Abstract
With the increasing prevalence of legal cannabis use and availability, there is an urgent need to identify cognitive impairments related to its use. It is widely believed that cannabis, or its main psychoactive component Δ9-tetrahydrocannabinol (THC), impairs working memory, i.e., the ability to temporarily hold information in mind. However, our review of the literature yielded surprisingly little empirical support for an effect of THC or cannabis on working memory. We thus conducted a study with three main goals: (1) quantify the effect of THC on visual working memory in a well-powered sample, (2) test the potential role of cognitive effects (mind wandering and metacognition) in disrupting working memory, and (3) demonstrate how insufficient sample size and task duration reduce the likelihood of detecting a drug effect. We conducted two double-blind, randomized crossover experiments in which healthy adults (N = 23, 23) performed a reliable and validated visual working memory task (the “Discrete Whole Report task”, 90 trials) after administration of THC (7.5 and/or 15 mg oral) or placebo. We also assessed self-reported “mind wandering” (Exp 1) and metacognitive accuracy about ongoing task performance (Exp 2). THC impaired working memory performance (d = 0.65), increased mind wandering (Exp 1), and decreased metacognitive accuracy about task performance (Exp 2). Thus, our findings indicate that THC does impair visual working memory, and that this impairment may be related to both increased mind wandering and decreased monitoring of task performance. Finally, we used a down-sampling procedure to illustrate the effects of task length and sample size on power to detect the acute effect of THC on working memory.
Similar content being viewed by others
Introduction
Cannabis and its main psychoactive constituent, Δ9-tetrahydrocannabinol (THC), are widely believed to impair working memory, the mental workspace used to hold information “in mind” that is needed for everyday behaviors such as driving and problem solving [1, 2]. Disruptions to working memory can thus disrupt ongoing behavior and lead to negative outcomes, from the innocuous (e.g., forgetting that your turn-signal is on) to the dire (e.g., hitting the cyclist you forgot was in your blind spot). Thus, understanding the acute effects of THC on working memory and cognition is of critical importance for public health and safety.
Despite the popular belief that THC impairs working memory, this impairment has been difficult to demonstrate under controlled conditions. Here we first reviewed 40 within-subjects, randomized, placebo-controlled studies that tested the acute effect of THC on working memory performance and then conducted a controlled laboratory study. Examining published Digit Span studies from 1970 to 2019, we found that >70% of 57 study conditions failed to detect an effect of the drug (p > 0.05). This difficulty in demonstrating the effects of THC on working memory in controlled studies [3,4,5,6,7,8] is especially notable given the bias toward publishing “positive” results [9,10,11], which should inflate reports of positive effects in the literature. We hypothesized that the lack of empirical support for impairment of working memory after THC reflects limitations of previous studies related to insufficient sample sizes and trial counts. We present the results of a study that addresses these problems.
Prior studies have predominantly used one of a few canonical working memory tasks in which subjects must remember verbal material such as letters or numbers over a short delay (e.g., “Digit Span” [12]). However, over the past 20 years, tasks that test memory for visual information (e.g., colors, shapes, and objects) have emerged as popular and robust measures of working memory functioning that are less susceptible to chunking or other strategic factors that are known to impact verbal working memory measures [13, 14]. These visual working memory tasks have been demonstrated to be highly reliable [15, 16] (specific to the Discrete Whole Report task, previously reported reliabilities include: Cronbach’s α for stability of performance across blocks > 0.9 [17], split-half reliability, with training sessions over multiple weeks, r = 0.60–0.86 [18], current study split-half reliability r ≥ 0.90), correlate well with other measures of working memory [19, 20], and predict individual differences in general fluid intelligence [19]. Further, these tasks have been deployed with a variety of patient populations [21,22,23,24], have been extensively examined using electroencephalography (EEG) and functional magnetic resonance imaging (fMRI) [25,26,27,28,29], and are simple enough to lend themselves to cross-species translational studies [30]. Here we used a specific variant of a visual working memory task, the “Discrete Whole Report” task [17, 31], to investigate the acute effects of THC on visual working memory performance. A particular advantage of this task over other highly similar tasks (e.g., partial-report [32, 33] and change detection [34, 35]) and other more distantly related visual WM tasks (e.g., spatial N-back [36]) is that it provides sensitive trial-by-trial measurements of performance [17, 37, 38] and is thus well-suited for characterizing deficits in the ability to store information.
We hypothesized that two key factors (sample size and task duration) affect power to detect working memory drug effects in a task-generalizable manner. To test this hypothesis, we performed simulations in which we tested the effect of sample size and task duration (i.e., number of trials) on power in our visual working memory sample and we compared the predictions of these simulations with results observed in the literature (specifically, the Digit Span literature). Our simulations focus primarily on understanding the task-generalizable effect of sample size and task duration on power. However, the working memory tasks in the reviewed literature differ in other regards, which we later discuss.
We also examined self-reports of mind wandering (Exp 1) and metacognition (Exp 2), processes that may be modulated by the acute effect of THC. In Experiment 1, we tested whether a tendency to have one’s mind “off-task” (i.e., “mind wandering” or “zoning out”) [39] co-occurs with decreased working memory performance and the acute administration of THC. Whereas mind wandering and decreased awareness of mind wandering are known to occur during nicotine withdrawal [40] and alcohol intoxication [41], little is known about the effect of THC on mind wandering during the ongoing task performance. In Experiment 2, we tested whether poor metacognition (i.e., performance monitoring) likewise co-occurs with behavioral decrements and the acute administration of THC. Prior work has found acute administration of THC decreased performance monitoring in a simple visual attention task [42, 43]. Here we tested whether acute administration of THC likewise disrupts performance monitoring during a working memory task using metacognitive accuracy of task performance [44] as an index of performance monitoring.
The current study had three key goals that together test the effect of THC on visual working memory. First, we sought to characterize the effects of THC on visual working memory using a longer than typical task (90 trials vs. ~15 trials) and higher than typical sample size (combined n = 46 vs. n = ~15). Second, we tested visual working memory performance in relation to other ongoing cognitive processes, specifically increased mind wandering (Exp 1) and decreased metacognitive accuracy of task performance (Exp 2). Finally, we examined previous studies on the effect of THC on working memory, to determine whether previous failures to detect effects could be related to insufficient power. To this end, we combined our literature review with a down-sampling procedure on our own, well-powered sample (achieved power > 0.99), to determine the consequence of inadequate sample sizes and task lengths on the ability to detect a working memory impairment (d = 0.65).
Methods
Participants
Healthy occasional (non-daily) cannabis users, aged 18–35 years, were recruited for two experiments. Sample sizes were set a priori to n = 24 per study. Procedures were approved by the University of Chicago Institutional Review Board and participants provided written, informed consent. Studies took place within the Human Behavioral Pharmacology lab at the University of Chicago. Participants were screened with a physical examination, an electrocardiogram, and a semi-structured interview by a clinical psychologist. Exclusion criteria included any current Axis I DSM-IV disorder including substance dependence, current use of >5 tobacco cigarettes per day, history of psychosis or mania, less than a high school education, lack of English fluency, a body mass index outside 19–33 kg/m2, high blood pressure (>140/90), abnormal electrocardiogram, daily use of any medication other than birth control, pregnancy, or lactating. Cannabis use was assessed in an in-person interview. Inclusion criteria for Experiment 1 were lifetime use between 4 and 100 times and non-daily use, and for Experiment 2 some lifetime use but not daily use. Equal numbers of men and women participated in both Experiments and their mean ages were 23.0 years (SD = 3.6) in Experiment 1 and 23.4 years (SD = 4.3) in Experiment 2. Data from one subject in each study were excluded because of extreme values (>3 SDs below the mean).
Drug
THC (Marinol®; Solvay Pharmaceuticals) was placed in opaque, size 00 capsules with dextrose filler. Placebo capsules contained only dextrose (0 mg THC). Chosen doses produce reliable subjective and cardiovascular effects without adverse effects [45, 46]. Experiment 1: Participants received a placebo capsule and a 15 mg capsule in two randomized, counterbalanced sessions. Experiment 2: Participants received a placebo capsule, a 7.5 mg capsule, and a 15 mg capsule in three randomized, counterbalanced sessions.
Design
Subjects participated in two (Experiment 1) or three (Experiment 2) sessions, conducted at least 1 week apart, in a comfortable laboratory setting. Both experiments used double-blind, within-subjects, counterbalanced designs. A lab member that knew the design of the study but was not running participants performed randomization (via a computerized list shuffle method) and sorted placebo and THC capsules into bags (i.e., bags labeled “first capsule” and “second capsule” in Experiment 1; bags labeled “first capsule”, “second capsule” and “third capsule” in Experiment 2).
Procedures
Pre-session
During an orientation session subjects received instructions, signed a consent form, and practiced the tasks. They were instructed to consume their normal amount of caffeine and nicotine, but to abstain from alcohol, prescription drugs (except contraceptives), over-the-counter-drugs, cannabis, and other illicit drugs for at least 48 h before session. Participants were informed that they would be tested for recent drug use at the beginning of each session and positive tests would result in rescheduling or dismissal. Finally, they were advised to get their normal amounts of sleep and to not eat for 2 h prior to each experimental session. To minimize expectancy effects, participants were informed that they may receive a stimulant, sedative, cannabinoid, or placebo during the sessions.
Experimental sessions
At the beginning of each laboratory visit, subjects provided breath and urine samples for breath alcohol level (Alco-sensor III, Intoximeters, St. Louis, MO), a urine drug test (ToxCup, Branan Medical Co., Irvine, CA), and a pregnancy test (females only; Aimstrip, Craig Medical, Vista, CA). Those testing positive were rescheduled or dropped from the study. Baseline cardiovascular and mood measures were taken, then participants consumed the capsule (placebo or THC, double-blind). During the first 120 min, participants relaxed with magazines and music while the drug was absorbed. Cardiovascular and mood measures were taken at regular intervals (every 30–60 min) throughout the session. Cognitive testing was conducted from 120 to 220 min post capsule intake. These time points fall in the time range where subjective and behavioral effects of the drug have reached their peak and remain elevated [47]. The key cognitive test of interest was the Discrete Whole Report task (see section “Visual Working Memory Task”, [17, 26, 31]). During the Whole Report Task, participants also reported ratings of their level of mind wandering (Experiment 1) or metacognition (Experiment 2). In Experiment 1 the working memory task was performed at around 160 min post capsule intake (M = 159.4, SD = 14.5, Range = [126,193]) and in Experiment 2 it was performed 220 min post capsule intake (exact time not recorded). In both experiments, subjects also completed other tasks that are reported elsewhere [48,49,50] that were not expected to interfere with the task reported here.
Physiological and subjective measures
In both experiments, heart rate and blood pressure were recorded at regular time points (every ~30 min, Supplementary Methods) with portable monitors (Experiment 1: A&D Medical/Life Source, San Jose, CA; Experiment 2: Omron 10 Plus, Omron Healthcare). Self-report measures of the drug effects were obtained at the same times. These have been reported elsewhere and included the Addiction Research Center Inventory (ARCI) [51, 52], the Visual Analog Scales [VAS] [53], the Drug Effects Questionnaire [DEQ] [54], and an End of Session Questionnaire (Experiment 1 only). The results of all subjective measures are reported in the main text and/or Supplementary Results, and descriptions of the subjective measures are given in the Supplementary Methods.
Visual working memory task
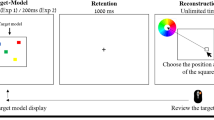
The visual working memory (“Discrete Whole Report”) task consisted of 90 trials (3 blocks of 30) [17, 31]. On each trial, participants briefly viewed (200 ms) an array of six brightly colored squares and remembered the colors and locations of the squares across a delay with a blank screen (1000 ms; see Fig. 1). Colors for each trial were chosen without replacement from a set of nine highly discriminable colors [17]. At test, “response grids” appeared at each location (3 × 3 grid of all nine colors). Participants freely recalled the color–location pairing of each item by clicking the color in each response grid that corresponded to the color remembered at that location. They were required to make a response to all six squares before moving on to the next trial. In both experiments, participants also provided self-reported measures about their performance throughout the task (“Task-Unrelated Thoughts” or “Item-level Confidence Judgments”).
On each trial, participants briefly view a memory array containing six colored squares (Memory array). Participants remember the colors across a 1000 ms delay and report them at test (Response). Participants may report the colors of the items in any order that they choose and they must make a response for all six locations. In the figure, the participant is clicking the magenta sub-section of the response grid to indicate that the remembered square in the bottom right corner was magenta.
Task-unrelated thoughts
In Experiment 1, participants were asked, on 20% of trials, about the contents of their thoughts “at the moment”, choosing between three categories as follows: “on task”, “mind wandering” or “zoning out”. Participants were given instructions and examples of each category during the orientation pre-session. In the instructions, the categories were defined as follows: (1) “on task” indicates that the subject was focused on the task at hand, (2) “mind wandering” indicates that the subject was internally focused on something other than the task, and (3) “zoning out” indicates that the subject was withdrawn and not allocated to anything in particular. If subjects endorsed “mind wandering”, they were asked to classify whether their mind had wandered toward the future, the past, “other”, or “I don’t know”. Although the terms “mind wandering” and “zoning out” are sometimes used interchangeably (e.g., see ref. [40]), here we distinguished between periods of internally directed attention (“mind wandering”) and periods where there is a complete absence of attention to anything in particular (“zoning out”, also referred to as “mind blanking” [55]). The zoning-out rating thus differentiates an increase in internally directed attention from a total disengagement of attention.
Item-level confidence judgments
In Experiment 2, in addition to reporting the color, participants made a binary confidence judgment for each response. While making their responses about the color of each object, they indicated confidence by clicking on the chosen color with either the left or right mouse button. If they felt they had “some information” in mind about the color of the item they were reporting, they should click the color with the left mouse button. If they felt they had “no information” in mind about the color of the item they were reporting, they should click the color with the right mouse button. The number of confident items was calculated for each trial by summing the number of left click responses (ranging from 0 to 6 confident responses per trial). To calculate metacognitive accuracy, we correlated the number of confident responses per trial with the number of accurate responses per trial (e.g., number of items where the correct color was chosen on each trial, ranging from 0 to 6 correct items per trial).
Statistical analysis
Data analysis was performed using JASP (Version 0.11.1) [56] and custom scripts in MATLAB 2018A (The MathWorks, Natick, MA, USA). Data sets for all experiments are available on the Open Science Framework at https://osf.io/5heur/. To assess subjective and physiological measures at the time of the working memory test, we calculated a change score from baseline (time point closest to the working memory test minus the time point immediately before consuming the capsule). In Experiment 1, One participants’ heart rate and blood pressure could not be collected due to device malfunction, leaving 22 participants, and one participants’ subjective measures could not be collected due to a computer malfunction, leaving 22 participants. Significance of placebo vs. THC was tested by paired t-test (two-tailed) in Experiment 1 (placebo vs. 15 mg THC) and tested by one-way repeated-measures analysis of variance (ANOVA) in Experiment 2 (within-subjects factor Drug containing three levels: Placebo, 7.5 mg THC, and 15 mg THC).
Literature review and power analysis
We reviewed the literature to find within-subjects, randomized, placebo-controlled studies testing the acute effect of THC on working memory performance (Supplementary Methods). By far, the most common test of working memory was the Digit Span (Forward/Backward) Task. We found 15 papers meeting our inclusion criteria that reported the results of a standard Digit Span task [45, 57,58,59,60,61,62,63,64,65,66,67,68,69,70]. Together, these papers reported a total of 57 different conditions that were tested (e.g., Forward vs. Backward span, differing doses of THC). We did not include conditions from papers that reported only combined Digit Span (i.e., forward and reverse not separately reported) [71] or conditions measuring Digit Recall instead of Span [72,73,74,75,76]. These and other tasks show consistent patterns (Supplementary Results), but we chose to focus on only Digit Span conditions for our core arguments, because this task is the single most-used task and is administered in a highly consistent manner. See Supplementary Results for the largely nonsignificant p-values across conditions for other working measures in the literature.
Results
Demographic information, subjective measures, and physiological measures
Demographic characteristics of participants are shown in Table 1. Mean values for each of the physiological and subjective measures are shown in Supplementary Tables S4 and S5.
THC produced its expected effects on physiological and subjective measures. THC (15 mg) increased heart rate, including at the time of the working memory test in both Exp 1, t(21) = 3.55, p = 0.002, d = 0.76, and Exp 2, F(2,44) = 12.16, p < 0.001. Only one dose (15 mg) was used in Exp 1. In Exp 2 (15 mg, 7.5 mg), there was a linear effect of dose (p < 0.001), but only the high dose was significantly different from placebo (high vs. placebo p < 0.001, low vs. placebo p = 0.25). The drug did not affect systolic or diastolic blood pressure (p > 0.3). See Supplementary Results for tables of all values. THC also increased scores on the “marijuana scale” of the ARCI (p < 0.001) and the “Feel” (p < 0.001), “Like” (p ≤ 0.005), “Dislike” (p ≤ 0.03), and “High” (p < 0.001) questions of the DEQ (Bonferroni-corrected for the five DEQ measures). The drug increased “Want more” ratings of the DEQ (p = 0.002; p = 0.197) and VAS measures “Sociable” and “Friendly” (p < 0.05, Bonferroni-corrected for 13 VAS measures) in Exp 1, but not in Exp 2. See Supplementary Results for tables of all values.
Mean working memory performance
THC impaired working memory performance relative to placebo in both Exp 1 (Fig. 2a) and Exp 2 (Fig. 2b). In Exp 1, participants correctly reported an average of 3.11 (SD = 0.49) items in the placebo condition and 2.77 (SD = 0.50) items in the 15 mg THC condition, t(22) = 3.72, p = 0.001, d = 0.78. In Exp 2, participants correctly reported an average of 3.02 (SD = 0.53) items in the placebo condition, 2.84 (SD = 0.44) items in the 7.5 mg THC condition, and 2.78 (SD = 0.54) items in the 15 mg THC condition, F(1.52,33.42) = 4.58, p = 0.026, ηp2 = 0.17 (Greenhouse–Geisser-corrected values are reported whenever the assumption of sphericity is violated). Although polynomial contrasts revealed a linear effect of dose in Exp 2 (p = 0.005), only the high dose was significantly different from placebo (placebo vs. high, p = 0.018; placebo vs. low, p = 0.07).
Mean values for the number of items correctly identified in Exp 1 (a; N = 23), Exp 2 (b; N = 23), and the two experiments combined (c; N = 46). Here and elsewhere, violin plots show the distribution of participants, black error bars represent 1 SEM, and transparent gray lines show individual participants. THC significantly reduced the number of remembered items in the 15 mg conditions.
In a separate analysis, we combined the working memory performance data from Experiments 1 and 2. A unique set of participants was recruited for Experiments 1 and 2; hence, this pooling did not result in multiple data points from the same participants. As only Experiment 2 had the 7.5 mg THC condition, this condition was discarded from pooled analyses. We observed the same main effect of THC on working memory performance (comparing the combined placebo condition with the combined 15 mg THC condition; Fig. 2c). A mixed ANOVA with within-subjects factor Drug and between-subjects factor Experiment revealed no main effect of Experiment, F(1,44) = 0.09, p = 0.76, ηp2 = 0.002, and no interaction between Drug and Experiment, F(1,44) = 0.52, p = 0.48, ηp2 = 0.01; hence, this combination of experiments is justified. This combination of experiments yields a total sample size of 46 subjects and a robust effect of Drug on working memory performance (Fig. 2c), t(45) = 4.43, p < 0.001, d = 0.65. With this larger sample size, we quantified reliability, the effect of experimental block, and the effects of response number on both accuracy and response time. Task reliability (even–odd correlation) was excellent during both the placebo (r = 0.91) and the THC (r = 0.90) conditions, and individual differences in performance were preserved across the THC and Placebo conditions, as shown by a positive correlation (Fig. 3a; r = 0.63, p < 0.001, 95% confidence interval [0.41, 0.78]).
a Correlation between mean number correct on the working memory task after placebo and THC (15 mg) conditions. This shows that individual differences are reliable across the drug and placebo conditions, but that most individuals are impaired by the drug. b Mean number of items correctly identified during the three blocks of the task after placebo and THC (15 mg). Performance was consistently poorer after THC across all three blocks. c Working memory performance as a function of response number and drug. Responses were overall less accurate for THC vs. placebo, particularly early in the trial. d Cumulative response time as a function of response number and drug indicates that impaired performance was not due to a speed-accuracy trade-off; participants were overall slower for THC vs. placebo.
Changes in working memory performance across experimental blocks and individual responses
To determine whether the effect of THC on working memory performance was related to a decline in effort or task engagement over the course of the experiment, we compared performance across the three blocks of the experiment (30 trials per block). The difference between THC and placebo scores was constant over time (Fig. 3b). A repeated-measures ANOVA with factors Drug and Block revealed no main effect of Block, F(2,90) = 2.69, p = 0.074, ηp2 = 0.06, and no interaction between Drug and Block, F(1.73,77.98) = 1.60, p = 0.210, ηp2 = 0.03.
To determine whether the effect of THC on working memory was related to careless responding and a speed-accuracy trade-off, we examined response time and accuracy for each response in the trial. If participants were simply more careless at responding in the THC condition, then they may have responded quickly and with poor accuracy. Thus, if the THC-related working memory decrement is driven by a speed-accuracy trade-off, we should observe faster response times for trials where participants showed lower accuracy. The empirical data did not support a speed-accuracy trade-off account. Accuracy was overall lower in the Drug condition, particularly for the first three responses. There was a main effect of Drug, F(1,45) = 19.64, p < 0.001, ηp2 = 0.30, a main effect of Response Number, F(2.44,109.58) = 909.07, p < 0.001, ηp2 = 0.95, and an interaction between Drug and Response Number, F(3.68,165.62) = 4.38, p = 0.003, ηp2 = 0.09. However, poorer accuracy was not associated with faster response times. Instead, response times were actually slower overall. A repeated-measures ANVOA examining response times showed a main effect of Drug, F(1,45) = 21.68, p = 0.014, ηp2 = 0.13, a main effect of Response Number, F(1.13,50.99) = 1026.79, p < 0.001, ηp2 = 0.96, and a significant interaction between Response Number and Drug, F(1.13,50.64 = 11.61, p < 0.001, ηp2 = 0.21.
Effects of THC on mind wandering during the task (Exp 1)
In Exp 1 only, we used “thought probes” to assay the contents of participants’ thoughts while performing the working memory task (Fig. 4a). THC significantly reduced reports of being On Task t(22) = 5.08, p < 0.001, d = 1.06 and increased frequency of both Mind Wandering t(22) = 4.42, p < 0.001, d = 0.92, and Zoning Out, t(22) = 2.13, p = 0.044, d = 0.45. In a separate ANOVA looking at the drug’s effects on type of mind wandering (Past, Future, Other, or “I Don’t Know”), there was no interaction between Drug and Mind Wandering Type, F(3,48) = 2.49, p = 0.07, ηp2 = 0.135.
Effect of THC on metacognitive accuracy (Exp 2)
In Exp 2 only, we examined subjects’ ability to accurately monitor ongoing task performance (i.e., metacognitive accuracy), by providing confidence ratings for each response. To measure metacognitive accuracy, we calculated the correlation between the number of correct items on each trial and the number of confident responses on each trial (separately for each individual using Spearman’s r). Higher, positive correlation values correspond with more accurate monitoring of task performance (e.g., the participant got N correct and reported N confident responses). We tested whether metacognitive accuracy declined as a function of Drug. A repeated-measures ANOVA with factors Drug and Dose revealed a main effect of Drug, F(2,44) = 3.43, p = 0.04, ηp2 = 0.14. Consistent with the impairment to overall performance, post-hoc t-tests for each dose revealed that this measure of metacognitive accuracy was impaired for the high dose (p = 0.03) but not the low dose (p = 0.07). We did not see an effect of the drug on metacognitive bias (mean number of confident items minus the mean number of correct items; positive numbers indicate overconfidence and negative numbers represent under-confidence). Participants were slightly overconfident in all three drug conditions (M = 0.51 items overconfident, SD = 0.97) and a repeated-measures ANOVA revealed no significant main effect of drug condition on overconfidence, F(1.14,25.09) = 0.49, p = 0.52).
Literature review and power analysis
Although the Digit Span task has been widely used, we found that the drug had no effect on this task in >70% (73.68%) of the 57 conditions that met the inclusion criteria of our Digit Span review (Fig. 5a). A lack of sensitivity was evident for both Forward and Backward span, as well as other working memory tasks, such as Spatial N-Back (Supplementary Results). The lack of effect was observed in studies using a range of doses, including higher doses than what was used here, and multiple modes of administration (Supplementary Table S1). The apparently weak effect of THC on working memory could indicate that the drug does not affect performance but, alternatively, it could reflect a lack of power in most prior studies. Although it was not possible to calculate effect sizes for the reviewed studies, we were able to demonstrate the effects of task time and sample size on power new empirical data.
a Histogram of reported p-values for 57 conditions of forward- and backward-digit span. b Down-sampling analysis of the current data. Each factorial combination of trial number (x-axis) and sample size (y-axis) contains the average power of 250 iterations sampled from the full data set (collapsed across Exp 1 and the 15 mg of THC condition in Exp 2). c, d The black line shows the distribution of digit span p-values from the literature for conditions with fewer (c) or more (d) than the median number of subjects. Dotted lines show that this distribution of p-values from sampling the current visual working memory data set with equivalent, insufficient power (e.g., 7 subjects and 15 trials).
To test whether the distribution of p-values in the literature review was related to insufficient statistical power or a lack of an effect of THC on working memory performance, we performed a down-sampling procedure [15] on the combined data from Exp 1 and Exp 2 (15 mg THC; n = 46, trials = 90). When we reduced the sample size and task length of our data set to match those in the literature, we could nearly perfectly predict the distribution of p-values that was observed in the literature.
With 46 subjects and 90 trials per subject, the achieved power (1 − β) for our main effect of THC on working memory performance was in excess of 0.99. Figure 5b reveals the results of iterative down-sampling of this data (e.g., randomly choosing N subjects and T trials, calculating power). Each cell in this figure contains the average power for 250 random iterations. When down-sampling to a typical sample size and task duration for the Digit Span literature (e.g., 15 subjects, 15 trials), power plummets to only 0.47. In Fig. 5c, d, we have plotted the distribution of p-values for Digit Span studies above and below the median sample size found in the literature. To compare predictions from our down-sampling procedure, we chose the cell from Fig. 5b that most closely matched the number of subjects (5 subjects for the reviewed studies with below the median number of subjects overall, 15 subjects for the reviewed studies with above the median number of subjects overall), and then discretized the p-value outcomes for each of the 250 iterations (<0.001, <0.01, <0.05, or NS). The digit span typically comprises 3 trials of each of 4–5 set sizes (12–15 trials) and takes approximately 4–5 minutes; 15 trials of the whole report task likewise takes ~5 min. As shown in Fig. 5, with 5 minutes of task time and fewer subjects per “experiment”, we obtain distributions of p-values that are nearly identical to those in the empirical literature (Fig. 5c, d).
Discussion
The present study demonstrated that a single 15 mg dose of THC impairs working memory when tested under rigorous, placebo-controlled, double-blind conditions. THC, the main psychoactive constituent in cannabis, is commonly thought to impair working memory, but this effect has been difficult to demonstrate in controlled studies. We review previous studies assessing the effects of THC, and conclude that the failures to detect effects were quite likely due to inadequate statistical power. Using a well-powered sample (combined n = 46, [1 − β] > 0.99), we found that a single 15 mg dose of THC reliably impairs visual working memory. This effect was not, however, apparent for a lower 7.5 mg dose (Experiment 2, n = 23).
We found a robust behavioral effect of THC on working memory performance, but more work is needed to understand the neural mechanisms underlying this behavioral deficit. For example, disruption to working memory is a key cognitive deficit in people with schizophrenia [77], and this deficit is hypothesized to be related to disruptions of the endocannabinoid system [78], more specifically to the dorsolateral prefrontal cortex (DLPFC) [79,80,81], but see ref. [82]. The DLPFC is laden with CB1 receptors, the primary target of THC, and is a critical component of WM maintenance [83,84,85]. Studies of the neural mechanisms underlying WM disruption under the effects of THC may thus provide a reversible demonstration of WM deficits related to disruptions of the endocannabinoid system.
In addition to decreasing WM performance, subjective thought probes revealed that THC increased rates of mind wandering and zoning out (Exp 1) and decreased metacognitive accuracy (Exp 2). To our knowledge, our work provides the first demonstration of THC’s effects on mind wandering during a concurrent cognitive task. These finding are consistent with prior work on THC, including task-independent reports of mind wandering in structured interviews [86, 87], failure to de-activate the default mode network during task performance [88] (but see [89]), and decreased error monitoring [42, 43, 90]. Similar to the effects of nicotine cravings [40] and alcohol [41], THC appears to increase mind wandering and other off-task mental states (e.g., “zoning out” or “mind blanking” [55]), and decrease awareness of task performance. These broad effects on conscious experience are likely to drive performance decrements in a broad range of cognitive tasks.
Limitations and implications for future studies of THC and cognition
There in an intense public interest in the effects of cannabis on cognition and an urgent need for practical information that will guide use. Despite the importance of this study in providing new information on how THC affects memory, the study also had limitations that suggest future avenues for research. First, we were able to test working memory at only one, relatively late time point after oral consumption of a THC capsule (160–220 min), and at two moderate doses [91]. It will be important to characterize the effects of THC on working memory performance over the full time-course of the drug, and, importantly at higher doses and by different routes of administration (especially smoked and vaped). In addition, further work is needed to understand relationships between THC-related working memory impairments and other task and participant factors, such as recent and lifetime exposure to THC, and generalizability to other cognitive constructs. Second, we need more information on the severity of working memory disruption and the extent to which the effect depends on initial performance. Although the effect we observed was relatively large (d = 0.65), this effect is smaller than, for example, normal variation in working memory performance across individuals. The behavioral performance difference between the placebo and drug conditions was 0.29 items, but the difference between the top- and bottom-half of individuals within the placebo condition was 0.80 items. Further, the effects of the drug may be especially pronounced in certain at-risk populations, including those with initially poor working memory performance.
Our finding that power curves for a down-sampled visual working memory task closely matched the observed power in the Digit Span literature suggests that task length is a strong driving factor in the low rate of positive drug effects in the literature. However, more methodological work is needed to determine if directly manipulating the task length of standard tasks (e.g., a 25 min rather than 5 min version of the Digit Span task) will rescue statistical power as our simulations suggest. Alternatively, it is possible that other task differences (e.g., factor loadings) also contribute to the low rate of positive drug effects in the literature. For example, simple span tasks (e.g., the Forward Digit Span) do not load well onto a general working memory factor at the latent level [20] and often fail to predict individual differences in general fluid intelligence [19, 92,93,94,95], but also see ref. [96]. Other common tasks (e.g., spatial N-back, Supplementary Table S2) likewise have the potential differences from the specific visual working memory task used here. N-back tasks load well onto a general WM factor at the latent level [20] and are useful for investigating the executive function component of WM (vs. the storage component). However, N-back tasks are not highly correlated with other working memory tasks [97,98,99] and often have relatively poor statistical reliability [95], potentially making it difficult to detect effects across treatment conditions.
Our literature search revealed the importance of statistical power in studies of THC on cognition. Here, we used one relatively long task (~30 min, 90 trials). In contrast, many earlier studies used several shorter tasks (e.g., ten 3 min tasks), presumably to assess a range of potential deficits. However, because task length and statistical power have direct tradeoffs, this approach may miss important effects. Similar problems of inadequate power may exist in other studies of effects of drugs on working memory and other aspects of cognition. Thus, we recommend that longer tasks be used to determine the effects of drugs on cognition.
Funding and disclosure
This research was supported by grants awarded to HdW. (National Institute on Drug Abuse grant 5R01-DA002812) and to EV (National Institute of Mental Health grant 5R01-MH087214 and Office of Naval Research grant N00014–12–1–0972). KA was supported by National Institute of Mental Health grant 5T32-MH020002. EP was supported by National Institute on Drug Abuse grant 5T32-DA043469. M.D. was supported by National Institute on Drug Abuse grant T32-DA007209. The authors have no conflicts of interest to disclose.
References
Baddeley AD, Hitch G. Working Memory. In: Psychology of Learning and Motivation. Elsevier, pp. 47–89. 1974. http://linkinghub.elsevier.com/retrieve/pii/S0079742108604521. Accessed 24 Aug 2016.
Cowan N, Elliott EM, Scott Saults J, Morey CC, Mattox S, Hismjatullina A, Conway ARA. On the capacity of attention: Its estimation and its role in working memory and cognitive aptitudes. Cogn Psychol. 2005;51:42–100.
Vadhan NP, Serper MR, Haney M. Effects of Δ-THC on working memory: implications for schizophrenia? Prim Psychiatry. 2009;16:51–99.
Ranganathan M, D’Souza DC. The acute effects of cannabinoids on memory in humans: a review. Psychopharmacology. 2006;188:425–44.
Curran HV, Freeman TP, Mokrysz C, Lewis DA, Morgan CJA, Parsons LH. Keep off the grass? Cannabis, cognition and addiction. Nat Rev Neurosci. 2016;17:293–306.
Chait LD, Pierri J. Effects of smoked marijuana on human performance: a critical review. In: Murphy L and Bartke A, editors. Marijuana/cannabinoids: neurobiology and neurophysiology. Boca Raton, FL, USA: CRC Press; 1992. p. 387–423.
Broyd SJ, van Hell HH, Beale C, Yücel M, Solowij N. Acute and chronic effects of cannabinoids on human cognition—a systematic review. Biol Psychiatry. 2016;79:557–67.
Zuurman L, Ippel AE, Moin E, van Gerven JMA. Biomarkers for the effects of cannabis and THC in healthy volunteers. Br J Clin Pharmacol. 2009;67:5–21.
Easterbrook PJ, Gopalan R, Berlin JA, Matthews DR. Publication bias in clinical research. Lancet. 1991;337:867–72.
Malički M, Marušić A. Is there a solution to publication bias? Researchers call for changes in dissemination of clinical research results. J Clin Epidemiol. 2014;67:1103–10.
Rosenthal R. The file drawer problem and tolerance for null results. Psychological Bull. 1979;86:638–41.
Wechsler D. The measurement of adult intelligence. 1st ed. Baltimore, MD, USA: Williams & Wilkins Co.; 1939.
Broadbent DE. The magic number seven after fifteen years. In: Kennedy A and Wilkes A, editors. Studies In Long Term Memory. London: John Wiley & Sons; 1975.
Miller GA. The magical number seven, plus or minus two: some limits on our capacity for processing information. Psychological Rev. 1956;63:81–97.
Xu Z, Adam KCS, Fang X, Vogel EK. The reliability and stability of visual working memory capacity. Behav Res Methods. 2017;50:576–88.
Pailian H, Halberda J. The reliability and internal consistency of one-shot and flicker change detection for measuring individual differences in visual working memory capacity. Mem Cognition. 2015;43:397–420.
Adam KCS, Mance I, Fukuda K, Vogel EK. The contribution of attentional lapses to individual differences in visual working memory capacity. J Cogn Neurosci. 2015;27:1601–16.
Adam KCS, Vogel EK. Improvements to visual working memory performance with practice and feedback. PLoS ONE. 2018;13:e0203279.
Unsworth N, Fukuda K, Awh E, Vogel EK. Working memory and fluid intelligence: Capacity, attention control, and secondary memory retrieval. Cogn Psychol. 2014;71:1–26.
Waris O, Soveri A, Ahti M, Hoffing RC, Ventus D, Jaeggi SM et al. A latent factor analysis of working memory measures using large-scale data. Front Psychol. 2017. http://journal.frontiersin.org/article/10.3389/fpsyg.2017.01062/full. Accessed 12 Nov 2018.
Gold JM, Hahn B, Zhang WW, Robinson BM, Kappenman ES, Beck VM, Luck SJ. Reduced capacity but spared precision and maintenance of working memory representations in schizophrenia. Arch Gen Psychiatry. 2010;67:570.
Lee E-Y, Cowan N, Vogel EK, Rolan T, Valle-Inclan F, Hackley SA. Visual working memory deficits in patients with Parkinson’s disease are due to both reduced storage capacity and impaired ability to filter out irrelevant information. Brain. 2010;133:2677–89.
Gold JM, Wilk CM, McMahon RP, Buchanan RW, Luck SJ. Working memory for visual features and conjunctions in schizophrenia. J Abnorm Psychol. 2003;112:61–71.
Jeneson A, Wixted JT, Hopkins RO, Squire LR. Visual working memory capacity and the medial temporal lobe. J Neurosci. 2012;32:3584–9.
McCollough AW, Machizawa MG, Vogel EK. Electrophysiological measures of maintaining representations in visual working memory. Cortex. 2007;43:77–94.
Adam KCS, Robison MK, Vogel EK. Contralateral delay activity tracks fluctuations in working memory performance. J Cogn Neurosci. 2018;30:1229–40.
Xu Y, Chun MM. Dissociable neural mechanisms supporting visual short-term memory for objects. Nature. 2006;440:91–5.
Todd JJ, Marois R. Capacity limit of visual short-term memory in human posterior parietal cortex. Nature. 2004;428:751–4.
Vogel EK, Machizawa MG. Neural activity predicts individual differences in visual working memory capacity. Nature. 2004;428:748–51.
Reinhart RMG, Heitz RP, Purcell BA, Weigand PK, Schall JD, Woodman GF. Homologous mechanisms of visuospatial working memory maintenance in macaque and human: properties and sources. J Neurosci. 2012;32:7711–22.
Huang L. Visual working memory is better characterized as a distributed resource rather than discrete slots. J Vis. 2010;10:8–8.
Wilken P, Ma WJ. A detection theory account of change detection. J Vis. 2004;4:1120–35.
Zhang W, Luck SJ. Discrete fixed-resolution representations in visual working memory. Nature. 2008;453:233–5.
Pashler H. Familiarity and visual change detection. Percept Psychophys. 1988;44:369–78.
Luck SJ, Vogel EK. The capacity of visual working memory for features and conjunctions. Nature. 1997;390:279–81.
Kirchner WK. Age differences in short-term retention of rapidly changing information. J Exp Psychol. 1958;55:352–8.
Adam KCS, Vogel EK. Reducing failures of working memory with performance feedback. Psychonomic Bull Rev. 2016;23:1520–7.
deBettencourt MT, Keene PA, Awh E, Vogel EK. Real-time triggering reveals concurrent lapses of attention and working memory. Nat Hum Behav. 2019;3:808–16.
Smallwood J, Schooler JW. The restless mind. Psychol Bull. 2006;132:946–58.
Sayette MA, Schooler JW, Reichle ED. Out for a smoke: the impact of cigarette craving on zoning out during reading. Psychol Sci. 2010;21:26–30.
Sayette MA, Reichle ED, Schooler JW. Lost in the sauce: the effects of alcohol on mind wandering. Psychological Sci. 2009;20:747–52.
Spronk D, Dumont GJH, Verkes RJ, de Bruijn ERA. Acute effects of delta-9-tetrahydrocannabinol on performance monitoring in healthy volunteers. Front. Behav. Neurosci. 2011. http://journal.frontiersin.org/article/10.3389/fnbeh.2011.00059/abstract. Accessed 31 March 2020].
Kowal MA, van Steenbergen H, Colzato LS, Hazekamp A, van der Wee NJA, Manai M, Durieux J, Hommel B. Dose-dependent effects of cannabis on the neural correlates of error monitoring in frequent cannabis users. Eur Neuropsychopharmacol. 2015;25:1943–53.
Adam KCS, Vogel EK. Confident failures: lapses of working memory reveal a metacognitive blind spot. Atten, Percept, Psychophys. 2017;79:1506–23.
McDonald J, Schleifer L, Richards JB, de Wit H. Effects of THC on behavioral measures of impulsivity in humans. Neuropsychopharmacology. 2003;28:1356–65.
Curran V, Brignell C, Fletcher S, Middleton P, Henry J. Cognitive and subjective dose-response effects of acute oral Δ 9 -tetrahydrocannabinol (THC) in infrequent cannabis users. Psychopharmacology. 2002;164:61–70.
Wachtel SR, ElSohly MA, Ross SA, Ambre J, de Wit H. Comparison of the subjective effects of Δ9-tetrahydrocannabinol and marijuana in humans. Psychopharmacology. 2002;161:331–9.
Pabon E, de Wit H. Developing a phone-based measure of impairment after acute oral ∆9 -tetrahydrocannabinol. J Psychopharmacol. 2019;33:1160–9.
Doss MK, Weafer J, Gallo DA, de Wit H. Δ9-Tetrahydrocannabinol at retrieval drives false recollection of neutral and emotional memories. Biol Psychiatry. 2018;84:743–50. http://linkinghub.elsevier.com/retrieve/pii/S000632231831477X.
Doss MK, Weafer J, Gallo DA, de Wit H. Δ9-Tetrahydrocannabinol during encoding impairs perceptual details yet spares context effects on episodic memory. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;5:110–8.
Chait LD, Fischman MW, Schuster CR. ‘Hangover’ effects the morning after marijuana smoking. Drug Alcohol Depend. 1985;15:229–38.
Martin WR, Sloan JW, Sapira JD, Jasinski DR. Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther. 1971;12:245–58.
Folstein MF, Luria R. Reliability, validity, and clinical application of the visual analogue mood scale. Psychological Med. 1973;3:479.
Morean ME, de Wit H, King AC, Sofuoglu M, Rueger SY, O’Malley SS. The drug effects questionnaire: psychometric support across three drug types. Psychopharmacology. 2013;227:177–92.
Ward AF, Wegner DM. Mind-blanking: when the mind goes away. Front Psychol. 2013. http://journal.frontiersin.org/article/10.3389/fpsyg.2013.00650/abstract. Accessed 26 March 2020.
JASP Team. 2019. JASP. Available at: https://jasp-stats.org. Accessed 1 Jan 2020.
Tinklenberg JR, Melges FT, Hollister LE, Gillespie HK. Marijuana and immediate memory. Nature. 1970;226:1171–2.
Melges FT, Tinklenberg JR, Hollister LE, Gillespie HK. Marihuana and temporal disintegration. Science. 1970;168:1118–20.
Ballard ME, de Wit H. Combined effects of acute, very-low-dose ethanol and delta(9)-tetrahydrocannabinol in healthy human volunteers. Pharmacol Biochem Behav. 2011;97:627–31.
Casswell S, Marks DF. Cannabis and temporal disintegration in experienced and naive subjects. Science. 1973;179:803–5.
Hooker WD, Jones RT. Increased susceptibility to memory intrusions and the Stroop interference effect during acute marijuana intoxication. Psychopharmacology. 1987;91:20–4.
Chait LD, Corwin RL, Johanson CE. A cumulative dosing procedure for administering marijuana smoke to humans. Pharmacol Biochem Behav. 1988;29:553–7.
Heishman SJ, Stitzer ML, Yingling JE. Effects of tetrahydrocannabinol content on marijuana smoking behavior, subjective reports, and performance. Pharmacol Biochem Behav. 1989;34:173–9.
Zacny JP, Chait LD. Response to marijuana as a function of potency and breathhold duration. Psychopharmacology. 1991;103:223–6.
Azorlosa JL, Heishman SJ, Stitzer ML, Mahaffey JM. Marijuana smoking: effect of varying delta 9-tetrahydrocannabinol content and number of puffs. J Pharmacol Exp Ther 1992;261:114–22.
Chait LD, Perry JL. Acute and residual effects of alcohol and marijuana, alone and in combination, on mood and performance. Psychopharmacology. 1994;115:340–9.
Azorlosa JL, Greenwald MK, Stitzer ML. Marijuana smoking: effects of varying puff volume and breathhold duration. J Pharmacol Exp Ther 1995;272:560–9.
Hart C. Effects of acute smoked marijuana on complex cognitive performance. Neuropsychopharmacology. 2001;25:757–65.
Morrison PD, Zois V, McKeown DA, Lee TD, Holt DW, Powell JF, Kapur S, Murray RM. The acute effects of synthetic intravenous Δ9-tetrahydrocannabinol on psychosis, mood and cognitive functioning. Psychological Med. 2009;39:1607.
Dornbush RL, Kokkevi A. Acute effects of cannabis on cognitive, perceptual, and motor performance in chronic Hashish users. Ann NY Acad Sci. 1976;282:313–22.
Greenwald MK, Stitzer ML. Antinociceptive, subjective and behavioral effects of smoked marijuana in humans. Drug Alcohol Depend. 2000;59:261–75.
Tinklenberg JR. Marihuana and alcohol: time production and memory functions. Arch Gen Psychiatry. 1972;27:812.
Galanter M. δ9-Transtetrahydrocannabinol and natural marihuana: a controlled comparison. Arch Gen Psychiatry. 1973;28:278.
Cappell HD, Pliner PL. Volitional control of marijuana intoxication: a study of the ability to “come down” on command. J Abnorm Psychol. 1973;82:428–34.
Fant RV, Heishman SJ, Bunker EB, Pickworth WB. Acute and residual effects of marijuana in humans. Pharmacol Biochem Behav. 1998;60:777–84.
Ramesh D, Haney M, Cooper ZD. Marijuana’s dose-dependent effects in daily marijuana smokers. Exp Clin Psychopharmacol. 2013;21:287–93.
Lee J, Park S. Working memory impairments in schizophrenia: a meta-analysis. J Abnorm Psychol. 2005;114:599–611.
Carter E, Wang X-J. Cannabinoid-mediated disinhibition and working memory: dynamical interplay of multiple feedback mechanisms in a continuous attractor model of prefrontal cortex. Cereb Cortex. 2007;17:i16–26.
Manoach DS. Prefrontal cortex dysfunction during working memory performance in schizophrenia: reconciling discrepant findings. Schizophrenia Res. 2003;60:285–98.
Glahn DC, Ragland JD, Abramoff A, Barrett J, Laird AR, Bearden CE, Velligan DI. Beyond hypofrontality: a quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Hum Brain Mapp. 2005;25:60–9.
Van Snellenberg JX, Torres IJ, Thornton AE. Functional neuroimaging of working memory in schizophrenia: task performance as a moderating variable. Neuropsychology. 2006;20:497–510.
Hahn B, Robinson BM, Leonard CJ, Luck SJ, Gold JM. Posterior parietal cortex dysfunction is central to working memory storage and broad cognitive deficits in schizophrenia. J Neurosci. 2018;38:8378–87.
Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–61.
D’Esposito M, Postle BR. The cognitive neuroscience of working memory. Annu Rev Psychol. 2015;66:115–42.
Barbey AK, Koenigs M, Grafman J. Dorsolateral prefrontal contributions to human working memory. Cortex. 2013;49:1195–205.
Hathaway AD. Cannabis effects and dependency concerns in long-term frequent users: a missing piece of the public health puzzle. Addiction Res Theory. 2003;11:441–58.
Osborne GB, Fogel C. Understanding the motivations for recreational marijuana use among adult Canadians. Subst Use Misuse. 2008;43:539–72.
Bossong MG, Jansma JM, van Hell HH, Jager G, Kahn RS, Ramsey NF. Default mode network in the effects of Δ9-tetrahydrocannabinol (THC) on human executive function. PLoS ONE. 2013;8:e70074.
Wall MB, Pope R, Freeman TP, Kowalczyk OS, Demetriou L, Mokrysz C, Hindocha C, Lawn W, Bloomfield MA, Freeman AM, et al. Dissociable effects of cannabis with and without cannabidiol on the human brain’s resting-state functional connectivity. J Psychopharmacol. 2019;33:822–30.
Hester R, Nestor L, Garavan H. Impaired error awareness and anterior cingulate cortex hypoactivity in chronic cannabis users. Neuropsychopharmacol. 2009;34:2450–8.
Vandrey R, Herrmann ES, Mitchell JM, Bigelow GE, Flegel R, LoDico C, Cone EJ. Pharmacokinetic profile of oral cannabis in humans: blood and oral fluid disposition and relation to pharmacodynamic outcomes. J Anal Toxicol. 2017;41:83–99.
Engle RW, Tuholski SW, Laughlin JE, Conway AR. Working memory, short-term memory, and general fluid intelligence: a latent-variable approach. J Exp Psychol Gen. 1999;128:309–31.
Conway ARA, Cowan N, Bunting MF, Therriault DJ, Minkoff SRB. A latent variable analysis of working memory capacity, short-term memory capacity, processing speed, and general fluid intelligence. Intelligence. 2002;30:163–83.
Unsworth N, Fukuda K, Awh E, Vogel EK. Working memory delay activity predicts individual differences in cognitive abilities. J Cogn Neurosci. 2015;27:853–65.
Jaeggi SM, Buschkuehl M, Perrig WJ, Meier B. The concurrent validity of the N-back task as a working memory measure. Memory. 2010;18:394–412.
Unsworth N, Engle RW. Simple and complex memory spans and their relation to fluid abilities: evidence from list-length effects. J Mem Lang. 2006;54:68–80.
Miller KM, Price CC, Okun MS, Montijo H, Bowers D. Is the N-back task a valid neuropsychological measure for assessing working memory? Arch Clin Neuropsychol. 2009;24:711–7.
Redick TS, Lindsey DRB. Complex span and n-back measures of working memory: a meta-analysis. Psychonomic Bull Rev. 2013;20:1102–13.
Kane MJ, Conway ARA, Miura TK, Colflesh GJH. Working memory, attention control, and the n-back task: a question of construct validity. J Exp Psychol: Learn Mem Cognition. 2007;33:615–22.
Author information
Authors and Affiliations
Contributions
MD and EP collected data. KA performed analyses and drafted the manuscript. KA, MD, EP, EV, and HdW planned the experiments and revised the manuscript.
Corresponding author
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Adam, K.C.S., Doss, M.K., Pabon, E. et al. Δ9-Tetrahydrocannabinol (THC) impairs visual working memory performance: a randomized crossover trial. Neuropsychopharmacol. 45, 1807–1816 (2020). https://doi.org/10.1038/s41386-020-0690-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-020-0690-3
This article is cited by
-
The why behind the high: determinants of neurocognition during acute cannabis exposure
Nature Reviews Neuroscience (2021)
-
Consensus recommendations on dosing and administration of medical cannabis to treat chronic pain: results of a modified Delphi process
Journal of Cannabis Research (2021)