exhibited differential fear conditioning-related responses in the bilateral anterior insula, dmPFC, caudate nucleus, and thalamus (with CS+ > CS− responses), as well as in the precuneus and angular gyrus (with CS− > CS+ responses) (all ps < 0.05; see Supplementary Table 1). Direct comparisons between the Pers and NoPers groups in the magnitude of fear conditioning-related responses in these regions showed that there were significant between-group differences in the left caudate nucleus and bilateral default mode network regions (i.e., angular gyrus and precuneus; ps < 0.05; see Supplementary Table 1), but not in the anterior insula, thalamus or dmPFC (all ps > 0.05; see Supplementary Table 1).
Symptom correlations
In the full cohort (n = 64), there was a significant negative correlation between the severity of psychotic experiences (total PDI score) and the magnitude of differential conditioning (CS− > CS+) responses of the bilateral amygdala (b = −0.026, t = 2.550, p = 0.013) and bilateral hippocampus (b = −0.013, t = 2.180, p = 0.033). There were no such correlations in the Pers and NoPers groups alone, with the separate responses of the right or left amygdala or hippocampus, or between levels of anxiety or depression and MTL fear conditioning-related responses in the full cohort or either of the two groups (all ps > 0.05).
Discussion
Summary of main findings
In this study, non-help-seeking young adults who endorsed persecutory beliefs showed abnormal neural responses during Pavlovian fear conditioning when compared to demographically matched adults without such beliefs. Specifically, the expected pattern (CS− > CS+) of learned responses36,37 was observed in the participants without persecutory beliefs in the right amygdala and hippocampus, but not in those with persecutory beliefs. In addition, the severity of psychotic experiences across the full sample correlated with the magnitude of this abnormality.
Fear conditioning responses in the human medial temporal lobe
We found that the participants without persecutory beliefs (the control group) showed greater responses to the CS− than the CS+ in the amygdala and hippocampus, which is consistent with the results of some but not all prior studies. In particular, prior fMRI studies of fear conditioning conducted in healthy humans have shown mixed findings with respect to the role of the amygdala, with early studies showing greater response to the CS+ than the CS−34,35, but later studies detecting only transient or inconsistent amygdala responses during fear conditioning36. A meta-analysis of fMRI studies of fear conditioning in humans (including 27 studies, N = 677) did not detect significant amygdala responses during fear conditioning but identified robust CS− > CS+ responses within the hippocampus and closely connected areas such as the posterior cingulate cortex36. A second meta-analysis found similar results, observing strong CS− > CS+ responses in both the amygdala and hippocampus during fear conditioning37. Moreover, another recent study aiming to resolve these questions about the role of the amygdala in human fear conditioning (N = 601) suggested that the responses of the amygdala during fear conditioning likely depends on temporal factors and varies across subnuclei of the amygdala, with late (versus early) conditioning in the basolateral nucleus of the amygdala favoring “safety” (CS− > CS+) over “threat” (CS+ > CS−) signaling70. Thus, the authors of this study concluded that, due to the functional heterogeneity of the amygdala, robust CS+ > CS− amygdala responses may only be detectable with large sample sizes70. Despite the variability of prior findings, the overall literature suggests that the amygdala and hippocampus are involved in both types of responses, i.e., threat and safety -related signaling39,40. In a non-threatening context, safety signaling may dominate39, and safety-selective neurons of the amygdala and hippocampus may respond preferentially to the CS− in Pavlovian fear conditioning paradigms38,39,41. Prior studies have also shown that default network regions show greater responses to the CS− compared to the CS+ 71, consistent with overall evidence that there is suppression of default network activity in the presence of behaviorally-salient stimuli in the environment72,73, including those associated with an electric shock (a CS+).
Impaired safety signaling in psychosis
Thus, taken together, one interpretation of the current results is that individuals with (or at risk for developing) persecutory beliefs are impaired in their capacity to generate one type of safety signal, which may render them vulnerable to worsening psychotic symptoms, particularly in certain environmental contexts associated with elevated levels of stress. This finding is generally consistent with prior evidence that another form of associative memory-based safety signaling, the recall of extinction memory traces, is impaired in individuals diagnosed with schizophrenia42, particularly those with delusions30. Thus, the function of a network of brain regions involved in both the encoding and retrieval of safety-related information may be altered in individuals who are vulnerable to or experiencing psychotic symptoms, such as persecutory delusions.
Abnormal responses of the default network
In addition to a reduction in the CS− > CS+ responses of the amygdala and hippocampus, the participants with persecutory beliefs also demonstrated a similar pattern of impaired CS− > CS+ responses of two default network areas, the precuneus and angular gyrus, compared to the control group. These findings are consistent with those of a prior fear conditioning fMRI study of a combined sample of individuals with attenuated psychotic symptoms and clinical psychosis, which detected lower CS− > CS+ responses of another default network region, the ventromedial prefrontal cortex (a region showing significant CS− > CS+ responses in the healthy control subjects), in the psychosis spectrum group compared to the control group74. This pattern of poor engagement of default network areas during the less behaviorally salient condition (the CS− in this case) may be related to observations of impaired task-induced suppression of default network areas reported previously in studies using cognitively demanding tasks (e.g., working memory paradigms) in schizophrenia72,73 and psychosis risk75. Thus, an alternative interpretation of the current findings is that attentional impairment, or some other cognitive deficit, may have led to reduced engagement of default network and medial temporal lobe areas during the non-threatening condition (the CS−). Although there were no differences between the groups in accuracy in the low-level attentional task that was performed during the fear conditioning paradigm (detecting brief head nods of the face stimuli), this task may not have been engaging enough to consistently capture the attention of participants throughout data collection.
Alternatively, given that the hippocampus and precuneus are well-established components of an extended episodic memory network, the CS− > CS+ response may reflect the encoding of an episodic memory trace related to contingency awareness (the CS—shock or no shock association), and this memory encoding may be disrupted in individuals with persecutory beliefs30,36. Future studies which measure default network and medial temporal lobe responses using both fear conditioning and other types of tasks can determine whether the changes observed in the current study represent a specific safety signaling deficit related to impaired associative learning or another type of cognitive impairment, a broader abnormality in default network and medial temporal lobe functioning, or both.
CS+ > CS− responses
In contrast to the pattern observed in the amygdala, hippocampus, and default network, the caudate nucleus showed significantly greater responses to the CS+ compared to the CS−, and the subjects with persecutory beliefs exhibited blunting of this response in the left caudate nucleus. This pattern is consistent with a finding of reduced differential conditioning (lower CS+ > CS−) in the physiological expression (skin conductance responses) of fear conditioning in a recent meta-analysis that compared 77 schizophrenia patients to 74 controls29. Given that a deficient skin conductance response during fear conditioning was not evident in the subjects with persecutory beliefs in the current study, we speculate that an abnormality in this peripheral measure of fear conditioning may only arise at a later stage of illness, or may be present in only those who will later develop psychotic illness.
However, this pattern of results is reminiscent of those of a number of prior studies of associative learning in psychotic disorders that found abnormalities in neural responses accompanied by intact behavioral responses indicating successful associative learning in the psychosis group24,76. Murray and colleagues found evidence for several possible explanations for this dissociation24, including that low-level engagement of the relevant neural circuitry, or compensatory recruitment of areas outside of the hypothesized brain network, may have been sufficient for normal task performance in these prior studies. Also, fMRI may be more sensitive to subtle differences in associative learning mechanisms than behavioral outcomes. In the current study, the absence of differences between the two groups in the responses during fear conditioning of the majority of the salience network regions, including the insula, dorsomedial prefrontal cortex and thalamus, is consistent with these explanations; autonomic responses during fear conditioning may be generated primarily by this network26, and may be relatively intact in non-help-seeking young people with persecutory beliefs.
The reduction in striatal responses in the group with persecutory beliefs during fear conditioning is consistent with a number of prior fMRI studies showing reduced striatal responsiveness in individuals with attenuated psychotic symptoms across a number of experimental paradigms77,78,79, suggesting that this finding may be task-independent, or that a fundamental associative learning deficit underlies the striatal dysfunction observed across these studies.
Relationship of these findings to animal models of psychosis
The findings of this study are in line with a well-known model of psychosis, based on pre-clinical studies conducted primarily in rodents, which posits that overactivity of the hippocampus, due to impaired functioning of hippocampal inhibitory interneurons, leads to disruption of the function of the striatum and closely connected regions of the basal ganglia and midbrain80. Findings of several imaging studies conducted in individuals with schizophrenia80 or with attenuated psychosis6 have provided support for this model. For example, overactivity of the hippocampus in individuals who are at risk for psychosis (who have attenuated psychotic symptoms) has been linked to overactivity of regions involved in dopamine signaling, including the midbrain and basal ganglia6,7,9. The current results suggest that psychotic symptoms, particularly persecutory beliefs, may arise from impairments in associative learning that are linked to dysfunction of this hippocampal-striatal circuitry.
Limitations
There are several limitations of this work that must be considered when interpreting these findings. First, the participants of this study did not undergo diagnostic evaluations for psychiatric disorders; thus we cannot exclude the possibility that individuals with diagnoses of psychotic disorders or other psychiatric illnesses were included in this sample. However, the fact that no participants received a score of 6 on any of the positive symptom items of the assessment of psychosis risk (indicating the presence of active clinical psychosis) confirms that no actively psychotic participants were included. Also, no participants were being treated with psychotropic medications, and all were enrolled in college as full-time students at the time of the study, suggesting that, if psychopathology was present, it was not severe or disabling.
In addition, in future studies, the inclusion of a clinical comparison group, i.e., those with schizophrenia or a related psychotic illness, would allow for a comparison of fear conditioning responses of those with mild persecutory beliefs with those with more severe and impairing persecutory beliefs that are held with conviction. We elected not to include such a group in this initial study because of the potentially confounding effects of treatment with antipsychotic medication and the effects on the brain of an ongoing disabling illness that can be present in a clinical psychosis sample. Future studies in larger samples can investigate the degree of overlap between medial temporal lobe-based associative memory abnormalities found in those with subclinical versus clinical levels of psychotic symptoms.
Additional future directions
In this study, deficient responses of regions of a medial temporal lobe-default-striatal network were observed during fear conditioning in a non-help-seeking sample of young adults who endorsed persecutory beliefs, when compared to a well-matched group of young adults without such beliefs. These findings could provide further justification for specific mechanistic studies of psychosis. For example, studies of a GAD65 gene knock-out mouse, which lacks the capacity for GAD65-mediated GABA synthesis, have shown that this alteration leads to abnormal fear conditioning-related responses81,82 and hyperactivity of the amygdala, hippocampus, and medial hypothalamus83. These findings suggest that deficits in GABAergic functioning could potentially account for the abnormalities observed in the current study. Future studies could directly test this model, by measuring levels of GABA and fear conditioning responses of the medial temporal lobe-default-striatal circuit in individuals with and without persecutory beliefs.
In addition, the specific pattern of disrupted fear and safety learning observed in this study could be tested as a potential marker of risk for clinical psychosis in longitudinal studies that measure the development of psychotic disorders in those at elevated risk for such disorders. A subset or combination of risk factors (e.g., attenuated psychotic symptoms/persecutory beliefs, polygenic risk score for psychosis or transdiagnostic conditions, environmental stressors) may be linked to such deficits in associative memory processes. The identification of a neural correlate of a psychotic symptom in an unmedicated, treatment-free sample, consistent with a neural systems model of psychosis, represents a rational candidate for further testing as a potential biomarker of psychosis risk.
Data availability
The datasets used in the analyses of this study are available from the corresponding author by request.
Change history
23 April 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41598-024-60109-3
References
Heckers, S. Neuroimaging studies of the hippocampus in schizophrenia. Hippocampus 11, 520–528 (2001).
Shenton, M. E., Dickey, C. C., Frumin, M. & McCarley, R. W. A review of MRI findings in schizophrenia. Schizophr. Res. 49, 1–52 (2001).
Holt, D. & Phillips, M. The human amygdala in schizophrenia. In The human amygdala (eds Phelps, E. A. & Whalen, P. J.) (Guilford Press, 2009).
Karlsgodt, K. H., Sun, D. & Cannon, T. D. Structural and functional brain abnormalities in schizophrenia. Curr. Dir. Psychol. Sci. 19, 226–231 (2010).
Ho, N. F. et al. Progression from selective to general involvement of hippocampal subfields in schizophrenia. Mol. Psychiatry 22, 142–152 (2017).
Allen, P. et al. Resting hyperperfusion of the hippocampus, midbrain, and basal ganglia in people at high risk for psychosis. Am. J. Psychiatry 173(4), 392–399 (2016).
Allen, P. et al. Increased resting hippocampal and basal ganglia perfusion in people at ultra high risk for psychosis: Replication in a second cohort. Schizophr. Bull. 44(6), 1323–1331 (2018).
Modinos, G. et al. Neural circuitry of novelty salience processing in psychosis risk: Association with clinical outcome. Schizophr. Bull. 46(3), 670–679 (2020).
Modinos, G. et al. Interactions between hippocampal activity and striatal dopamine in people at clinical high risk for psychosis: Relationship to adverse outcomes. Neuropsychopharmacology 46(8), 1468–1474 (2021).
Liddle, P. F. et al. Patterns of cerebral blood flow in schizophrenia. Br. J. Psychiatry 160, 179–186 (1992).
Schobel, S. A. et al. Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Arch. Gen. Psychiatry 66, 938–946 (2009).
Whalley, H. C. et al. Correlations between fMRI activation and individual psychotic symptoms in un-medicated subjects at high genetic risk of schizophrenia. BMC Psychiatry 7, 1–10 (2007).
Thoresen, C. et al. Frontotemporal hypoactivity during a reality monitoring paradigm is associated with delusions in patients with schizophrenia spectrum disorders. Cogn. Neuropsychiatry 19(2), 97–115 (2014).
Stegmayer, K. et al. Limbic interference during social action planning in schizophrenia. Schizophr. Bull. 44(2), 359–368 (2018).
Alho, J. et al. Hippocampus-centered network is associated with positive symptom alleviation in patients with first-episode psychosis. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 8(12), 1197–1206 (2023).
Pinkham, A. E. et al. Amygdala hyperactivity at rest in paranoid individuals with schizophrenia. Am. J. Psychiatry 172, 784–792 (2015).
Fan, L., Klein, H., Bass, E., Springfield, C. & Pinkham, A. Amygdala hyperconnectivity in the paranoid state: A transdiagnostic study. J. Psychiatr. Res 138, 117–124 (2021).
Grace, A. A. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat. Rev. Neurosci. 17, 524–532 (2016).
Kapur, S. Psychosis as a state of aberrant salience: A framework linking biology, phenomenology, and pharmacology in schizophrenia. Am. J. Psychiatry 160, 13–23 (2003).
Allen, P. et al. Abnormal relationship between medial temporal lobe and subcortical dopamine function in people with an ultra high risk for psychosis. Schizophr. Bull. 38, 1040–1049 (2012).
Suzuki, W. A. Associative learning signals in the brain. Prog. Brain Res. 169, 305–320 (2008).
Jensen, J. et al. The formation of abnormal associations in schizophrenia: Neural and behavioral evidence. Neuropsychopharmacology 33, 473–479 (2008).
Corlett, P. R. et al. Frontal responses during learning predict vulnerability to the psychotogenic effects of ketamine: Linking cognition, brain activity, and psychosis. Arch. Gen. Psychiatry 63, 611–621 (2006).
Murray, G. K., Corlett, P. R. & Fletcher, P. C. The neural underpinnings of associative learning in health and psychosis: How can performance be preserved when brain responses are abnormal?. Schizophr. Bull. 36, 465–471 (2010).
LeDoux, J. E. Emotion circuits in the brain. Annu. Rev. Neurosci. 23, 155–184 (2000).
Sehlmeyer, C. et al. Human fear conditioning and extinction in neuroimaging: a systematic review. PloS One 4(6), e5865 (2009).
Shechner, T., Hong, M., Britton, J. C., Pine, D. S. & Fox, N. A. Fear conditioning and extinction across development: evidence from human studies and animal models. Biol Psychol. 100, 1–12 (2014).
Delgado, M. R., Olsson, A. & Phelps, E. A. Extending animal models of fear conditioning to humans. Biol Psychol. 73, 39–48 (2006).
Tuominen, L. et al. Impairment in acquisition of conditioned fear in schizophrenia. Neuropsychopharmacology. 47, 681–686 (2022).
Holt, D. J., Coombs, G., Zeidan, M. A., Goff, D. C. & Milad, M. R. Failure of neural responses to safety cues in schizophrenia. Arch Gen Psychiatry. 69, 893–903 (2012).
Pina, M. M. & Cunningham, C. L. Effects of dopamine receptor antagonists on the acquisition of ethanol-induced conditioned place preference in mice. Psychopharmacology (Berl). 231, 459–468 (2014).
Holt, D. J. et al. A parametric study of fear generalization to faces and non-face objects: relationship to discrimination thresholds. Front Hum Neurosci. 8, 624 (2014).
Tuominen, L. et al. The relationship of perceptual discrimination to neural mechanisms of fear generalization. Neuroimage. 188, 445–455 (2019).
LaBar, K. S., Gatenby, J. C., Gore, J. C., LeDoux, J. E. & Phelps, E. A. Human amygdala activation during conditioned fear acquisition and extinction: A mixed-trial fMRI study. Neuron 20, 937–945 (1998).
Phelps, E. A., Delgado, M. R., Nearing, K. I. & LeDoux, J. E. Extinction learning in humans: Role of the amygdala and vmPFC. Neuron 43, 897–905 (2004).
Fullana, M. A. et al. Neural signatures of human fear conditioning: An updated and extended meta-analysis of fMRI studies. Mol. Psychiatry 21, 500–508 (2015).
Visser, R. M., Bathelt, J., Scholte, H. S. & Kindt, M. Robust BOLD responses to faces but not to conditioned threat: Challenging the amygdala’s reputation in human fear and extinction learning. J. Neurosci. 41, 10278–10292 (2021).
Meyer, H. C. et al. Ventral hippocampus interacts with prelimbic cortex during inhibition of threat response via learned safety in both mice and humans. Proc. Natl. Acad. Sci. 116, 26970–26979 (2019).
Rogan, M. T., Leon, K. S., Perez, D. L. & Kandel, E. R. Distinct neural signatures for safety and danger in the amygdala and striatum of the mouse. Neuron 46, 309–320 (2005).
Sangha, S., Chadick, J. Z. & Janak, P. H. Safety encoding in the basal amygdala. J. Neurosci. 33, 3744–3751 (2013).
Genud-Gabai, R., Klavir, O. & Paz, R. Safety signals in the primate amygdala. J. Neurosci. 33, 17986–17994 (2013).
Holt, D. J. et al. Extinction memory is impaired in schizophrenia. Biol. Psychiatry 65, 455–463 (2009).
Binbay, T. et al. Testing the psychosis continuum: Differential impact of genetic and nongenetic risk factors and comorbid psychopathology across the entire spectrum of psychosis. Schizophr. Bull. 38, 992–1002 (2012).
DeCross, S. N., Farabaugh, A. H., Holmes, A. J., Ward, M., Boeke, E. A., Wolthusen, R. P. F. et al. Increased amygdala-visual cortex connectivity in youth with persecutory ideation. Psychol. Med. 1–11 (2019).
Satterthwaite, T. D. et al. Structural brain abnormalities in youth with psychosis spectrum symptoms. JAMA Psychiatry 73(5), 515–524 (2016).
Modinos, G. et al. Prefrontal GABA levels, hippocampal resting perfusion and the risk of psychosis. Neuropsychopharmacology 43(13), 2652–2659 (2018).
Papanastasiou, E. et al. Examination of the neural basis of psychoticlike experiences in adolescence during reward processing. JAMA Psychiatry 75, 1043–1051 (2018).
Freeman, D. Persecutory delusions: A cognitive perspective on understanding and treatment. Lancet Psychiatry 3, 685–692 (2016).
Shapero, B. G. et al. Understanding the effects of emotional reactivity on depression and suicidal thoughts and behaviors: Moderating effects of childhood adversity and resilience. J. Affect. Disord. 245, 419–427 (2019).
Wright, A. C. et al. The impact of childhood trauma, hallucinations, and emotional reactivity on delusional ideation. Schizophr. Bull. Open 1(1), sgaa021 (2020).
Burke, A. S. et al. Rationale, methods, feasibility, and preliminary outcomes of a transdiagnostic prevention program for at-risk college students. Front. Psychiatry 10, 1030 (2020).
DeTore, N. R. et al. Efficacy of a transdiagnostic, prevention-focused program for at-risk young adults: A waitlist-controlled trial. Psychol. Med. 53, 3490–3499 (2023).
DeTore, N. R., Burke, A., Nyer, M. & Holt, D. J. A brief resilience-enhancing intervention and loneliness in at-risk young adults: A Secondary analysis of a randomized clinical trial. JAMA Netw. Open 7(2), e2354728 (2024).
Barbour, T. et al. Elevated amygdala activity in young adults with familial risk for depression: A potential marker of low resilience. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 5, 194–202 (2020).
Peters, E., Joseph, S., Day, S. & Garety, P. Measuring delusional ideation: The 21-item Peters et al. Delusions Inventory (PDI). Schizophr. Bull. 30, 1005–1022 (2004).
Beck, A. T. & Steer, R. A. Internal consistencies of the original and revised Beck Depression Inventory. J. Clin. Psychol. 40, 1365–1367 (1984).
Farabaugh, A. et al. Depression and suicidal ideation in college students. Psychopathology 45(4), 228–234 (2012).
Preti, A. et al. The psychometric discriminative properties of the Peters et al. Delusions Inventory: A receiver operating characteristic curve analysis. Compr. Psychiatry 48(1), 62–69 (2007).
Sullivan, S. A. et al. Longitudinal associations between adolescent psychotic experiences and depressive symptoms. PLoS One 9, e105758 (2014).
Häfner, H., Maurer, K., Trendler, G., an der Heiden, W. & Schmidt, M. The early course of schizophrenia and depression. Eur. Arch. Psychiatry Clin. Neurosci. 255, 167–173 (2005).
Ferris, F. L. 3rd., Kassoff, A., Bresnick, G. H. & Bailey, I. New visual acuity charts for clinical research. Am. J. Ophthalmol. 94, 91–96 (1982).
Spielberger, C. D. & Vagg, P. R. Psychometric properties of the STAI: A reply to Ramanaiah, Franzen, and Schill. J. Pers. Assess. 48, 95–97 (1984).
Miller, T. J. et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: Predictive validity, interrater reliability, and training to reliability. Schizophr. Bull. 29, 703–715 (2003).
Scott, J. et al. Psychopathology during childhood and adolescence predicts delusional-like experiences in adults: A 21-year birth cohort study. Am. J. Psychiatry 166, 567–574 (2009).
Verdoux, H. & van Os, J. Psychotic symptoms in non-clinical populations and the continuum of psychosis. Schizophr. Res. 54, 59–65 (2002).
Peters, E. R., Joseph, S. A. & Garety, P. A. Measurement of delusional ideation in the normal population: Introducing the PDI (Peters et al. Delusions Inventory). Schizophr. Bull. 25, 553–576 (1999).
Lincoln, T. M., Ziegler, M., Lüllmann, E., Müller, M. J. & Rief, W. Can delusions be self-assessed? Concordance between self-and observer-rated delusions in schizophrenia. Psychiatry Res. 178, 249–254 (2010).
Holt, D. J., Coombs, G., Zeidan, M. A., Goff, D. C. & Milad, M. R. Failure of neural responses to safety cues in schizophrenia. Arch. Gen. Psychiatry 69(9), 893–903 (2012).
Fischl, B. et al. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron 33, 341–355 (2002).
Wen, Z. et al. Temporally and anatomically specific contributions of the human amygdala to threat and safety learning. Proc. Natl. Acad. Sci. U. S. A. 119, e2204066119 (2022).
Marstaller, L., Burianova, H. & Reutens, D. C. Adaptive contextualization: A new role for the default mode network in affective learning. Hum. Brain Mapp. 38, 1082–1091 (2017).
Whitfield-Gabrieli, S. & Ford, J. M. Default mode network activity and connectivity in psychopathology. Annu. Rev. Clin. Psychol. 8, 49–76 (2012).
Buckner, R. L. The brain’s default network: Origins and implications for the study of psychosis. Dialogues Clin. Neurosci. 15, 351–358 (2013).
Quarmley, M. et al. Reduced safety processing during aversive social conditioning in psychosis and clinical risk. Neuropsychopharmacology 44(13), 2247–2253 (2019).
Falkenberg, I. et al. Failure to deactivate medial prefrontal cortex in people at high risk for psychosis. Eur. Psychiatry 30(5), 633–640 (2015).
Corlett, P. R. et al. Disrupted prediction-error signal in psychosis: Evidence for an associative account of delusions. Brain 130(9), 2387–2400 (2007).
Schmidt, A. et al. Longitudinal alterations in motivational salience processing in ultra-high-risk subjects for psychosis. Psychol. Med. 47(2), 243–254 (2017).
Karcher, N. R., Hua, J. P. & Kerns, J. G. Probabilistic category learning and striatal functional activation in psychosis risk. Schizophr. Bull. 45(2), 396–404 (2019).
Davies, C. et al. A single dose of cannabidiol modulates medial temporal and striatal function during fear processing in people at clinical high risk for psychosis. Transl. Psychiatry 10(1), 311 (2020).
Sonnenschein, S. F., Gomes, F. V. & Grace, A. A. Dysregulation of midbrain dopamine system and the pathophysiology of schizophrenia. Front. Psychiatry 11, 613 (2020).
Stork, O., Yamanaka, H., Stork, S., Kume, N. & Obata, K. Altered conditioned fear behavior in glutamate decarboxylase 65 null mutant mice. Genes Brain Behav. 2, 65–70 (2003).
Bergado-Acosta, J. R., Muller, I., Richter-Levin, G. & Stork, O. The GABA-synthetic enzyme GAD65 controls circadian activation of conditioned fear pathways. Behav Brain Res. 260, 92–100 (2014).
Muller, I., Caliskan, G. & Stork, O. The GAD65 knock out mouse—a model for GABAergic processes in fear- and stress-induced psychopathology. Genes Brain Behav. 14, 37–45 (2015).
Funding
This study was supported by the National Institute of Mental Health (R01MH095904, DJH).
Author information
Authors and Affiliations
Contributions
D.H. and L.T. designed the study; L.L. and W.D. collected the data; W.D. and L.T. analyzed the data; W.D. and D.H. wrote the main manuscript text; R.S. and L.V. prepared the figures; all authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of this Article contained errors. Full information regarding the corrections made can be found in the correction for this Article.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Deng, W., Tuominen, L., Sussman, R. et al. Changes in responses of the amygdala and hippocampus during fear conditioning are associated with persecutory beliefs. Sci Rep 14, 8173 (2024). https://doi.org/10.1038/s41598-024-57746-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-57746-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.