Abstract
Anxiety disorders are the most common mental disorders in adolescents. However, only 50% of pediatric patients with anxiety disorders respond to the first-line pharmacologic treatments—selective serotonin reuptake inhibitors (SSRIs). Thus, identifying the neurofunctional targets of SSRIs and finding pretreatment or early-treatment neurofunctional markers of SSRI treatment response in this population is clinically important. We acquired pretreatment and early-treatment (2 weeks into treatment) functional magnetic resonance imaging during a continuous processing task with emotional and neutral distractors in adolescents with generalized anxiety disorder (GAD, N = 36) randomized to 8 weeks of double-blind escitalopram or placebo. Generalized psychophysiological interaction analysis was conducted to examine the functional connectivity of the amygdala while patients viewed emotional pictures. Full-factorial analysis was used to investigate the treatment effect of escitalopram on amygdala connectivity. Correlation analyses were performed to explore whether pretreatment and early (week 2) treatment-related connectivity were associated with treatment response (improvement in anxiety) at week 8. Compared to placebo, escitalopram enhanced emotional processing speed and enhanced negative right amygdala-bilateral ventromedial prefrontal cortex (vmPFC) and positive left amygdala-right angular gyrus connectivity during emotion processing. Baseline amygdala-vmPFC connectivity and escitalopram-induced increased amygdala-angular gyrus connectivity at week 2 predicted the magnitude of subsequent improvement in anxiety symptoms. These findings suggest that amygdala connectivity to hubs of the default mode network represents a target of acute SSRI treatment. Furthermore, pretreatment and early-treatment amygdala connectivity could serve as biomarkers of SSRI treatment response in adolescents with GAD. The trial registration for the study is ClinicalTrials.gov Identifier: NCT02818751.
Similar content being viewed by others
Introduction
Generalized anxiety disorder (GAD) frequently emerges in adolescence and is among the most common psychiatric disorders in adolescents [1]. However, first-line pharmacologic treatments for pediatric anxiety disorders—selective serotonin reuptake inhibitors (SSRIs)—produce remission in only 50% of youth [2, 3]. In addition, it usually takes 6–8 weeks to evaluate the efficacy of an SSRI in an individual patient [2, 4, 5]. Thus, identifying the neurofunctional targets of SSRIs in adolescents with GAD might facilitate the development of new medications. Finding pretreatment or early-treatment neurofunctional markers of SSRI response could reduce the time that a patient is treated with an ineffective SSRI and hasten a switch to an alternative treatment.
GAD is associated with abnormal emotion processing; patients with GAD are hypervigilant to threat, unable to regulate threat responses, and consequently show exaggerated reactions to threat [6]. Functional neuroimaging studies examining emotion processing in adults with anxiety disorders consistently demonstrate hyperactivation of amygdala and ventral prefrontal cortex (PFC) in response to negative emotional stimuli [7, 8]. The amygdala findings are particularly of interest due to the importance of this structure in anxiety, anxiety disorders, and in preclinical models of anxiety [9, 10]. To date, only a handful of (n = 6) cross-sectional studies have investigated the brain activation and connectivity to emotional faces (e.g., angry or fearful) during attentional tasks in children and adolescents with anxiety disorders. These studies reveal increased amygdala and ventrolateral PFC (vlPFC) activation [11, 12], reduced ventromedial PFC (vmPFC) and anterior cingulate cortex (ACC) activation [13, 14], and weaker amygdala-vlPFC negative connectivity [15]. However, attentional tasks frequently do not segregate emotional and attentional functions. In addition, emotional processing abnormalities in patients with anxiety disorders, especially those with GAD, are not limited to facial expressions, but relate to daily life, performance, perceived self-competence (e.g., being good enough), and safety (e.g., car accidents, physical illness) [16]. With these considerations in mind, we employed a continuous performance task with emotional and neutral distractors (CPT-END)—not faces—to probe emotional processing in adolescents with GAD. This task enabled a dissociation of attentional and emotional operations into their constituent brain networks. With this task, our previous study (non-overlapping sample) revealed increased activation during emotional processing in both vmPFC and vlPFC in adolescents with GAD compared to healthy youths [17]. In addition, we observed altered functional coupling between the above regions and hubs of the default mode network (DMN) including increased amygdala-posterior cingulate cortex and decreased amygdala-precuneus and vlPFC-vmPFC connectivity [17]. These prior findings are consistent with resting-state functional connectivity (FC) studies that also reveal aberrant amygdala-DMN connectivity in adolescents with GAD [18, 19]. Collectively, these findings suggest that, in addition to altered functional activation and connectivity in amygdala-PFC circuitry, altered connectivity between this circuit and DMN may better characterize the emotion-related functional alterations in pediatric patients with GAD.
However, the clinical utility of these alterations for tracking and predicting treatment response remains unexplored in patients with anxiety disorders. Such studies can clarify how effective treatment impacts GAD-related neurofunctional alterations and may serve as biomarkers for translational drug development. To date, only one open-label study, using vlPFC as the region of interest (ROI), reported increased vlPFC activation to angry faces following 8 weeks of fluoxetine in adolescents with GAD (n = 7) compared to healthy youth [20]. This finding suggests that SSRIs normalize GAD-related regional brain alterations. However, larger samples and double-blind, placebo-controlled trials—which can avoid nonspecific placebo effects—are needed to validate this [3, 21, 22]. In addition, brain regions do not work independently; instead, they cooperate with each other to accomplish various neurocognitive functions including emotion processing [23]. The mechanism of how SSRIs change FC between GAD-related brain regions remains unknown.
Moreover, it is important to know whether functional activation and connectivity before treatment or their changes early in the course of treatment could serve as biomarkers for identifying patients who are more or less likely to be treatment responders. In this regard, one recent study reported that lower pretreatment ACC and PFC activation in response to fearful faces predicted SSRI response in children and adolescents with generalized and/or social anxiety disorder [24]. SSRI-related functional alterations that occur early in the course of treatment might provide additional information compared to pretreatment neurofunctional fingerprints [25], yet they have not been examined as predictors of SSRI treatment outcome.
With these considerations in mind, we recruited adolescents with GAD, randomized them to escitalopram and placebo, performed functional neuroimaging before and 2 weeks after beginning escitalopram or placebo (forced titration to 15 mg), and evaluated the change in anxiety severity over 6 subsequent weeks (total treatment duration 8 weeks) [26]. The aims of this study were to examine the impact of acute SSRI treatment on amygdala connectivity and activation during emotional processing using the CPT-END, and to investigate whether pretreatment and early-treatment-related amygdala connectivity and activation are associated with treatment response in adolescents with GAD. While our primary focus is on the emotion condition, this task (CPT-END) allows secondary analysis of other neurocognitive processes. Based on prior work in adolescents with anxiety disorders [17, 20, 27,28,29], we hypothesized that escitalopram—compared with placebo—would enhance amygdala connectivity with DMN regions and altered their regional activation, and that SSRI-related connectivity and activation prior to treatment (baseline) and early in the course of treatment (week 2) would predict endpoint treatment response in escitalopram-treated patients.
Methods
Patients
This randomized clinical trial (ClinicalTrials.gov Identifier: NCT02818751) was approved by the Institutional Review Board of the University of Cincinnati and conducted in accordance with Good Clinical Practice guidelines. Outpatients aged 12–17 years with GAD (DSM-IV-TR criteria, assessed using the Anxiety Disorders Interview Schedule) and a Pediatric Anxiety Rating Scale (PARS) score ≥15 [30], and a Clinical Global Impression score ≥4 at screening and baseline visits were eligible [31]. Additional inclusion and exclusion criteria have been previously described [26].
Fifty-one adolescents with GAD were randomized to escitalopram or placebo (1:1), and 36 patients completed both the baseline and week 2 magnetic resonance (MR) scans and were included in the current study (Supplementary Fig. 1). Patients who received escitalopram (n = 19) and those who received placebo (n = 17) did not differ statistically in age (mean age: 14.5 ± 1.6 years for escitalopram group, and 15.2 ± 1.5 years for placebo group, p = 0.18), gender (4 males in escitalopram group, 6 in placebo group, p = 0.46), or baseline anxiety severity (PARS score 18 for escitalopram and placebo groups, p = 0.77). Informed consent was obtained from all adolescents with GAD and their guardians.
Treatment and assessments
Patients were randomized to 8 weeks of double-blind escitalopram or placebo (1:1), which was delivered in identically appearing capsules. Treatment was assigned by investigational pharmacists using a random number generator and randomization was stratified by sex. Patients, caregivers, and investigational staff were blind to treatment assignment. Escitalopram was initiated at 5 mg daily for 2 days, then 10 mg daily for 7 days and then 15 mg daily. At the week 4 and 6 visits, escitalopram could be titrated to 20 mg daily.
The PARS was used to measure anxiety symptom severity and was administered at each study visit (week 0, 1, 2, 4, 6, and 8) to assess the trajectory of symptom change. The risks of randomization to placebo were carefully considered. Patients were carefully monitored at study visits by a board-certified child and adolescent psychiatrist and any patient with a significant increase in PARS score at 2 consecutive visits or who developed of a mood disorder or significant development/worsening of suicidality was discontinued. In addition, an external data safety monitoring board oversaw the conduct of the study. For patients who discontinued treatment prior to week 8 (endpoint), a last observation carried forward approach was utilized for missing PARS scores (three participants in each group discontinued). The anxiety severity was significantly decreased in escitalopram-treated patients at week 2 (PARSbaseline =17 ± 2, PARSweek 2 = 12 ± 4, paired-t = 5.788, p < 0.001), while it remained unchanged in patients who received placebo (PARSbaseline =17 ± 3, PARSweek 2 = 16 ± 3, paired-t = 1.89, p = 0.077).
fMRI task
Participants performed the CPT-END in the scanner [32], during which they pressed button 1 for emotional, neutral, and square pictures and pressed button 2 for circle pictures. This rapid, fixed event-related task consisted of two runs, each run containing four conditions and a total of 158 pictures (squares 70%, circles 10%, emotional pictures 10%, neutral pictures 10%). Each picture was presented for 2750 ms, following which a fixation cross presented for 250 ms (Fig. 1).
MR image acquisition and preprocessing
MR images were acquired on a 3-Tesla scanner (Achieva; Philips, USA) with a 32-channel phased-array head coil at baseline and 2 weeks after beginning treatment. Scanner noise was attenuated with earplugs and headphones; head motion was restricted with foam padding. Functional images were obtained using a single shot, fast Fourier echo, echo planar sequence with the following parameters: repetition time (TR) = 2000 ms; echo time (TE) = 30 ms; number of axial slices = 40; in plane resolution = 2.7875 mm × 2.7875 mm; slice thickness = 3 mm; slice gap = 0 mm; flip angle = 75°; matrix = 80 × 80; field of view = 223 mm × 223 mm, duration of each run = 8 min 38 s. High-resolution anatomical images were obtained using a three-dimensional T1-weighted Turbo field echo sequence with the following parameters: TR = 6.8 ms; TE = 2.9 ms; number of sagittal slices = 160; resolution = 1 mm × 1 mm; slice thickness = 1 mm; slice gap = 0 mm; flip angle = 9°; matrix = 256 × 256; field of view = 256 mm × 256 mm. Images were reviewed by a pediatric neuroradiologist to exclude cases with gross structural abnormalities or image artifacts.
Standard preprocessing procedures within the CONN toolbox (v18.b) were used: realignment, slice-timing, co-registration to individual structural T1 images, segmentation, normalization to Montreal Neurological Institute space, resampling at 2 × 2 × 2 mm3, spatial smoothing (8 mm full width at the half maximum Gaussian kernel), and temporal band-pass filtering (0.008–0.09 Hz) [33]. Five orthogonal time-series from white matter and cerebrospinal fluid, 12 head motion parameters from realignment, and outlier volumes from scrubbing (the difference in framewise displacement between two consecutive volumes exceeded 1 mm) were included as covariates to reduce physiological and movement confounds. A priori, we planned to exclude patients with mean motion >0.5 mm or outlier volumes >15% (76 volumes) but no patient was excluded according to these criteria. In addition, the mean head motion did not differ between escitalopram (baseline = 0.24 mm, week 2 = 0.28 mm) and placebo (baseline = 0.19 mm, week 2 = 0.23 mm) groups at baseline (t = 0.70, p = 0.49) or week 2 (t = 0.62, p = 0.54). The outlier volumes did not differ between escitalopram (baseline = 31, week 2 = 37) and placebo (baseline = 16, week 2 = 20) groups at baseline (t = 0.95, p = 0.35) or week 2 (t = 0.99, p = 0.33) either. We also calculated the percentage of amygdala-based connections that are significantly related to head motion at the voxel level, and plotted the relationship between connectivity strength and Euclidean distance. The results of these analyses clearly support our results not being driven by motion-related artifact (Supplementary Figs. 3 and 4).
Amygdala-based whole-brain functional connectivity during emotion processing
To assess amygdala-based whole-brain FC during emotional processing, a generalized psychophysiological interaction analysis (PPI) was performed using the preprocessed images described above [34]. Generalized PPI allowed us to study how brain regions interact in a task-dependent manner, which is often used in fast event-related task designs. Specifically, it convolves the task regressor (generated with specific onset time of each trial) with the hemodynamic response functions, and computes the interaction regressor between the resulting task regressor and the time-series of a seed region (left and right amygdala separately as defined in the Harvard–Oxford cortical atlas). Then bivariate regression analysis was used to examine the relationship between interaction regressor and the time-series of every other voxel in the brain (seed-to-voxel connectivity analyses). In this first-level analysis, the whole-brain FC (β weights) of left and right amygdala for each condition was determined for each patient. Amygdala-based FC images during viewing of emotional pictures, our primary condition of interest, were then passed to full-factorial analysis to examine the treatment-by-time interaction. Connectivity images were dependent variables, treatment (escitalopram vs. placebo) and time (baseline vs. week 2) were independent variables, and age and sex were covariates. Family-wise error (FWE) was applied to correct for multiple comparisons, with thresholds of p < 0.005 at the voxel level to preserve p < 0.05 at the cluster level. The FC value (β weights) was extracted from clusters with significant treatment-by-time interactions to explore their relationship with treatment response.
To further understand the effects of SSRIs on emotion processing, we explored changes in functional activation in regions wherein we observed treatment-related effects on FC. Whole-brain functional activation analysis in response to emotional pictures was also conducted for compensatory. The functional activation (analyzed with SPM 12 as shown in the Supplementary information), while viewing emotional pictures, in bilateral amygdala and its coupling regions were extracted. Two-sample t-tests were used to compare activity changes of bilateral amygdala and its coupling regions between patients who received escitalopram and those who received placebo.
To identify the effects of escitalopram on behavioral performance, reaction times to each stimuli type were analyzed using repeated-measures ANOVA in SPSS 22, with treatment (escitalopram vs. placebo) as the between-group variable and time (baseline vs. week 2) as the within-group variable, p < 0.05 was considered statistically significant.
We finally examined the potential value of pretreatment and early-treatment (week 2) amygdala connectivity, activation, and behavior performance during emotion processing in predicting treatment response (reduction in symptom severity) at endpoint with correlation analyses.
Results
Escitalopram effect on behavioral performance
Repeated-measures ANOVA for reaction times showed no group-by-time interaction or main effect of time. However, following 2 weeks of treatment, escitalopram-treated patients had significantly faster reaction times relative to baseline for all stimulus types. No changes in reaction times were observed in patients who received placebo (Table 1 and Supplementary Fig. 2). At baseline, reaction times to all stimulus types did not differ between escitalopram and placebo groups and were not related to response. In addition, the change in reaction time from baseline to week 2 was not significantly associated with the change in anxiety severity at week 2 or endpoint in escitalopram-treated patients.
Escitalopram effects on amygdala-based functional connectivity during emotion processing
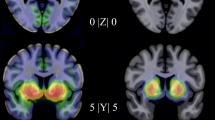
Controlling for age and sex, escitalopram—relative to placebo—decreased connectivity between the right amygdala and a region that included bilateral vmPFC and subgenual ACC during emotion processing from baseline to week 2 (cluster size = 418 voxel, MNI coordinate: x = –2, y = 38, z = 0; F = 13.13, p = 0.023 FWE-corrected) (Fig. 2). In addition, greater negative amygdala-vmPFC connectivity at baseline predicted more improvement in anxiety at endpoint in patients who received escitalopram (r = –0.46, p = 0.047), but not placebo (r = 0.19, p = 0.468).
In addition, escitalopram—relative to placebo—increased connectivity between left amygdala and right angular gyrus from baseline to week 2 during emotional processing (cluster size = 428 voxel, MNI coordinate: x = 46, y = –58, z = 46; F = 16.18, p = 0.018 FWE-corrected) (Fig. 2). Importantly, greater amygdala-angular gyrus connectivity at week 2 predicted greater improvement in anxiety at week 8 (r = 0.55, p = 0.015).
Escitalopram effect on functional activation during emotion processing
At the whole-brain level, escitalopram was not associated with changes in functional activation. To further explore brain activation alterations underlying the escitalopram-related connectivity changes described above, we extracted the average functional activation value from bilateral amygdala, right angular gyrus, and vmPFC during the viewing of emotional pictures. Left amygdala activation significantly decreased in patients who received escitalopram relative to those who received placebo, but right amygdala activation remained unchanged (left: t = –2.08, p = 0.045; right: t = –0.20, p = 0.843). While connectivity of amygdala-vmPFC and amygdala-angular gyrus changed significantly following escitalopram, the local activation in right angular gyrus (t = 0.82, p = 0.420) and bilateral vmPFC (t = 0.33, p = 0.743) did not change with treatment. These regional activation changes did not correlate with improvement in anxiety. In addition, these regional activation changes did not significantly associate with FC changes. Moreover, we explored the escitalopram-associated changes on circle and neutral conditions respectively and during attentional processing (circles vs. squares), and found no significant differences in activation or connectivity between the two groups.
Discussion
This is the first double-blind, placebo-controlled evaluation of an SSRI on FC and behavioral performance during emotional processing in adolescents. Within 2 weeks of beginning treatment, escitalopram improved task performance (decreased the reaction time) in adolescents with GAD, while placebo did not. Beyond this, escitalopram, compared to placebo, altered FC between amygdala and two critical nodes of DMN (vmPFC and angular gyrus) in response to emotional pictures. Specifically, escitalopram increased the connectivity between left amygdala and right angular gyrus, and the magnitude of this amygdala-angular gyrus FC after 2 weeks of treatment predicted the degree of improvement in anxiety at the end of the study. In addition, escitalopram decreased the connectivity between right amygdala and bilateral vmPFC. The pretreatment amygdala-vmPFC connectivity also predicted treatment response. Exploratory analysis revealed that escitalopram, compared to placebo, decreased the left amygdala activation during the presentation of emotional pictures, but no changes in right amygdala, bilateral vmPFC, or right angular gyrus activation were observed. The laterality of the activation results may reflect that the left amygdala is frequently activated by emotional stimuli more so than is the right amygdala [35], potentially providing greater sensitivity for detecting SSRI-related treatment changes. Consistent with our hypothesis, these results suggest that, compared to placebo, escitalopram alters the amygdala-DMN connectivity and dampens amygdala activation during emotion processing within the first 2 weeks of treatment. Our findings also raise the possibility that pretreatment- and early-treatment-associated amygdala connectivity could predict treatment response. Such neurofunctional predictors that present at baseline or emerge early in the course of treatment could allow clinicians to select alternative treatments for adolescents with GAD who are less likely to respond to SSRIs.
In the current study, escitalopram—relative to placebo—significantly decreased amygdala-vmPFC/ACC connectivity resulting in a greater negative coupling between these two regions. ACC and vmPFC have a regulatory role with regard to amygdala, which control the amygdala to generate exaggerated emotion response [36, 37]. This automatic emotion regulation may result from their inhibitory connections with the amygdala [38]. Deficient structural and functional amygdala-PFC connectivity at rest and during emotion processing/regulation have been previously described in anxiety disorders [11, 18, 39], and predicted trait anxiety in healthy young adults [40]. Our findings extend the results of these previous studies and demonstrate that escitalopram, like other SSRIs [41,42,43], may exert its effect by increasing this weaker amygdala-PFC FC. The increased amygdala-PFC connectivity enhances the PFC-driven emotion regulation of the amygdala and attenuates its exaggerated activation to negative emotions. This putative mechanism is supported by our exploratory finding of decreased amygdala activity after 2 weeks of escitalopram treatment. Importantly, we also found that greater negative amygdala-vmPFC connectivity at baseline predicted greater improvement in anxiety at endpoint in escitalopram-treated patients (but not in those who received placebo). Collectively, these findings indicate amygdala-vmPFC connectivity as an important target for SSRIs and raise the possibility that it could guide clinicians to tailor or select from evidence-based treatments and serve as a biomarker of treatment response. Future studies are needed to determine whether SSRIs also change the structural connectivity of this circuitry and how structural and FC are coupled and mutually influenced by treatment—both psychopharmacologic and psychotherapeutic.
In addition, escitalopram, compared to placebo, increased connectivity between left amygdala and right angular gyrus, an important node of the DMN that recently is implicated in emotion regulation [27]. This resulted in a positive coupling between amygdala and angular gyrus after 2 weeks treatment. Further, escitalopram significantly reduced left amygdala activity consistent with previous studies that demonstrate decreased amygdala activity after received SSRIs (sertraline and citalopram) [42, 44, 45]. Inadequate deactivation of amygdala and DMN during emotional processing and regulation are critical characteristics in anxiety disorders [46, 47], which predict later treatment response [48]. Ineffective suppression of DMN activity may reduce emotional regulation during the task and consequently produce amygdala hyperactivation. In this study, SSRI treatment produced greater synchronized activity decreases in amygdala and angular gyrus, which could facilitate emotional regulation. Moreover, this escitalopram-enhanced amygdala-angular gyrus connectivity at week 2 was associated with greater subsequent clinical improvement. Our findings provide a promising translational pathway, for stratifying cases in clinical trials, for improving selection of lower animal models for treatment development, and potentially in the longer term for improving clinical outcomes. Potentially, early neurofunctional changes could classify patients who recently initiated treatment as having a high, intermediate, or low likelihood of responding to a “full” treatment trial for a given intervention.
Interestingly, in the current study, escitalopram increased the functional coupling between amygdala and both vmPFC and angular gyrus but in different directions (i.e., negative connectivity in amygdala-vmPFC, positive connectivity in amygdala-angular gyrus). These differences in the directionality of coupling during emotion processing might relate to the distinct roles of the vmPFC and angular gyrus in regulating the amygdala. The vmPFC and angular gyrus represent anterior and posterior components of the DMN that are involved in different types of processing [49, 50]. With regard to the role of the DMN in anxiety, anterior DMN connectivity has been positively correlated with anxiety severity, while the posterior DMN connectivity has been negatively correlated with anxiety in healthy individuals [49]. This different role of DMN subsystems in anxiety is consistent with our observation that escitalopram treatment is associated with decreased connectivity in anterior DMN and increased connectivity in posterior DMN with corresponding improvement in anxiety.
Besides greater functional coupling of brain regions, better performance during the emotional processing task was also observed with acute SSRI treatment. Specifically, reaction times to all stimulus pictures were significantly shortened by escitalopram, but not in patients who received placebo. This observation contrasts with some previous open-label treatment studies with SSRIs (sertraline and fluoxetine) in adolescents and adults with anxiety disorders that failed to detect treatment effects on behavior performance [20, 42]. This discrepancy might due to our particular task, or the more serotonergically selective effects of escitalopram that has greater--allosterically mediated--affinity for the serotonin transporter. Our findings provide evidence that escitalopram improves the efficiency of cognitive function, which is reduced and correlated with anxiety severity [51], in adolescents with anxiety disorders when they perform emotional tasks. Future, larger studiess could further explore the potential value of baseline task performance or its early change in predicting treatment response.
Finally, while this is the first clinical trial to examine the effect of SSRI treatment on FC during emotional processing in adolescents with GAD, our findings should be interpreted in the context of several limitations. First, the sample size was small and did not provide the power to examine second- and third-order interaction effects, although the sample size is typical of neuroimaging studies with double-blind, placebo-controlled design in adolescents. Larger trials are needed to explore the age, sex, and comorbidity effects on the FC changes observed herein. Second, consistent with prior studies [15, 46], we examined brain connectivity changes during emotional processing using an emotion condition and the features of stimulus pictures (luminance, chroma, and complexity) and button pressing did not differ between groups and did not change between baseline and week 2. Thus, effects of processing faces, generic stimulus, or the button press are unlikely to account for our results. Third, we focused on amygdala connectivity however, examining whole-brain connectivity changes will require a future, larger studies. Fourth, future studies could benefit from longer fMRI scans or more emotional stimuli to increase reliability of measurement and confirm our study findings. Fifth, our task is a fixed event-related design paradigm, future task-based fMRI studies with jitter could enhance modeling of the evoked BOLD response. Finally, the FC analysis registered in clinicaltrials.gov did not specify PPI nor did it specifically define the boundaries of the ROI. Analytic approaches and atlases have developed and improved rapidly in the 8 years following the funding of this project and we sought to use the most up-to-date and appropriate analysis and anatomic ROI.
Conclusion
In adolescents with GAD, escitalopram compared to placebo not only improved the behavioral performance, but enhanced the functional coupling between amygdala and hubs of the DMN during emotional processing within the first 2 weeks of treatment. Specifically, escitalopram increased negative amygdala-vmPFC connectivity and increased positive amygdala-angular gyrus connectivity. Importantly, more negative amygdala-vmPFC connectivity at baseline and escitalopram-increased amygdala-angular gyrus connectivity at week 2 both predicted the magnitude of clinical improvement in anxiety at the end of the trial. These findings suggest that amygdala connectivity with DMN regions might be a target of acute SSRI treatment, and that pretreatment and early-treatment amygdala-based connectivity could serve as biomarkers for predicting SSRI response in adolescents with GAD, which if replicated may facilitate adjusting treatment approaches for those who are unlikely to respond.
References
Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication-Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2010;49:980–9.
Walkup JT, Albano AM, Piacentini J, Birmaher B, Compton SN, Sherrill JT, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359:2753–66.
Dobson ET, Bloch MH, Strawn JR. Efficacy and tolerability of pharmacotherapy for pediatric anxiety disorders: a network meta-analysis. J Clin Psychiatry. 2019;80:17r12064.
Strawn JR, Mills JA, Sauley BA, Welge JA. The impact of antidepressant dose and class on treatment response in pediatric anxiety disorders: a meta-analysis. J Am Acad Child Adolesc Psychiatry. 2018;57:235–44.e2.
Tulisiak AK, Klein JA, Harris E, Luft MJ, Schroeder HK, Mossman SA, et al. Antidepressant prescribing by pediatricians: a mixed-methods analysis. Curr Probl Pediatr Adolesc Health Care. 2017;47:15–24.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington VA: American Psychiatric Association; 2013.
Weber-Goericke F, Muehlhan M. A quantitative meta-analysis of fMRI studies investigating emotional processing in excessive worriers: application of activation likelihood estimation analysis. J Affect Disord. 2019;243:348–59.
McTeague LM, Rosenberg BM, Lopez JW, Carreon DM, Huemer J, Jiang Y, et al. Identification of common neural circuit disruptions in emotional processing across psychiatric disorders. Am J Psychiatry. 2020;177:411–21.
LeDoux JE, Pine DS. Using neuroscience to help understand fear and anxiety: a two-system framework. Am J Psychiat. 2016;173:1083–93.
Campese VD, Kim IT, Hou M, Gupta S, Draus C, Kurpas B, et al. Chemogenetic inhibition reveals that processing relative but not absolute threat requires basal amygdala. J Neurosci. 2019;39:8510–16.
Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HM, et al. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry. 2008;65:568–76.
Williams LE, Oler JA, Fox AS, McFarlin DR, Rogers GM, Jesson MA, et al. Fear of the unknown: uncertain anticipation reveals amygdala alterations in childhood anxiety disorders. Neuropsychopharmacology. 2015;40:1428–35.
Swartz JR, Phan KL, Angstadt M, Klumpp H, Fitzgerald KD, Monk CS. Altered activation of the rostral anterior cingulate cortex in the context of emotional face distractors in children and adolescents with anxiety disorders. Depress Anxiety. 2014;31:870–79.
Carlisi CO, Hilbert K, Guyer AE, Ernst M. Sleep-amount differentially affects fear-processing neural circuitry in pediatric anxiety: a preliminary fMRI investigation. Cogn Affect Behav Neurosci. 2017;17:1098–113.
Monk CS, Nelson EE, McClure EB, Mogg K, Bradley BP, Leibenluft E, et al. Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. Am J Psychiatry. 2006;163:1091–7.
Hirsch CR, Mathews A, Lequertier B, Perman G, Hayes S. Characteristics of worry in generalized anxiety disorder. J Behav Ther Exp Psychiatry. 2013;44:388–95.
Strawn JR, Bitter SM, Weber WA, Chu WJ, Whitsel RM, Adler C, et al. Neurocircuitry of generalized anxiety disorder in adolescents: a pilot functional neuroimaging and functional connectivity study. Depress Anxiety. 2012;29:939–47.
Roy AK, Fudge JL, Kelly C, Perry JS, Daniele T, Carlisi C, et al. Intrinsic functional connectivity of amygdala-based networks in adolescent generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry. 2013;52:290–99 e2.
Toazza R, Franco AR, Buchweitz A, Molle RD, Rodrigues DM, Reis RS, et al. Amygdala-based intrinsic functional connectivity and anxiety disorders in adolescents and young adults. Psychiat Res-Neuroim. 2016;257:11–16.
Maslowsky J, Mogg K, Bradley BP, McClure-Tone E, Ernst M, Pine DS, et al. A preliminary investigation of neural correlates of treatment in adolescents with generalized anxiety disorder. J Child Adolesc Psychopharmacol. 2010;20:105–11.
Strawn JR, Dobson ET, Mills JA, Cornwall GJ, Sakolsky D, Birmaher B, et al. Placebo response in pediatric anxiety disorders: results from the child/adolescent anxiety multimodal study. J Child Adolesc Psychopharmacol. 2017;27:501–08.
Lu L, Li H, Mills JA, Schroeder H, Mossman SA, Varney ST, et al. Greater dynamic and lower static functional brain connectivity prospectively predict placebo response in pediatric generalized anxiety disorder. J Child Adolesc Psychopharmacol. 2020;30:606–16.
Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, et al. Functional network organization of the human brain. Neuron. 2011;72:665–78.
Burkhouse KL, Kujawa A, Klumpp H, Fitzgerald KD, Monk CS, Phan KL. Neural correlates of explicit and implicit emotion processing in relation to treatment response in pediatric anxiety. J Child Psychol Psychiatry. 2017;58:546–54.
Bartlett EA, DeLorenzo C, Sharma P, Yang J, Zhang MR, Petkova E, et al. Pretreatment and early-treatment cortical thickness is associated with SSRI treatment response in major depressive disorder. Neuropsychopharmacology. 2018;43:2221–30.
Strawn JR, Mills JA, Schroeder H, Mossman SA, Varney ST, Ramsey LB, et al. Escitalopram in adolescents with generalized anxiety disorder: a double-blind, randomized, placebo-controlled study. J Clin Psychiatry. 2020;81:20m13396.
Sylvester CM, Corbetta M, Raichle ME, Rodebaugh TL, Schlaggar BL, Sheline YI, et al. Functional network dysfunction in anxiety and anxiety disorders. Trends Neurosci. 2012;35:527–35.
Lu L, Mills JA, Li H, Schroeder HK, Mossman SA, Varney ST, et al. Acute neurofunctional effects of escitalopram in pediatric anxiety: a double-blind, placebo-controlled trial. J Am Acad Child Adolesc Psychiatry. 2021;60:1309–18.
Perino MT, Yu Q, Myers MJ, Harper JC, Baumel WT, Petersen SE, et al. Attention alterations in pediatric anxiety: evidence from behavior and neuroimaging. Biol Psychiatry. 2021;89:726–34.
The Research Units on Pediatric Psychopharmacology Anxiety Study Group. The Pediatric Anxiety Rating Scale (PARS): development and psychometric properties. J Am Acad Child Adolesc Psychiatry. 2002;41:1061–9.
Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont). 2007;4:28–37.
Yamasaki H, LaBar KS, McCarthy G. Dissociable prefrontal brain systems for attention and emotion. Proc Natl Acad Sci USA. 2002;99:11447–51.
Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–41.
McLaren DG, Ries ML, Xu G, Johnson SC. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage. 2012;61:1277–86.
Baas D, Aleman A, Kahn RS. Lateralization of amygdala activation: a systematic review of functional neuroimaging studies. Brain Res Brain Res. Rev. 2004;45:96–103.
Rosenkranz JA, Moore H, Grace AA. The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli. J Neurosci. 2003;23:11054–64.
Etkin A, Prater KE, Hoeft F, Menon V, Schatzberg AF. Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. Am J Psychiatry. 2010;167:545–54.
Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13:33–57. 829
Baur V, Bruhl AB, Herwig U, Eberle T, Rufer M, Delsignore A, et al. Evidence of frontotemporal structural hypoconnectivity in social anxiety disorder: a quantitative fiber tractography study. Hum Brain Mapp. 2013;34:437–46.
Kim MJ, Whalen PJ. The structural integrity of an amygdala-prefrontal pathway predicts trait anxiety. J Neurosci. 2009;29:11614–8.
Townsend JD, Torrisi SJ, Lieberman MD, Sugar CA, Bookheimer SY, Altshuler LL. Frontal-amygdala connectivity alterations during emotion downregulation in bipolar I disorder. Biol Psychiatry. 2013;73:127–35.
Phan KL, Coccaro EF, Angstadt M, Kreger KJ, Mayberg HS, Liberzon I, et al. Corticolimbic brain reactivity to social signals of threat before and after sertraline treatment in generalized social phobia. Biol Psychiatry. 2013;73:329–36.
Outhred T, Das P, Felmingham KL, Bryant RA, Nathan PJ, Malhi GS, et al. Facilitation of emotion regulation with a single dose of escitalopram: a randomized fMRI study. Psychiatry Res. 2015;233:451–7.
Harmer CJ, Mackay CE, Reid CB, Cowen PJ, Goodwin GM. Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biol Psychiatry. 2006;59:816–20.
Godlewska BR, Browning M, Norbury R, Cowen PJ, Harmer CJ. Early changes in emotional processing as a marker of clinical response to SSRI treatment in depression. Transl Psychiat. 2016;6:e957.
Gentili C, Ricciardi E, Gobbini MI, Santarelli MF, Haxby JV, Pietrini P, et al. Beyond amygdala: default mode network activity differs between patients with social phobia and healthy controls. Brain Res Bull. 2009;79:409–13.
Zilverstand A, Parvaz MA, Goldstein RZ. Neuroimaging cognitive reappraisal in clinical populations to define neural targets for enhancing emotion regulation. A systematic review. Neuroimage. 2017;151:105–16.
Spies M, Kraus C, Geissberger N, Auer B, Klobl M, Tik M, et al. Default mode network deactivation during emotion processing predicts early antidepressant response. Transl Psychiatry. 2017;7:e1008.
Coutinho JF, Fernandesl SV, Soares JM, Maia L, Goncalves OF, Sampaio A. Default mode network dissociation in depressive and anxiety states. Brain Imaging Behav. 2016;10:147–57.
Zidda F, Andoh J, Pohlack S, Winkelmann T, Dinu-Biringer R, Cavalli J, et al. Default mode network connectivity of fear- and anxiety-related cue and context conditioning. Neuroimage. 2018;165:190–99.
Fujii Y, Kitagawa N, Shimizu Y, Mitsui N, Toyomaki A, Hashimoto N, et al. Severity of generalized social anxiety disorder correlates with low executive functioning. Neurosci Lett. 2013;543:42–6.
Acknowledgements
We thank the patients and their families for participating in this study, the Data Safety Monitoring Board for their oversight of the study, and the MR technologists and pediatric radiologist from the Imaging Research Center at Cincinnati Children’s Hospital Medical Center. We thank James C. Eliasson, PhD, for developing and optimizing the CPT-END task. We also thank Li Xue, MS, for her help with the calculation of the percentage of amygdala-based connections that are related to head motion at the voxel level.
Funding
This work was supported by the National Institute of Mental Health (K23 MH106037, JRS), the National Institute of Child Health and Development (R01 HD098757, JRS), the National Institute of Environmental Health Sciences (R01 ES027224, KMC), and the Chinese National Natural Science Foundation (Grant No. 81820108018, XH/QG/JAS; Grant No. 81621003, No. 8202780056, and No. 82027808, XH/QG/LL). LL received a Chinese Government Scholarship. JAM has received research support from the Yung Family Foundation. XH and QG received research support from the Functional and Molecular Imaging Key Laboratory of Sichuan Province (FMIKLSP), China. JAS consults to VeraSci. JRS has received research support from AbbVie, Neuronetics, Lundbeck, Otsuka, PCORI, and the National Institutes of Health. He has provided consultation to Intracellular Therapeutics and the Food and Drug Administration in 2020. He receives royalties from Springer Publishing for two texts and received material support from Myriad. He has also received honoraria from CMEology and Genomind. The remaining authors have nothing to disclose.
Author information
Authors and Affiliations
Contributions
LL performed the analyses and drafted the manuscript. HL helped with imaging analysis. JAM participated in statistical analysis. KMC implemented the imaging sequence and oversaw data acquisition. HKS and SAM coordinated the study and evaluated patients. WTB, XH, QG, and JAS reviewed and revised the manuscript. JRS obtained grant funding, designed the protocol, evaluated the study participants, obtained the data, and drafted/revised the manuscript. All authors read, modified, and approved the final version of the submitted manuscript.
Corresponding authors
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Lu, L., Li, H., Baumel, W.T. et al. Acute neurofunctional effects of escitalopram during emotional processing in pediatric anxiety: a double-blind, placebo-controlled trial. Neuropsychopharmacol. 47, 1081–1087 (2022). https://doi.org/10.1038/s41386-021-01186-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-021-01186-0
This article is cited by
-
Gastrointestinal Symptoms in Pediatric Patients with Anxiety Disorders and Their Relationship to Selective Serotonin Reuptake Inhibitor Treatment or Placebo
Child Psychiatry & Human Development (2023)