Abstract
Compulsion-like alcohol drinking (CLAD), where consumption continues despite negative consequences, is a major obstacle to treating alcohol use disorder. The locus coeruleus area in the brainstem and norepinephrine receptor (NER) signaling in forebrain cortical regions have been implicated in adaptive responding under stress, which is conceptually similar to compulsion-like responding (adaptive responding despite the presence of stress or conflict). Thus, we examined whether anterior insula (aINS)-to-brainstem connections and alpha-1 NERs regulated compulsion-like intake and alcohol-only drinking (AOD). Halorhodopsin inhibition of aINS–brainstem significantly reduced CLAD, with no effect on alcohol-only or saccharin intake, suggesting a specific aINS–brainstem role in aversion-resistant drinking. In contrast, prazosin inhibition of alpha-1 NERs systemically reduced both CLAD and AOD. Similar to systemic inhibition, intra-aINS alpha-1-NER antagonism reduced both CLAD and AOD. Global aINS inhibition with GABAR agonists also strongly reduced both CLAD and AOD, without impacting saccharin intake or locomotion, while aINS inhibition of calcium-permeable AMPARs (with NASPM) reduced CLAD without impacting AOD. Finally, prazosin inhibition of CLAD and AOD was not correlated with each other, systemically or within aINS, suggesting the possibility that different aINS pathways regulate CLAD versus AOD, which will require further study to definitively address. Together, our results provide important new information showing that some aINS pathways (aINS–brainstem and NASPM-sensitive) specifically regulate compulsion-like alcohol consumption, while aINS more generally may contain parallel pathways promoting CLAD versus AOD. These findings also support the importance of the adaptive stress response system for multiple forms of alcohol drinking.
Similar content being viewed by others
Introduction
Alcohol use disorder (AUD) ranks among the most prevalent mental disorders [1, 2], and is characterized by loss of control over intake, negative emotional state, and compulsive alcohol use where consumption persists despite negative consequences, which is a major obstacle for treating AUD [2,3,4,5]. The molecular mechanisms and neural circuits that drive compulsion-like intake have begun to be investigated but are still not well understood [6, 7]. However, preclinical rodent studies where alcohol drinking occurs in concert with footshock or bitter-tasting quinine are considered to model some aspects of human compulsion-like intake (see Supplementary Discussion), and thus have been used to better understand mechanisms that promote compulsion-like alcohol consumption (reviewed in refs. [7, 8]).
One area of particular interest is the anterior insula (aINS), a potent regulator of emotional states and strong contributor to many aspects of addiction behavior [9, 10]. In rodents, aINS signaling can regulate compulsion-like but not alcohol-only drinking (AOD) [11, 12], and also promotes operant alcohol intake [13] (see “Discussion”). In addition, we found that compulsion-like drinking requires medial prefrontal cortex and aINS inputs to nucleus accumbens (NAcb), with no role in alcohol-only consumption [12, 14]. Interestingly, a related aINS circuit is implicated in compulsion-like responding for alcohol in heavy human drinkers [15, 16]. In this regard, clinical studies have proposed that compulsion during addiction is characterized by conflict (between the drive to consume intoxicant and the drive to avoid negative consequence), which recruits cortical conflict-processing circuits that promote compulsion-like responding (but play a lesser role in non-conflict intake) [9, 15, 17].
The aINS also projects to stress-regulating regions [10, 18]. Inputs to the locus coeruleus (LC) area are of particular interest, since LC and downstream norepinephrine (NE) signaling are considered critical for adaptive responding to stressors [19, 20], which may be considered conceptually similar to compulsion-like responding since both involve overcoming stress/conflict to allow efficient responding. Rats possess a small but detectable insula-LC input [21,22,23,24] (Fig. 1C), and, as noted in ref. [21] and elsewhere, projections lateral to LC may contact LC dendrites lateral to LC soma; central amygdala inputs are also primarily lateral to LC neurons, and can act through LC cells to promote adaptive stress responses [19]. In addition, LC/NE likely contribute to reinforcing effects of alcohol [20]. Further, preclinical and clinical alcohol studies have focused on pharmacological compounds that reduce NE receptor (NER) signaling, including alpha-1-NER blockers such as prazosin (see refs. [25, 26]), which preferentially impact human drinkers with greater withdrawal-related negative affect [25]. Rodent studies also implicate alpha-1 NERs in alcohol behaviors [20], including anxiety-related drinking [27], stress-induced reinstatement [28], operant intake in dependent rats [29], and drinking in alcohol-preferring rats [30, 31]. Together, these support the importance of the NE system in maladaptive drives for alcohol.
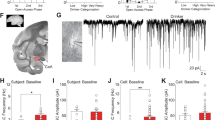
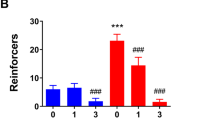
(A) Schematic of an alcohol-drinking study: 3mo intermittent intake, then 5d/week 20-min access; test sessions for “Opto” (optogenetics) involved “laser” or “no-las” (no laser) tests during alcohol-only (AOD) and compulsion-like alcohol-drinking (CLAD) sessions; “Pharm” (pharmacology) experiments were similar except with “drug” or “veh” (vehicle) (details in “Materials and Methods”). (B) Cartoon of optogenetic methods targeting AINS to LC Area (LCA). (C) Horizontal section showing aINS inputs (green) overlap LC (red for TH + staining) but are primarily lateral to LC. (D, E) Examples of fiber-optic placement in (F) eYFP rat and (G) halorhodopsin rat (TH + LC is torn in E). Note that eYFP expression is less strong in brainstem relative to halorhodopsin–eYFP, which likely reflects that the latter has targeting to the surface membrane. (F) 488 nanometer laser light in brainstem significantly reduced CLAD in halorhodopsin- but not eYFP-expressing rats. * indicates p < 0.05 difference between CLAD/Halorh/Laser and both CLAD/eYFP/Laser and AOD/Halorh/Laser. For the 3-way ANOVA, there was a significant interaction for drinkXlaser (F1,52 = 8.982, p = 0.004) but no interaction for drinkXvirus (F1,52 = 2.306, p = 0.135) or laserXvirus (F1,52 = 0.011, p = 0.917), and significant main effect of drink (F1,52 = 7.766, p = 0.007) but not laser (F1,52 = 3.538, p = 0.066) or virus (F1,52 = 0.171, p = 0.681). Two-way ANOVAs showed a significant effect of laser stimulation with halorhodopsin [F(drinking-condition;1,6) = 8.653, p = 0.007; F(laser;1,6) = 1.841, p = 0.187; F(interaction;1,6) = 11.811, p = 0.002] but not eYFP [F(drinking-condition;1,7) = 1.952, p = 0.205; F(laser;1,7) = 4.559, p = 0.070; F(interaction;1,7) = 1.060, p = 0.337]. Two-way ANOVAs determined post hocs. (G) No effect of halorhodopsin inhibition of aINS–brainstem on saccharin intake. Scale bars indicate 1, 2, and 0.6 mm in (C), (D), and (E). PB parabrachium, TH tyrosine hydroxylase.
Since aINS is implicated in compulsion-like alcohol drinking in rodents and humans [9, 11, 12, 15, 16], CLAD can be conceptually similar to adaptive stress responses, and adaptive stress responses can involve NER activation in forebrain cortical areas [19], we set out to examine how the NE system, including interactions with insula, might regulate alcohol consumption. While aINS–brainstem inputs regulated compulsion-like but not alcohol-only consumption, prazosin systemically or within aINS impacted both compulsion-like and AOD. Along with other findings, these results support the importance of aINS–brainstem and aINS-NAcb inputs for compulsion-like consumption, where aINS alpha-1 NERs and global activity can, unexpectedly, regulate both compulsion-like and alcohol-only drinking.
Materials and methods
Complete Methods are in Supplementary Material.
Subjects and drinking methods
All experiments were conducted following NIH guidelines and approved by UCSF and Indiana University IACUCs. P45-50 male Wistar rats (Harlan/Envigo, n = 90 total) were singly housed with ad libitum food and water. After 2 weeks, rats drank under intermittent two-bottle choice (I2BC) for alcohol (20% v/v) or water, with 16–24-h access starting Monday, Wednesday, and Friday afternoons. After ~12-week I2BC, rats began drinking under limited-daily-access, two-bottle choice (LDA) for alcohol or water, with 20-min access Monday to Friday. These brief daily alcohol-only intake days are indicated below by “AOD,” with compulsion-like alcohol-drinking days indicated by “CLAD.” After 3–4-week LDA, rats had 2–3 quinine–alcohol sessions (10-mg/L quinine, CLAD) to habituate to the novelty of quinine, then returned to AOD. We have used these methods to assess CLAD mechanisms [12, 14, 32, 33]. Figure 1A shows schematic of an alcohol-drinking study. All studies were performed in the home cage.
Before test sessions, rats were handled daily for 1 week, with a single vehicle injection at the end. Experimental days were generally 2/week, with at least one AOD day between tests. Conditions were randomized across animals and sessions using a Latin-square design. For most groups, animals underwent each experimental condition twice: all conditions (e.g., vehicle vs. drug) were randomized for one round, then conditions randomized again for the second round. For each experimental condition from a given animal, drinking data from the two rounds were averaged to give a single intake value; this reduces variability in drinking measures [12, 14, 34]. Where specifically noted below, animals underwent each experimental condition only once. Test sessions were 20 min, except 5 min for optogenetic experiments to reduce laser exposure; shorter sessions yield about the same intake as 20-min sessions [12] due to front-loading [32, 33].
Separate cohorts were used for halorhodopsin or eYFP, with within-animal tests of AOD or CLAD and with or without 488-nm laser stimulation (during the entire drinking session). Five halorhodopsin-transfected animals were tested for saccharin intake; these involved only one laser and one without-laser test session. Two implanted rats began to have some looseness in their headcaps, and even though their alcohol-drinking levels on non-test days were not reduced, we removed these rats from studies. These alcohol-drinking animals were trained to drink saccharin (500 mg/l) using a modified LDA (20 min/day, 5days/week), with saccharin intake in morning and alcohol drinking in the afternoon [12, 14, 34] for 3–4 weeks before test sessions.
All drug/vehicle intracranial injections were bilateral and 30 min before test sessions. Systemic prazosin was tested with 0.75 or 1.5 mg/kg intraperitoneally (i.p.) versus vehicle, with some animals tested with both doses (initial plans were to test all rats with both doses, which was disrupted by Covid, see Supplementary Methods). A separate cohort received intra-aINS prazosin (0.3 µg/0.6 µl per side, or vehicle), with six rats then tested for saccharin intake (as above, two of eight rats in the second aINS cohort began to have some looseness in their headcaps, and even though alcohol drinking was not reduced, we removed these rats); an additional cohort received 0.1 µg/side aINS prazosin. To test global inhibition, rats were injected with muscimol/baclofen (M/B, 250 ng/side for each inhibitor), with 7 of 8 rats tested for locomotor effects in open field (details in Supplementary Methods), which occurred after all alcohol-drinking studies were performed, and, on testing day, locomotor behavior was assessed midday before alcohol drinking had occurred that day. Controls in separate cohorts examined aINS M/B for saccharin intake, and orbitofrontal cortex (OFC, targeting lateral) M/B for CLAD. In a final cohort, 1-naphthylacetyl spermine (NASPM; 20 µg/0.6 µl/side), or vehicle was injected intra-aINS. Also, OFC M/B, aINS NASPM/vehicle, aINS low-praz/vehicle, and some i.p. prazosin (Covid-related, Supplementary Material) were tested with only a single round of injections.
In vivo optogenetics
After ~12-week I2BC and ~4-week LDA, rats were transfected (0.6 µl/side) bilaterally in aINS (AP +2.80, ML ±4.80 DV −5.2), with adeno-associated virus encoding halorhodopsin (eNpHR3.0-eYFP) or eYFP alone, under Camk2a promoter (Supplementary Method). Rats were also implanted bilaterally with chronic fiber-optic ferrules (CFOs, described in ref. [12]) targeting LC area of the brainstem (AP −13.9, ML ±1.90, DV −8.26, 30° tilt).
Test sessions began after ~8–9 weeks to allow for viral expression [12, 14]. During test sessions, CFOs were attached by zirconium sleeve to a fiber-optic cable connected to 488-nm laser (Shanghai Laser & Optics Century, 13–15 mW). Due to proximity between the two CFOs, it was only possible to connect fiber optics on one side of brainstem in about half of animals; however, sides were alternated across tests, and light intensity was high enough to inhibit both sides (based on behavior), since unilateral high laser light did reduce CLAD, while lower levels (4–6 mW) inhibited CLAD bilaterally but not unilaterally (not shown). Also, we use the term brainstem since aINS projections overlap LC but also are strong lateral to LC (and elsewhere) (Fig. 1C), and one limitation of our study is that laser light likely shines broadly across the brainstem; thus, “brainstem” notes the broader region, which aINS inputs and laser light can impact (we also cannot rule out effects through aINS collaterals). However, analogously located inputs from central amygdala can act through LC during adaptive stress responses [19].
Histology and IHC verified placements (Supplementary Method).
Cannula implantation and microinfusions
After 3–4-week LDA, we bilaterally implanted guide cannulae (Plastics One) aimed at aINS (AP +2.8, ML ±4.8, DV −4.2 mm) or OFC (AP +3.2, ML ±3.2, DV −4.2 mm) [35, 36]. During test sessions, 33-guage injectors were connected to 10-μl microsyringes (701-RN, Hamilton) and lowered to 1 mm below cannulae. Drugs were injected bilaterally (0.6 μl, 0.2 μl/min), and left for an additional 2 min before removal.
Reagents
Prazosin hydrochloride, muscimol, baclofen, and NASPM trihydrochloride were from Sigma-Aldrich (USA). Prazosin was dissolved in sterile water, and NASPM and M/B in sterile saline (0.9%). 0.3-µg/side prazosin and 20-µg/side NASPM doses were determined from a large complement of previous studies (Supplementary Method).
Statistics and data analysis
Alcohol consumption was determined through changes in bottle weight before and after a drinking session and converted to grams-ethanol/kilograms-body-weight. Statistical comparisons were primarily within-subject, using one- or two-way ANOVA with repeated-measures followed by Bonferroni post hoc, when applicable. Some comparisons used paired t-test, Wilcoxon, or Pearson’s correlation. While the majority of our data were normally distributed with non-significantly different standard deviations (tested by the Shapiro–Wilk test), there were data which were not normal or had non-equivalent variance. We used ANOVAs for aggregated data, but, when analyzing particular pairs of treatment groups, use of nonparametric (Wilcoxon) versus parametric did not change our findings. These are detailed in Supplementary Methods. Also, the need for use of 2- and 3-way ANOVAs to examine association between the variables, which are parametric, is a limitation in our study, as is the use of Bonferroni correction, since this results in higher chance for type II errors with small samples.
For 20-min studies we also determined concurrent water intake, a potential internal control for nonspecific effects on consumption, which was not altered (Supplementary Fig. 1), although low water intake [14] makes it challenging to rule out floor effects. We also examined measures to assess whether AOD interspersed among test sessions was similar to pretest intake (Supplementary Fig. 2). To further examine drinking changes, across groups and for correlations, we determined a given treatment’s impact from log[100 × (intake during drug treatment)/(intake during vehicle)], as we previously described [34, 37] (see Supplementary Methods). Log value of 2 (log[100]) indicates no treatment effect. Analyses were performed using GraphPad Prism or SPSS. All data are mean ± SEM.
Results
Inhibition of aINS–brainstem inputs decreased CLAD but not AOD
To understand whether aINS inputs in brainstem regulate AOD or quinine–alcohol intake (CLAD), we transfected aINS with halorhodopsin (or eYFP control) and targeted brainstem with fiber optics (Fig. 1B–E; experimental timeline in Fig. 1A). Importantly, there was a significant three-way interaction of drink (AOD/CLAD) X laser (on/off) X virus (eYFP/Halo) (F1,52 = 4.133, p = 0.047, other comparisons in Fig. 1 legend). Laser stimulation to activate halorhodopsin in aINS–brainstem inputs inhibited CLAD, without reducing AOD (Fig. 1F; n = 7, p = 0.012 comparing level of CLAD intake with and without laser; p = 0.030 not significant with correction for multiple comparison, when comparing level of AOD intake with and without laser). However, in control animals expressing eYFP, laser stimulation did not alter CLAD or AOD (Fig. 1F; n = 8) (histology in Supplementary Fig. 3). In addition, halorhodopsin inhibition of aINS–brainstem inputs had no effect on saccharin intake (Fig. 1G; n = 5; t(4) = 0.714, p = 0.515). Further, while there was an apparent trend for laser reduction of CLAD in eYFP, the level of quinine–alcohol intake was significantly lower with halorhodopsin activated by laser relative to eYFP + laser (p = 0.010). Finally, control ex vivo studies demonstrated that halorhodopsin reduced glutamate release from aINS terminals in LC (Supplementary Fig. 4). Together, these results suggest that aINS–brainstem inputs promoted CLAD, with limited impact on alcohol-only or saccharin intake, indicating a specific aINS–brainstem role in conflict-resistant alcohol drinking.
Systemic administration of prazosin decreased both CLAD and AOD
Since alpha-1 NERs are implicated in stress-related drinking (see “Introduction”), we tested systemic injection of the widely used alpha-1-NER antagonist prazosin, with doses, timing, and administration route based on previous rat studies [29,30,31], including where moderate doses (0.5–1.0 mg/kg) impact drinking in alcohol-dependent but not nondependent rats, with higher doses (1.5–2.0 mg/kg) reducing intake in both [29]. 0.75-mg/kg prazosin significantly decreased both CLAD and AOD (Fig. 2A, n = 17; two-way ANOVA; F(treatment;1,16) = 55.656, p < 0.001; F(drinking-condition;1,16) = 5.470, p = 0.033; F(interaction;1,16) = 0.690, p = 0.418). Further, 1.5-mg/kg prazosin significantly decreased CLAD but not AOD (Fig. 2B, n = 18; two-way ANOVA; F(treatment;1,17) = 24.091, p < 0.001; F(drinking-condition;1,17) = 3.170, p = 0.093; F(interaction;1,17) = 3.415, p = 0.082; post hoc veh-vs-praz for AOD: p = 0.119; CLAD post hoc p < 0.001). However, the change in drinking with 1.5-mg/kg prazosin, normalized to consumption under vehicle, was not different between CLAD and AOD (p = 0.442 Mann–Whitney), and the change in drinking during AOD was not different between 0.75 and 1.5-mg/kg prazosin (p = 0.913 Mann–Whitney). Thus, systemic prazosin overall reduced CLAD and AOD, with more consistent effects at 0.75 mg/kg.
Although alpha-1-NER inhibition reduced both AOD and CLAD, there was some variability across individuals; one possibility is that some subjects had stronger alpha-1-NER regulation of alcohol drinking overall (with strong prazosin reduction of both AOD and CLAD), while other subjects had weaker alpha-1-NER regulation of intake (and thus demonstrated lower prazosin reduction of both AOD and CLAD). In this case, the impact of prazosin on AOD and CLAD would be correlated across animals, and would suggest that alpha-1 NERs were more important for driving intake (AOD or CLAD) in some individuals, and less important in others. However, in contrast to this hypothesis, systemic prazosin reduction in AOD was not correlated with prazosin reduction in CLAD, for either 0.75 g/kg (Fig. 3A, p = 0.378) or 1.5 g/kg (Fig. 3B, p = 0.768). These results suggested that prazosin regulation of AOD might occur through a different mechanism from alpha-1-NER promotion of CLAD (discussed further below). In addition, some of our previous work suggests that pharmacological impacts can vary with basal drinking levels [34, 37] (basal intake was determined from vehicle test days). 0.75-g/kg prazosin reduction of CLAD significantly correlated with basal compulsion-like drinking levels (Fig. 3C, p = 0.047), with a similar trend for 1.5-g/kg prazosin (Fig. 3D, p = 0.086), perhaps suggesting that alpha-1 NERs played a greater role in promoting CLAD in higher-drinking individuals. In contrast, prazosin impacts on AOD were not correlated with basal AOD for 0.75 g/kg (Fig. 3E, p = 0.211) or 1.5 g/kg (Fig. 3F, p = 0.317) prazosin.
Changes in intake were determined by taking a log transformation of drug intake/vehicle intake (see “Materials and methods”); dotted lines indicate when log of change in intake equals 2, indicating no change in drinking [log(100)]. Neither 0.75 (A) nor 1.5 (B) mg/kg prazosin changes in AOD were correlated with changes in CLAD. 0.75 mg/kg (C) but not 1.5-mg/kg (D) prazosin changes in CLAD were correlated with basal CLAD. Neither 0.75 (E) nor 1.5 (F) mg/kg prazosin changes in AOD were correlated with basal AOD. A-O alcohol-only (AOD), Praz prazosin, Q-A quinine–alcohol (CLAD). *p < 0.05.
Impacts of aINS inhibition on CLAD and AOD
Since (1) aINS is likely an important regulator of CLAD [9, 11, 12, 15, 16], (2) adaptive stress responses, which can be considered conceptually similar to CLAD, can involve NERs in forebrain cortical areas [19], and (3) systemic alpha-1 NERs regulated both CLAD and AOD (above), we next evaluated the potential role of aINS alpha-1 NERs for different forms of alcohol consumption, using a widely utilized dose of intracranial prazosin (Supplementary Methods). Intra-aINS prazosin (0.3 µg/side) significantly reduced both CLAD and AOD (Fig. 4A, n = 14; F(treatment;1,13) = 19.064, p = 0.001; F(drinking-condition;1,13) = 15.457, p = 0.002; F(interaction;1,13) = 0.619, p = 0.445) (histology in Supplementary Fig. 3). However, aINS prazosin did not impact saccharin intake (Fig. 4B, n = 6; t(5) = 0.109; p = 0.917), indicating that aINS alpha-1-NER alcohol regulation likely did not reflect nonspecific consumption effects. Also, a lower dose of prazosin (0.1 µg/side) had no impact on AOD or CLAD (Fig. 4C, n = 11, statistics in figure legend). Furthermore, similar to systemic prazosin, higher-dose intra-aINS prazosin regulation of AOD was not correlated with effects on CLAD (Fig. 4D, p = 0.979). Thus, we speculate that AOD and CLAD were regulated by different alpha-1-NER-related mechanisms within the insula (see below).
Prazosin (0.3 µg/side) in aINS reduced AOD and CLAD drinking (A) but not saccharin intake (B). (C) A lower dose of prazosin (0.1 µg/side) in aINS had no effect on AOD or CLAD drinking compared with vehicle (F(treatment;1,10) = 0.319, p = 0.585; F(drinking-condition;1,10) = 5.865, p = 0.036; F(interaction;1,10) = 0.588, p = 0.461), although there was small (~21.5%) but significant lower CLAD vs. AOD intake. (D) For 0.3-µg/side aINS prazosin, changes in AOD were not correlated with changes in CLAD. **,***: p < 0.01, p < 0.005.
After examining the impact of intra-aINS prazosin, we considered whether aINS activity more generally might regulate alcohol drinking. For example, if M/B in insula had the same impact of insula prazosin, this would suggest that insula alpha-1 NERs acted by activating aINS neurons (since insula alpha-1 NER and global inhibition would have similar effects). Like intra-aINS prazosin, aINS M/B reduced both CLAD and AOD (Fig. 5A, n = 8; F(treatment;1,7) = 97.537, p < 0.001; F(drinking-condition;1,7) = 0.415, p = 0.540; F(interaction;1,7) = 0.009, p = 0.929), with no difference in change in AOD versus CLAD (p = 0.383, Wilcoxon) (histology in Supplementary Fig. 3). However, intra-aINS M/B did not reduce saccharin intake (Fig. 5B, n = 6; t(5) = 0.296; p = 0.779) or locomotor distance in open-field test (Fig. 5C, n = 7; t(6) = 0.748; p = 0.483). Furthermore, OFC medial to aINS regulates some drug and alcohol behaviors [35, 38], and aINS M/B could act by diffusing into OFC; however, intra-OFC M/B did not affect CLAD (Fig. 5D, n = 5; t(4) = 0.622; p = 0.568). Also, similar to intra-aINS prazosin, aINS M/B changes in AOD versus changes in CLAD were not correlated across rats (Fig. 5E, p = 0.919). Together, these results strongly support that alpha-1 NER and global aINS activity were necessary for both alcohol-only and compulsion-like consumption, which did not reflect nonspecific effects on consumption or movement.
Inhibition of aINS with M/B strongly reduced AOD and CLAD (A) but not saccharin intake (B) or locomotion (C). (D) OFC M/B did not reduce CLAD. (E) Intra-aINS M/B changes in AOD were not correlated with changes in CLAD. (F) Intra-aINS NASPM reduced CLAD but not AOD. Bacl baclofen, Musc muscimol, Quin quinine. *,***: p < 0.05, p < 0.005.
Both M/B and prazosin within aINS reduced AOD and CLAD, suggesting that alpha-1 NERs activated aINS to promote AOD and CLAD. Thus, we next examined the impact of another method to more globally inhibit insula. In particular, we assessed NASPM, an inhibitor of calcium-permeable AMPARs, which drive many addictive behaviors [39], since cortical GABA neurons have NASPM-sensitive AMPARs [39, 40] and aINS GABA cells in mice regulate CLAD but not AOD [11]. Interestingly, NASPM reduced CLAD but not AOD (Fig. 5F, n = 7; F(treatment;1,6) = 62.438, p < 0.001; F(drinking-condition;1,6) = 0.133, p = 0.728; F(interaction;1,6) = 7.598, p = 0.033). These results provide useful context for our other aINS findings, suggesting that not all signaling mechanisms within aINS regulate both types of alcohol drinking (see below).
Discussion
Compulsion-like alcohol intake, where consumption continues despite negative consequences, is a major obstacle to treating AUD. Since LC/NE and cortical alpha-1 NERS have been implicated in adaptive stress responding, we examined whether aINS–brainstem connections and alpha-1 NERs were important for regulating CLAD. Optogenetic inhibition of halorhodopsin-transfected aINS–brainstem projections reduced CLAD but not AOD or saccharin intake. In contrast, and unexpectedly, prazosin inhibition of alpha-1 NERs systemically or within aINS reduced both CLAD and AOD. Global aINS inhibition with GABAR agonists also strongly reduced CLAD and AOD, without impacting saccharin intake or locomotor activity, suggesting regulation of alcohol drinking without nonspecific motivational or motor effects. However, aINS inhibition of CP-AMPARs reduced CLAD without impacting AOD, similar to aINS–brainstem (and aINS–NAcb [12]) inhibition, suggesting that not all aspects of aINS signaling regulated both AOD and CLAD. Finally, prazosin inhibition of CLAD and AOD was not correlated, systemically or within aINS, leading to the speculation that different alpha-1-NER-regulated aINS pathways might regulate CLAD versus AOD. Together, our results provide important new information that alpha-1 NERs in aINS supported both alcohol-only and compulsion-like drinking, while, in contrast, aINS projections to brainstem (and NAcb in a previous study [12]), and CP-AMPAR signaling within the aINS, all only drove compulsion-like intake. Further, since our results suggest the possibility that aINS alpha-1-NER regulation of AOD was not related to CLAD, our results provide a precedent that there may be multiple functional pathways within the aINS, which will be of value for future studies.
The aINS is considered a key part of the salience network, which often involves engaging with important events that may require action [9]. In addition, human and monkey studies implicate LC/NE signaling in energizing behavior [41,42,43], including in concert with aINS [44]. Alpha-1 NERs also contribute to cortical arousal to promote responding under some levels of challenge (see refs. [45, 46]). These observations are congruent with the suggested central role of LC/NE in adaptive responding, or maintaining cognitive flexibility, under stress [19, 47]. They are also conceptually related to compulsion-like responding, where intake persists despite negative challenge, and where aINS activity relates to persistent behavior and expected costs (see ref. [48]). A recent study also implicates NE signaling, including alpha-1 NERs, in rodent behaviors considered to reflect compulsion-like behavior (persistent motor actions) not related to addiction [49].
Given potential links between NE and aINS, it is interesting that aINS–brainstem projections regulated CLAD but not AOD, like aINS–NAcb projections [12]. In addition, neither aINS–brainstem (here) nor aINS–NAcb [12] impacted saccharin intake, suggesting limited nonspecific effects. Also, brainstem laser stimulation did not impact eYFP-transfected rats, suggesting that reduced intake did not reflect nonspecific effects of light (as in other areas [50]), even though laser was on throughout the session. It is also interesting that sustained attention involves LC and aINS co-activation in humans [51, 52], and alpha-1 NERS in rodents [47, 53]. We have proposed that CLAD reflects attention to a single, session-long strategy, based in part on CLAD showing reduced response variability that is maintained across a drinking session [32, 33]. Thus, we speculate that aINS connections to brainstem (and other regions) help maintain CLAD through greater attention and/or energization of responding. One important question is the relevance of acute aversion-resistant models to human AUD: as noted in Supplementary Discussion, we consider rodent CLAD models potentially relevant to treatment seekers, where negative consequences of intake are more acute. Also, one limitation of our study is that we did not directly assess whether manipulations performed altered quinine + saccharin intake, and thus we cannot strictly rule out an impact on aversion processing more generally. In previous studies, we found that other manipulations (aINS–NAc, NMDAR receptor modulation) did not alter quinine + saccharin intake [12, 14, 34], suggesting a specific behavioral effect on aversion-resistant alcohol drinking rather than aversion processing in those studies.
In contrast to aINS–brainstem inhibition, systemically blocking alpha-1 NERs reduced both CLAD and AOD. Alpha-1 NERs modulate many tasks in rodents, including drug reinstatement [54, 55] and fear memory [56], and the introduction describes systemic prazosin modulation of many rodent alcohol behaviors, including related to anxiety [27] and dependence [29]. In humans, prazosin reduces alcohol consumption in those with greater withdrawal-related negative mood (see refs. [25, 26]), and decreases stress- and cue-induced craving in AUD humans [57]. Thus, there is a considerable precedent for alpha-1 NERs driving alcohol drinking, especially related to negative conditions, while the contribution to AOD observed here is perhaps more challenging to explain (see below).
In parallel with systemic results, intra-aINS inhibition of alpha-1 NERs with 0.3 but not 0.1-µg/side prazosin reduced both AOD and CLAD. This raises the speculation that aINS alpha-1 NERs impacted AOD and CLAD with a reasonably similar dose-dependence. Thus, while alpha-1 NERs could act in several brain regions (e.g., [46, 58, 59]), our results suggest that aINS alpha-1 NER inhibition could be sufficient to mediate behavioral impacts of systemic alpha-1 NER inhibition. Also, global aINS inhibition with GABAR agonists, which here reduced AOD and CLAD but not saccharin intake or locomotion, also impacts drug reinstatement and impulsivity [60, 61], drug intake [62], anxiety [63], and behavioral control more generally [64], as well as punishment-related alcohol reinstatement [65] and alcohol interoception [66]. Also, similar to our results, aINS inhibition does not change locomotion [13, 61, 63], suggesting no nonspecific motor effects. However, there are some mixed findings, e.g., where aINS inhibition increases operant alcohol intake [13] and heroin responding [67], and does not impact drinking in an alcohol-preferring rat [68]; these could reflect multiple aINS pathways with differing contributions [69], differential aINS regulation across different behaviors, and/or strain differences. Also, OFC inhibition did not reduce CLAD, suggesting a specific role for aINS in alcohol-intake regulation. Lateral OFC inhibition reduces some drug behaviors [35] but not others [36], and increases CLAD in mice [38], which could reflect species variation, e.g., since AOD and CLAD do seem related in mice [70] unlike our rat findings here. Also, aINS alpha-1 NERs have received relatively little attention. They do not regulate heart rate responses at baseline or after tail pinch [58], but alpha-1 NER [71] or global [72] aINS prevent autonomic responses to restraint stress. Thus, our findings suggest that aINS alpha-1 NERs and broader activity can regulate multiple forms of alcohol drinking.
In contrast to aINS alpha-1 NER or global inhibition, inhibiting CP-AMPARs within aINS specifically regulated CLAD, without impacting AOD. CP-AMPARs are enriched on cortical GABAergic neurons [39, 40], and alcohol drinking in mice increases peri-neuronal nets around aINS GABAergic neurons, and dissolving these nets decreases CLAD but not AOD [11]. While future studies will be required to address the exact action of aINS CP-AMPARs, our findings, combined with ref. [11], indicate at least that not all intra-aINS manipulations cause broader effects on alcohol intake, unlike alpha-1 NER or M/B inhibition within aINS.
While alpha-1-NER inhibition systemically or intra-aINS reduced AOD and CLAD, the level of prazosin inhibition of CLAD was not correlated with the prazosin reduction of AOD across individuals, with relatively large sample sizes. Combined with aINS projections to brainstem (here) or NAcb [12], and aINS NASPM, specifically regulating CLAD, these findings suggest the possible speculation that different pathways within aINS mediate AOD versus CLAD. Identifying a possible alternate alpha-1-NER-regulated aINS AOD pathway will require considerable additional effort, since alpha-1 NERs in other cortical areas can be pre- and post-synaptic and regulate GABA neurons and glia [45, 59]. Additional studies should also assess alpha-1-NERs in other regions [20, 47, 73], other NER types in aINS [58, 74] and elsewhere, NE sources in aINS other than LC [75], and NE/alpha-1-NER regulation in females versus males [20].
Finally, alpha-1-NER regulation of AOD may seem paradoxical given the seemingly simple nature of such intake (relative to aversion-resistant consumption), especially with evidence implicating alpha-1 NERs for more stress-related intake. Indeed, prazosin reduces drinking in humans with greater negative mood [25, 26], and it is reasonable that negative affect arises during CLAD, but an AOD role seems more challenging to understand. Our findings could suggest that adult rat AOD involves some level of unease or ambivalence. Alternately, the alpha-1-NER role in drug-induced locomotion [76, 77] may implicate these receptors in reward-related energization more generally. However, protracted voluntary intermittent alcohol intake in adult males can lead to anxiety-like behavior ([78,79,80], but see [81]), moderate alcohol-dependence symptoms [82, 83], and greater anxiety inhibition by prazosin [27].
Conclusion
CLAD and AOD were both regulated by aINS alpha-1 NERs and GABAR-sensitive global activity, while aINS-to-brainstem projections and some within-aINS signaling (CP-AMPARs) only promoted CLAD. Also, prazosin effects on CLAD and AOD were not correlated, suggesting the possibility that separate alpha-1-NER-regulated aINS pathways modulate CLAD versus AOD. Thus, we provide important new information about how different and similar aspects of aINS/NE signaling regulate CLAD and AOD, with implications for other conflict- and stress-related behaviors.
Funding and disclosure
Supported by AA024109 (FWH) from the National Institute on Alcohol and Alcoholism. Raw data are available from communicating author. The authors declare no competing interests.
References
G.B.D. 2019 Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1223–49.
Carvalho AF, Heilig M, Perez A, Probst C, Rehm J. Alcohol use disorders. Lancet. 2019;394:781–92.
Epstein DH, Kowalczyk WJ. Compulsive seekers: our take. Two clinicians’ perspective on a new animal model of addiction. Neuropsychopharmacology. 2017;43:677–9.
Larimer ME, Palmer RS, Marlatt GA. Relapse prevention. An overview of Marlatt’s cognitive-behavioral model. Alcohol Res Health. 1999;23:151–60.
Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–38.
Everitt BJ, Robbins TW. Drug addiction: updating actions to habits to compulsions ten years on. Annu Rev Psychol. 2016;67:23–50.
Hopf FW, Lesscher HM. Rodent models for compulsive alcohol intake. Alcohol. 2014;48:253–64.
Hopf FW. Do specific NMDA receptor subunits act as gateways for addictive behaviors? Genes Brain Behav. 2017;16:118–38.
Naqvi NH, Gaznick N, Tranel D, Bechara A. The insula: a critical neural substrate for craving and drug seeking under conflict and risk. Ann N Y Acad Sci. 2014;1316:53–70.
Wilcox CE, Pommy JM, Adinoff B. Neural circuitry of impaired emotion regulation in substance use disorders. Am J Psychiatry. 2016;173:344–61.
Chen NM, Lasek AM. Perineuronal nets in the insula regulate aversion-resistant alcohol drinking. Addict Biol. 2020;25:e12821–32.
Seif T, Chang SJ, Simms JA, Gibb SL, Dadgar J, Chen BT, et al. Cortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intake. Nat Neurosci. 2013;16:1094–100.
Jaramillo AA, Randall PA, Stewart S, Fortino B, Van Voorhies K, Besheer J. Functional role for cortical-striatal circuitry in modulating alcohol self-administration. Neuropharmacology. 2018;130:42–53.
Seif T, Simms JA, Lei K, Wegner S, Bonci A, Messing RO, et al. D-serine and D-cycloserine reduce compulsive alcohol intake in rats. Neuropsychopharmacology. 2015;40:2357–67.
Grodin EN, Sussman L, Sundby K, Brennan GM, Diazgranados N, Heilig M, et al. Neural correlates of compulsive alcohol seeking in heavy drinkers. Biol Psych Cogn Neuro Neuroimag. 2018;2:1022–31.
Arcurio LR, Finn PR, James TW. Neural mechanisms of high-risk decisions-to-drink in alcohol-dependent women. Addict Biol. 2015;20:390–406.
Tiffany ST, Conklin CA. A cognitive processing model of alcohol craving and compulsive alcohol use. Addiction. 2000;95 Suppl 2:S145–53.
Gehrlach DA, Weiand C, Gaitanos TN, Cho E, Klein AS, Hennrich AA, et al. A whole-brain connectivity map of mouse insular cortex. Elife. 2020;9:e55585–610.
Valentino RJ, Van, Bockstaele E. Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur J Pharm. 2008;583:194–203.
Vazey EM, den Hartog CR, Moorman DE. Central noradrenergic interactions with alcohol and regulation of alcohol-related behaviors. Handb Exp Pharm. 2018;248:239–60.
Luppi PH, Aston-Jones G, Akaoka H, Chouvet G, Jouvet M. Afferent projections to the rat locus coeruleus demonstrated by retrograde and anterograde tracing with cholera-toxin B subunit and Phaseolus vulgaris leucoagglutinin. Neuroscience. 1995;65:119–60.
Clavier RM. Afferent projections to the self-stimulation regions of the dorsal pons, including the locus coeruleus, in the rat as demonstrated by the horseradish peroxidase technique. Brain Res Bull. 1979;4:497–504.
Yasui Y, Breder CD, Saper CB, Cechetto DF. Autonomic responses and efferent pathways from the insular cortex in the rat. J Comp Neurol. 1991;303:355–74.
Cedarbaum JM, Aghajanian GK. Afferent projections to the rat locus coeruleus as determined by a retrograde tracing technique. J Comp Neurol. 1978;178:1–16.
Sinha R, Wemm S, Fogelman N, Milivojevic V, Morgan PM, Angarita GA, et al. Moderation of Prazosin’s efficacy by alcohol withdrawal symptoms. Am J Psychiatry. 2020;178:447–58.
Wilcox CE, Adinoff B, Clifford J, Ling J, Witkiewitz K, Mayer AR, et al. Brain activation and subjective anxiety during an anticipatory anxiety task is related to clinical outcome during prazosin treatment for alcohol use disorder. Neuroimage Clin. 2020;26:102162.
Skelly MJ, Weiner JL. Chronic treatment with prazosin or duloxetine lessens concurrent anxiety-like behavior and alcohol intake: evidence of disrupted noradrenergic signaling in anxiety-related alcohol use. Brain Behav. 2014;4:468–83.
Le AD, Funk D, Juzytsch W, Coen K, Navarre BM, Cifani C, et al. Effect of prazosin and guanfacine on stress-induced reinstatement of alcohol and food seeking in rats. Psychopharmacology. 2012;218:89–99.
Walker BM, Rasmussen DD, Raskind MA, Koob GF. alpha1-noradrenergic receptor antagonism blocks dependence-induced increases in responding for ethanol. Alcohol. 2008;42:91–7.
Verplaetse TL, Rasmussen DD, Froehlich JC, Czachowski CL. Effects of prazosin, an alpha1-adrenergic receptor antagonist, on the seeking and intake of alcohol and sucrose in alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2012;36:881–6.
Rasmussen DD, Beckwith LE, Kincaid CL, Froehlich JC. Combining the alpha1-adrenergic receptor antagonist, prazosin, with the beta-adrenergic receptor antagonist, propranolol, reduces alcohol drinking more effectively than either drug alone. Alcohol Clin Exp Res. 2014;38:1532–9.
Darevsky D, Gill TM, Vitale KR, Hu B, Wegner SA, Hopf FW. Drinking despite adversity: behavioral evidence for a head down and push strategy of conflict-resistant alcohol drinking in rats. Addict Biol. 2019;24:426–37.
Darevsky D, Hopf FW. Behavioral indicators of succeeding and failing under higher-challenge compulsion-like alcohol drinking in rat. Behav Brain Res. 2020;393:112768.
Wegner SA, Hu B, De Oliveira Sergio T, Darevsky D, Kwok CC, Lei K, et al. A novel NMDA receptor-based intervention to suppress compulsion-like alcohol drinking. Neuropharmacology. 2019;157:107681.
Fuchs RA, Evans KA, Parker MP, See RE. Differential involvement of orbitofrontal cortex subregions in conditioned cue-induced and cocaine-primed reinstatement of cocaine seeking in rats. J Neurosci. 2004;24:6600–10.
Li X, Zeric T, Kambhampati S, Bossert JM, Shaham Y. The central amygdala nucleus is critical for incubation of methamphetamine craving. Neuropsychopharmacology. 2015;40:1297–306.
Lei K, Kwok C, Darevsky D, Wegner SA, Yu J, Nakayama L, et al. Nucleus accumbens shell Orexin-1 receptors are critical mediators of binge intake in excessive-drinking individuals. Front Neurosci. 2019;13:88.
den Hartog C, Zamudio-Bulcock P, Nimitvilai S, Gilstrap M, Eaton B, Fedarovich H, et al. Inactivation of the lateral orbitofrontal cortex increases drinking in ethanol-dependent but not non-dependent mice. Neuropharmacology. 2016;107:451–9.
Hopf FW, Mangieri RA. Do alcohol-related AMPA-type glutamate receptor adaptations promote intake? Handb Exp Pharm. 2018;248:157–86.
McBain CJ, Fisahn A. Interneurons unbound. Nat Rev Neurosci. 2001;2:11–23.
Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–50.
Varazzani C, San-Galli A, Gilardeau S, Bouret S. Noradrenaline and dopamine neurons in the reward/effort trade-off: a direct electrophysiological comparison in behaving monkeys. J Neurosci. 2015;35:7866–77.
Jahn CI, Gilardeau S, Varazzani C, Blain B, Sallet J, Walton ME, et al. Dual contributions of noradrenaline to behavioural flexibility and motivation. Psychopharmacology. 2018;235:2687–702.
Ullsperger M, Harsay HA, Wessel JR, Ridderinkhof KR. Conscious perception of errors and its relation to the anterior insula. Brain Struct Funct. 2010;214:629–43.
Datta D, Yang ST, Galvin VC, Solder J, Luo F, Morozov YM, et al. Noradrenergic alpha1-adrenoceptor actions in the primate dorsolateral prefrontal cortex. J Neurosci. 2019;39:2722–34.
Berridge CW, Shumsky JS, Andrzejewski ME, McGaughy JA, Spencer RC, Devilbiss DM, et al. Differential sensitivity to psychostimulants across prefrontal cognitive tasks: differential involvement of noradrenergic alpha(1)- and alpha(2)-receptors. Biol Psychiatry. 2012;71:467–73.
Berridge CW, Spencer RC. Differential cognitive actions of norepinephrine a2 and a1 receptor signaling in the prefrontal cortex. Brain Res. 2016;1641:189–96.
Porter BS, Li K, Hillman KL. Regional activity in the rat anterior cingulate cortex and insula during persistence and quitting in a physical-effort task. eNeuro. 2020;7:ENEURO.0243-20.2020.
Lustberg D, Iannitelli AF, Tillage RP, Pruitt M, Liles LC, Weinshenker D. Central norepinephrine transmission is required for stress-induced repetitive behavior in two rodent models of obsessive-compulsive disorder. Psychopharmacology. 2020;237:1973–87.
Owen SF, Liu MH, Kreitzer AC. Thermal constraints on in vivo optogenetic manipulations. Nat Neurosci. 2019;22:1061–5.
Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–24.
Alnaes D, Sneve MH, Espeseth T, Endestad T, van de Pavert SH, Laeng B. Pupil size signals mental effort deployed during multiple object tracking and predicts brain activity in the dorsal attention network and the locus coeruleus. J Vis. 2014;14:1–20.
Puumala T, Riekkinen P Sr, Sirvio J. Modulation of vigilance and behavioral activation by alpha-1 adrenoceptors in the rat. Pharm Biochem Behav. 1997;56:705–12.
Schmidt KT, Schroeder JP, Foster SL, Squires K, Smith BM, Pitts EG, et al. Norepinephrine regulates cocaine-primed reinstatement via alpha1-adrenergic receptors in the medial prefrontal cortex. Neuropharmacology. 2017;119:134–40.
Forget B, Wertheim C, Mascia P, Pushparaj A, Goldberg SR, Le Foll B. Noradrenergic alpha1 receptors as a novel target for the treatment of nicotine addiction. Neuropsychopharmacology. 2010;35:1751–60.
Do-Monte FH, Souza RR, Wong TT, Carobrez Ade P. Systemic or intra-prelimbic cortex infusion of prazosin impairs fear memory reconsolidation. Behav Brain Res. 2013;244:137–41.
Fox HC, Anderson GM, Tuit K, Hansen J, Kimmerling A, Siedlarz KM, et al. Prazosin effects on stress- and cue-induced craving and stress response in alcohol-dependent individuals: preliminary findings. Alcohol Clin Exp Res. 2012;36:351–60.
Funk D, Stewart J. Role of catecholamines in the frontal cortex in the modulation of basal and stress-induced autonomic output in rats. Brain Res. 1996;741:220–9.
Ye L, Orynbayev M, Zhu X, Lim EY, Dereddi RR, Agarwal A, et al. Ethanol abolishes vigilance-dependent astroglia network activation in mice by inhibiting norepinephrine release. Nat Commun. 2020;11:6157.
Venniro M, Caprioli D, Zhang M, Whitaker LR, Zhang S, Warren BL, et al. The anterior insular cortex->central amygdala glutamatergic pathway is critical to relapse after contingency management. Neuron. 2017;96:414–27.e8.
Arguello AA, Wang R, Lyons CM, Higginbotham JA, Hodges MA, Fuchs RA. Role of the agranular insular cortex in contextual control over cocaine-seeking behavior in rats. Psychopharmacology. 2017;234:2431–41.
Rotge JY, Cocker PJ, Daniel ML, Belin-Rauscent A, Everitt BJ, Belin D. Bidirectional regulation over the development and expression of loss of control over cocaine intake by the anterior insula. Psychopharmacology. 2017;234:1623–31.
Mendez-Ruette M, Linsambarth S, Moraga-Amaro R, Quintana-Donoso D, Mendez L, Tamburini G, et al. The role of the rodent insula in anxiety. Front Physiol. 2019;10:330.
Neill DB. Frontal–striatal control of behavioral inhibition in the rat. Brain Res. 1976;105:89–103.
Campbell EJ, Flanagan JPM, Walker LC, Hill M, Marchant NJ, Lawrence AJ. Anterior insular cortex is critical for the propensity to relapse following punishment-imposed abstinence of alcohol seeking. J Neurosci. 2019;39:1077–87.
Jaramillo AA, Agan VE, Makhijani VH, Pedroza S, McElligott ZA, Besheer J. Functional role for suppression of the insular-striatal circuit in modulating interoceptive effects of alcohol. Addict Biol. 2018;23:1020–31.
Joshi DD, Puaud M, Fouyssac M, Belin-Rauscent A, Everitt B, Belin D. The anterior insular cortex in the rat exerts an inhibitory influence over the loss of control of heroin intake and subsequent propensity to relapse. Eur J Neurosci. 2020;52:4115–26.
Haaranen M, Scuppa G, Tambalo S, Jarvi V, Bertozzi SM, Armirotti A, et al. Anterior insula stimulation suppresses appetitive behavior while inducing forebrain activation in alcohol-preferring rats. Transl Psychiatry. 2020;10:150.
Haaranen M, Schafer A, Jarvi V, Hyytia P. Chemogenetic stimulation and silencing of the insula, amygdala, nucleus accumbens, and their connections differentially modulate alcohol drinking in rats. Front Behav Neurosci. 2020;14:580849.
Siciliano CA, Noamany H, Chang CJ, Brown AR, Chen X, Leible D, et al. A cortical-brainstem circuit predicts and governs compulsive alcohol drinking. Science. 2019;366:1008–12.
Alves FH, Crestani CC, Resstel LB, Correa FM. Both alpha1- and alpha2-adrenoceptors in the insular cortex are involved in the cardiovascular responses to acute restraint stress in rats. PLoS ONE. 2014;9:e83900.
Alves FH, Crestani CC, Resstel LB, Correa FM. Cardiovascular effects of noradrenaline microinjected into the insular cortex of unanesthetized rats. Auton Neurosci. 2011;160:90–8.
Ventura R, De Carolis D, Alcaro A, Puglisi-Allegra S. Ethanol consumption and reward depend on norepinephrine in the prefrontal cortex. Neuroreport. 2006;17:1813–7.
Rojas S, Diaz-Galarce R, Jerez-Baraona JM, Quintana-Donoso D, Moraga-Amaro R, Stehberg J. The insula modulates arousal-induced reluctance to try novel tastes through adrenergic transmission in the rat. Front Behav Neurosci. 2015;9:164.
Robertson SD, Plummer NW, Jensen P. Uncovering diversity in the development of central noradrenergic neurons and their efferents. Brain Res. 2016;1641:234–44.
Drouin C, Blanc G, Trovero F, Glowinski J, Tassin JP. Cortical alpha 1-adrenergic regulation of acute and sensitized morphine locomotor effects. Neuroreport. 2001;12:3483–6.
Darracq L, Blanc G, Glowinski J, Tassin JP. Importance of the noradrenaline–dopamine coupling in the locomotor activating effects of D-amphetamine. J Neurosci. 1998;18:2729–39.
Kang S, Li J, Zuo W, Fu R, Gregor D, Krnjevic K, et al. Ethanol withdrawal drives anxiety-related behaviors by reducing M-type potassium channel activity in the lateral habenula. Neuropsychopharmacology. 2017;42:1813–24.
Fu R, Mei Q, Shiwalkar N, Zuo W, Zhang H, Gregor D, et al. Anxiety during alcohol withdrawal involves 5-HT2C receptors and M-channels in the lateral habenula. Neuropharmacology. 2020;163:107863.
Albrechet-Souza L, Schratz CL, Gilpin NW. Sex differences in traumatic stress reactivity in rats with and without a history of alcohol drinking. Biol Sex Differ. 2020;11:27.
George O, Sanders C, Freiling J, Grigoryan E, Vu S, Allen CD, et al. Recruitment of medial prefrontal cortex neurons during alcohol withdrawal predicts cognitive impairment and excessive alcohol drinking. Proc Natl Acad Sci U S A. 2012;109:18156–61.
Li J, Bian W, Dave V, Ye JH. Blockade of GABA(A) receptors in the paraventricular nucleus of the hypothalamus attenuates voluntary ethanol intake and activates the hypothalamic-pituitary-adrenocortical axis. Addict Biol. 2011;16:600–14.
Steensland P, Fredriksson I, Holst S, Feltmann K, Franck J, Schilstrom B, et al. The monoamine stabilizer (-)-OSU6162 attenuates voluntary ethanol intake and ethanol-induced dopamine output in nucleus accumbens. Biol Psychiatry. 2012;72:823–31.
Acknowledgements
We thank Sarah Wean and Drs Andrea Jones and Simon Katner for assistance with intracranial prazosin experiments, and Molly Sazer-Hopf for help with brain images.
Author information
Authors and Affiliations
Contributions
TDOS, KL, SAW, and FWH designed the experiments; TDOS, KL, CK, SG, SAW, MW, JW, and FWH performed the experiments; TDOS, KL, SAW, DD, and FWH analyzed the data; TDOS and FWH wrote a first draft of the manuscript, all authors edited the manuscript and approved the final version. Raw data are available upon request.
Corresponding author
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
De Oliveira Sergio, T., Lei, K., Kwok, C. et al. The role of anterior insula–brainstem projections and alpha-1 noradrenergic receptors for compulsion-like and alcohol-only drinking. Neuropsychopharmacol. 46, 1918–1926 (2021). https://doi.org/10.1038/s41386-021-01071-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-021-01071-w