Abstract
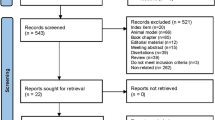
The elucidation of synaptic density changes provides valuable insights into the underlying brain mechanisms of substance use. In preclinical studies, synaptic density markers, like spine density, are altered by substances of abuse (e.g., alcohol, amphetamine, cannabis, cocaine, opioids, nicotine). These changes could be linked to phenomena including behavioral sensitization and drug self-administration in rodents. However, studies have produced heterogeneous results for spine density across substances and brain regions. Identifying patterns will inform translational studies given tools that now exist to measure in vivo synaptic density in humans. We performed a meta-analysis of preclinical studies to identify consistent findings across studies. PubMed, ScienceDirect, Scopus, and EBSCO were searched between September 2022 and September 2023, based on a protocol (PROSPERO: CRD42022354006). We screened 6083 publications and included 70 for meta-analysis. The meta-analysis revealed drug-specific patterns in spine density changes. Hippocampal spine density increased after amphetamine. Amphetamine, cocaine, and nicotine increased spine density in the nucleus accumbens. Alcohol and amphetamine increased, and cannabis reduced, spine density in the prefrontal cortex. There was no convergence of findings for morphine’s effects. The effects of cocaine on the prefrontal cortex presented contrasting results compared to human studies, warranting further investigation. Publication bias was small for alcohol or morphine and substantial for the other substances. Heterogeneity was moderate-to-high across all substances. Nonetheless, these findings inform current translational efforts examining spine density in humans with substance use disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Data will be provided upon reasonable request.
References
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association; 2013.
(SAMHSA) SA and MHSA. Key Substance Use and Mental Health Indicators in the United States: Results from the 2019 National Survey on Drug Use and Health. 2020.
(PAHO) PAHO. The burden of drug use disorders in the Region of the Americas, 2000-2019. Noncommunicable Diseases and Mental Health Data Portal. 2021. https://www.paho.org/en/enlace/burden-drug-use-disorders.
(NIDA) NI on DA. Trends and Statistics. 2022. https://nida.nih.gov/research-topics/trends-statistics.
Kuehn BM. Fentanyl drives startling increases in adolescent overdose deaths. JAMA 2023;329:280.
Citri A, Malenka RC. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology 2008;33:18–41.
Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci 2011;12:623–37.
Hyman SE, Malenka RC, Nestler EJ. NEURAL MECHANISMS OF ADDICTION: the role of reward-related learning and memory. Annu Rev Neurosci 2006;29:565–98.
Robinson TE, Kolb B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J Neurosci 1997;17:8491–7.
Robinson TE, Kolb B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci 1999;11:1598–604.
Robinson TE, Gorny G, Savage VR, Kolb B. Widespread but regionally specific effects of experimenter- versus self-administered morphine on dendritic spines in the nucleus accumbens, hippocampus, and neocortex of adult rats. Synapse 2002;46:271–9.
Nwabuisi-Heath E, LaDu MJ, Yu C. Simultaneous analysis of dendritic spine density, morphology and excitatory glutamate receptors during neuron maturation in vitro by quantitative immunocytochemistry. J Neurosci Methods 2012;207:137–47.
Alvarez VA, Sabatini BL. Anatomical and physiological plasticity of dendritic spines. Annu Rev Neurosci 2007;30:79–97.
Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology 2010;35:217–38.
Kalivas PW, O’Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology 2008;33:166–80.
Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci 2007;8:844–58.
Crombag HS. Opposite effects of amphetamine self-administration experience on dendritic spines in the medial and orbital prefrontal cortex. Cereb Cortex 2004;15:341–8.
Dumitriu D, LaPlant Q, Grossman YS, Dias C, Janssen WG, Russo SJ, et al. Subregional, dendritic compartment, and spine subtype specificity in cocaine regulation of dendritic spines in the nucleus accumbens. J Neurosci 2012;32:6957–66.
Shen H-W, Toda S, Moussawi K, Bouknight A, Zahm DS, Kalivas PW. Altered dendritic spine plasticity in cocaine-withdrawn rats. J Neurosci 2009;29:2876–84.
Siemsen BM, Giannotti G, McFaddin JA, Scofield MD, McGinty JF. Biphasic effect of abstinence duration following cocaine self-administration on spine morphology and plasticity-related proteins in prelimbic cortical neurons projecting to the nucleus accumbens core. Brain Struct Funct 2019;224:741–58.
Lescaudron L, Jaffard R, Verna A. Modifications in number and morphology of dendritic spines resulting from chronic ethanol consumption and withdrawal: a Golgi study in the mouse anterior and posterior hippocampus. Exp Neurol 1989;106:156–63.
Lee K, Dunwiddie T, Deitrich R, Lynch G, Hoffer B. Chronic ethanol consumption and hippocampal neuron dendritic spines: a morphometric and physiological analysis. Exp Neurol 1981;71:541–9.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021:n71.
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev 2016;5:210.
Yoder KK, Albrecht DS, Dzemidzic M, Normandin MD, Federici LM, Graves T, et al. Differences in IV alcohol-induced dopamine release in the ventral striatum of social drinkers and nontreatment-seeking alcoholics. Drug Alcohol Depend 2016;160:163–9.
Yoder KK, Morris ED, Constantinescu CC, Cheng T, Normandin MD, O’Connor SJ, et al. When what you see isn’t what you get: alcohol cues, alcohol administration, prediction error, and human striatal dopamine. Alcohol Clin Exp Res 2009;33:139–49.
Angarita GA, Worhunsky PD, Naganawa M, Toyonaga T, Nabulsi NB, Li CR, et al. Lower prefrontal cortical synaptic vesicle binding in cocaine use disorder: An exploratory 11 C‐UCB‐J positron emission tomography study in humans. Addict Biol 2022;27:e13123.
D’Souza DC, Radhakrishnan R, Naganawa M, Ganesh S, Nabulsi N, Najafzadeh S, et al. Preliminary in vivo evidence of lower hippocampal synaptic density in cannabis use disorder. Mol Psychiatry 2021;26:3192–3200.
Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol 2014;14:43.
Sedgwick P, Marston L. How to read a funnel plot in a meta-analysis. BMJ 2015;351:h4718.
Hooijmans CR, IntHout J, Ritskes-Hoitinga M, Rovers MM. Meta-analyses of animal studies: an introduction of a valuable instrument to further improve healthcare. ILAR J 2014;55:418–26.
Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012;9:671–5.
Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Wiley; 2008.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88.
Cohen J Statistical power analysis for the behavioral sciences. Academic press; 2013.
Alcantara AA, Lim HY, Floyd CE, Garces J, Mendenhall JM, Lyons CL, et al. Cocaine- and morphine-induced synaptic plasticity in the nucleus accumbens. Synapse 2011;65:309–20.
Jackson SJ, Andrews N, Ball D, Bellantuono I, Gray J, Hachoumi L, et al. Does age matter? The impact of rodent age on study outcomes. Lab Anim 2017;51:160–9.
McDonald RJ, Hong NS, Atwood A, Tyndall AV, Kolb B. An assessment of the functional effects of amphetamine-induced dendritic changes in the nucleus accumbens, medial prefrontal cortex, and hippocampus on different types of learning and memory function. Neurobiol Learn Mem 2021;180:107408.
Spiga S, Talani G, Mulas G, Licheri V, Fois GR, Muggironi G, et al. Hampered long-term depression and thin spine loss in the nucleus accumbens of ethanol-dependent rats. Proc Natl Acad Sci 2014;111:E3745–54.
Scallet AC, Uemura E, Andrews A, Ali SF, McMillan DE, Paule MG, et al. Morphometric studies of the rat hippocampus following chronic delta-9-tetrahydrocannabinol (THC). Brain Res 1987;436:193–8.
Staples MC, Kim A, Mandyam CD. Dendritic remodeling of hippocampal neurons is associated with altered NMDA receptor expression in alcohol dependent rats. Mol Cell Neurosci 2015;65:153–62.
Mitchell MC, Teigen EL, Ramchandani VA. Absorption and peak blood alcohol concentration after drinking beer, wine, or spirits. Alcohol Clin Exp Res 2014;38:1200–4.
Lee NK, Jenner L, Harney A, Cameron J. Pharmacotherapy for amphetamine dependence: a systematic review. Drug Alcohol Depend 2018;191:309–37.
Zimmerman JL. Cocaine intoxication. Crit Care Clin 2012;28:517–26.
Mohamadi A, Chan JJ, Lian J, Wright CL, Marin AM, Rodriguez EK, et al. Risk factors and pooled rate of prolonged opioid use following trauma or surgery. J Bone Jt Surg 2018;100:1332–40.
Le Houezec J. Role of nicotine pharmacokinetics in nicotine addiction and nicotine replacement therapy: a review. Int J Tuberc Lung Dis 2003;7:811–9.
Chye Y, Kirkham R, Lorenzetti V, McTavish E, Solowij N, Yücel M. Cannabis, cannabinoids, and brain morphology: a review of the evidence. Biol Psychiatry Cogn Neurosci Neuroimag 2021;6:627–35.
Marie N, Canestrelli C, Noble F. Transfer of neuroplasticity from nucleus accumbens core to shell is required for cocaine reward. PLoS One 2012;7:e30241.
Zehle S, Bock J, Jezierski G, Gruss M, Braun K. Methylphenidate treatment recovers stress-induced elevated dendritic spine densities in the rodent dorsal anterior cingulate cortex. Dev Neurobiol 2007;67:1891–1900.
Turner PV, Brabb T, Pekow C, Vasbinder MA. Administration of substances to laboratory animals: routes of administration and factors to consider. J Am Assoc Lab Anim Sci 2011;50:600–13.
O’Connor EC, Chapman K, Butler P, Mead AN. The predictive validity of the rat self-administration model for abuse liability. Neurosci Biobehav Rev 2011;35:912–38.
Chen BT, Bowers MS, Martin M, Hopf FW, Guillory AM, Carelli RM, et al. Cocaine but not natural reward self-administration nor passive cocaine infusion produces persistent LTP in the VTA. Neuron 2008;59:288–97.
Miguéns M, Kastanauskaite A, Coria SM, Selvas A, Ballesteros-Yañez I, DeFelipe J, et al. The effects of cocaine self-administration on dendritic spine density in the rat hippocampus are dependent on genetic background. Cereb Cortex 2015;25:56–65.
Yao D, Shi X, Wang L, Gosnell BA, Chen C. Characterization of differential cocaine metabolism in mouse and rat through metabolomics-guided metabolite profiling. Drug Metab Dispos 2013;41:79–88.
Bandeira F, Lent R, Herculano-Houzel S. Changing numbers of neuronal and non-neuronal cells underlie postnatal brain growth in the rat. Proc Natl Acad Sci 2009;106:14108–13.
Downes N, Mullins P. The development of Myelin in the brain of the juvenile rat. Toxicol Pathol 2014;42:913–22.
Fu Y, Rusznák Z, Herculano-Houzel S, Watson C, Paxinos G. Cellular composition characterizing postnatal development and maturation of the mouse brain and spinal cord. Brain Struct Funct 2013;218:1337–54.
Hauser SR, Mulholland PJ, Truitt WA, Waeiss RA, Engleman EA, Bell RL, et al. Adolescent intermittent ethanol (AIE) enhances the dopaminergic response to ethanol within the mesolimbic pathway during adulthood: alterations in cholinergic/dopaminergic genes expression in the nucleus accumbens shell. Int J Mol Sci 2021;22:11733.
Kipp BT, Nunes PT, Galaj E, Hitchcock B, Nasra T, Poynor KR, et al. Adolescent ethanol exposure alters cholinergic function and apical dendritic branching within the orbital frontal cortex. Neuroscience 2021;473:52–65.
Peterson VL, McCool BA, Hamilton DA. Effects of ethanol exposure and withdrawal on dendritic morphology and spine density in the nucleus accumbens core and shell. Brain Res 2015;1594:125–35.
Singer BF, Tanabe LM, Gorny G, Jake-Matthews C, Li Y, Kolb B, et al. Amphetamine-induced changes in dendritic morphology in rat forebrain correspond to associative drug conditioning rather than nonassociative drug sensitization. Biol Psychiatry 2009;65:835–40.
Li J, Liu N, Lu K, Zhang L, Gu J, Guo F, et al. Cocaine-induced dendritic remodeling occurs in both D1 and D2 dopamine receptor-expressing neurons in the nucleus accumbens. Neurosci Lett 2012;517:118–22.
Wissman AM, McCollum AF, Huang G-Z, Nikrodhanond AA, Woolley CS. Sex differences and effects of cocaine on excitatory synapses in the nucleus accumbens. Neuropharmacology 2011;61:217–27.
Pascual M, López-Hidalgo R, Montagud-Romero S, Ureña-Peralta JR, Rodríguez-Arias M, Guerri C. Role of mTOR-regulated autophagy in spine pruning defects and memory impairments induced by binge-like ethanol treatment in adolescent mice. Brain Pathol 2021;31:174–88.
Zhang L, Li J, Liu N, Wang B, Gu J, Zhang M, et al. Signaling via dopamine D1 and D3 receptors oppositely regulates cocaine-induced structural remodeling of dendrites and spines. Neurosignals 2012;20:15–34.
Rubino T, Prini P, Piscitelli F, Zamberletti E, Trusel M, Melis M, et al. Adolescent exposure to THC in female rats disrupts developmental changes in the prefrontal cortex. Neurobiol Dis 2015;73:60–69.
Hamilton DA, Kolb B. Differential effects of nicotine and complex housing on subsequent experience-dependent structural plasticity in the nucleus accumbens. Behav Neurosci 2005;119:355–65.
Grant BF. Prevalence and correlates of alcohol use and DSM-IV alcohol dependence in the United States: results of the National Longitudinal Alcohol Epidemiologic Survey. J Stud Alcohol 1997;58:464–73.
Serrano ME, Kim E, Petrinovic MM, Turkheimer F, Cash D. Imaging synaptic density: the next holy grail of neuroscience? Front Neurosci 2022;16:796129.
Towers EB, Williams IL, Qillawala EI, Rissman EF, Lynch WJ. Sex/gender differences in the time-course for the development of substance use disorder: a focus on the telescoping effect. Pharm Rev 2023;75:217–49.
Zhornitsky S, Oliva HNP, Jayne LA, Allsop ASA, Kaye AP, Potenza MN, et al. Changes in synaptic markers after administration of ketamine or psychedelics: a systematic scoping review. Front Psychiatry 2023;14:1197890.
Elliott AD. Confocal microscopy: principles and modern practices. Curr Protoc Cytom 2020;92:e68.
Kang HW, Kim HK, Moon BH, Lee SJ, Lee SJ, Rhyu IJ. Comprehensive review of golgi staining methods for nervous tissue. Appl Microsc 2017;47:63–69.
Clare R, King VG, Wirenfeldt M, Vinters HV. Synapse loss in dementias. J Neurosci Res 2010;88:2083–90.
Singh P, Jorgačevski J, Kreft M, Grubišić V, Stout RF, Potokar M, et al. Single-vesicle architecture of synaptobrevin2 in astrocytes. Nat Commun 2014;5:3780.
Shao L-X, Liao C, Gregg I, Davoudian PA, Savalia NK, Delagarza K, et al. Psilocybin induces rapid and persistent growth of dendritic spines in frontal cortex in vivo. Neuron 2021;109:2535–2544.e4.
Augustin SM, Lovinger DM. Synaptic changes induced by cannabinoid drugs and cannabis use disorder. Neurobiol Dis 2022;167:105670.
Mutch SA, Kensel-Hammes P, Gadd JC, Fujimoto BS, Allen RW, Schiro PG, et al. Protein quantification at the single vesicle level reveals that a subset of synaptic vesicle proteins are trafficked with high precision. J Neurosci 2011;31:1461–70.
Finnema SJ, Nabulsi NB, Mercier J, Lin S, Chen M-K, Matuskey D, et al. Kinetic evaluation and test–retest reproducibility of [11 C]UCB-J, a novel radioligand for positron emission tomography imaging of synaptic vesicle glycoprotein 2A in humans. J Cereb Blood Flow Metab 2018;38:2041–52.
Rossi R, Bærentzen SL, Thomsen MB, Real CC, Wegener G, Grassi-Oliveira R, et al. A single dose of cocaine raises SV2A density in hippocampus of adolescent rats. Acta Neuropsychiatr. 2023. https://doi.org/10.1017/neu.2023.14.
Lin L. Bias caused by sampling error in meta-analysis with small sample sizes. PLoS One 2018;13:e0204056.
Higgins JPT. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60.
Bonomi R, Naganawa M, Mignosa M, Skosnik P, Esterlis I, Nabulsi N, et al. Preliminary Evidence of Reduced Ventral Pallido-Striatal Synaptic Density in Opioid Use Disorder: A Pilot 11C-UCB-J PET Study. 61st Annual Meeting of the American College of Neuropsychopharmacology, 2022.
Russo SJ, Wilkinson MB, Mazei-Robison MS, Dietz DM, Maze I, Krishnan V, et al. Nuclear factor κB signaling regulates neuronal morphology and cocaine reward. J Neurosci 2009;29:3529–37.
Maze I, Covington HE, Dietz DM, LaPlant Q, Renthal W, Russo SJ, et al. Essential role of the histone methyltransferase G9a in cocaine-induced plasticity. Science (1979) 2010;327:213–6.
Russo SJ, Dietz DM, Dumitriu D, Morrison JH, Malenka RC, Nestler EJ. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci 2010;33:267–76.
Raval NR, Johansen A, Donovan LL, Ros NF, Ozenne B, Hansen HD, et al. A single dose of psilocybin increases synaptic density and decreases 5-HT2A receptor density in the pig brain. Int J Mol Sci 2021;22:835.
Kouba BR, Camargo A, Gil-Mohapel J, Rodrigues ALS. Molecular basis underlying the therapeutic potential of vitamin D for the treatment of depression and anxiety. Int J Mol Sci 2022;23:7077.
Latimer CS, Brewer LD, Searcy JL, Chen K-C, Popović J, Kraner SD, et al. Vitamin D prevents cognitive decline and enhances hippocampal synaptic function in aging rats. Proc Natl Acad Sci 2014;111:E4359–66.
Giza JI, Jung Y, Jeffrey RA, Neugebauer NM, Picciotto MR, Biederer T. The synaptic adhesion molecule SynCAM 1 contributes to cocaine effects on synapse structure and psychostimulant behavior. Neuropsychopharmacology 2013;38:628–38.
Tønnesen J, Katona G, Rózsa B, Nägerl UV. Spine neck plasticity regulates compartmentalization of synapses. Nat Neurosci 2014;17:678–85.
van Elburg RAJ, van Ooyen A. Impact of dendritic size and dendritic topology on burst firing in pyramidal cells. PLoS Comput Biol 2010;6:e1000781.
Abramoff MD, Magalhaes PJ, Ram SJ. Image processing with ImageJ. Biophoton Int 2004;11:36–42.
Holmes SE, Finnema SJ, Naganawa M, DellaGioia N, Holden D, Fowles K, et al. Imaging the effect of ketamine on synaptic density (SV2A) in the living brain. Mol Psychiatry 2022;27:2273–81.
Beroun A, Nalberczak-Skóra M, Harda Z, Piechota M, Ziółkowska M, Cały A, et al. Generation of silent synapses in dentate gyrus correlates with development of alcohol addiction. Neuropsychopharmacology. 2018;43:1989–19.
Cadete-Leite A, Amelia Tavares M, Uylings HBM, Paula-Barbosa M. Granule cell loss and dendritic regrowth in the hippocampal dentate gyrus of the rat after chronic alcohol consumption. Brain Res. 1988;473:1–14.
Frost ME, Peterson VL, Bird CW, McCool B, Hamilton DA. Effects of Ethanol Exposure and Withdrawal on Neuronal Morphology in the Agranular Insular and Prelimbic Cortices: Relationship with Withdrawal-Related Structural Plasticity in the Nucleus Accumbens. Brain Sci. 2019;9:180.
Galaj E, Guo C, Huang D, Ranaldi R, Ma Y-Y. Contrasting effects of adolescent and early-adult ethanol exposure on prelimbic cortical pyramidal neurons. Drug. Alcohol. Dependence. 2020;216:108309.
King MA, Hunter BE, Walker DW. Alterations and recovery of dendritic spine density in rat hippocampus following long-term ethanol ingestion. Brain Res. 1988;459:381–5.
Klenowski PM, Fogarty MJ, Shariff M, Belmer A, Bellingham MC, Bartlett SE. Increased Synaptic Excitation and Abnormal Dendritic Structure of Prefrontal Cortex Layer V Pyramidal Neurons following Prolonged Binge-Like Consumption of Ethanol. Eneuro. 2016;3:ENEURO.0248-16. 2016
Navarro AI, Mandyam CD. Protracted abstinence from chronic ethanol exposure alters the structure of neurons and expression of oligodendrocytes and myelin in the medial prefrontal cortex. Neuroscience. 2015;293:35–44.
Obray JD, Landin JD, Vaughan DT, Scofield MD, Chandler LJ. Adolescent alcohol exposure reduces dopamine 1 receptor modulation of prelimbic neurons projecting to the nucleus accumbens and basolateral amygdala. Addiction Neurosci. 2022;4:100044.
Riley JN, Walker DW. Morphological Alterations in Hippocampus After Long-Term Alcohol Consumption in Mice. Science. 1978;201:646–8.
Trantham-Davidson H, Centanni SW, Garr SC, New NN, Mulholland PJ, Gass JT, et al. Binge-Like Alcohol Exposure During Adolescence Disrupts Dopaminergic Neurotransmission in the Adult Prelimbic Cortex. Neuropsychopharmacology. 2017;42:1024–36.
Varodayan FP, Sidhu H, Kreifeldt M, Roberto M, Contet C. Morphological and functional evidence of increased excitatory signaling in the prelimbic cortex during ethanol withdrawal. Neuropharmacology. 2018;133:470–80.
Kolb B, Li Y, Robinson T, Parker LA. THC alters alters morphology of neurons in medial prefrontal cortex, orbital prefrontal cortex, and nucleus accumbens and alters the ability of later experience to promote structural plasticity. Synapse. 2018;72:e22020.
Landfield PW, Cadwallader LB, Vinsant S. Quantitative changes in hippocampal structure following long-term exposure to Δ9-tetrahydrocannabinol: possible mediation by glucocorticoid systems. Brain Res. 1988;443:47–62.
Miller ML, Chadwick B, Dickstein DL, Purushothaman I, Egervari G, Rahman T, et al. Adolescent exposure to Δ9-tetrahydrocannabinol alters the transcriptional trajectory and dendritic architecture of prefrontal pyramidal neurons. Mol. Psychiatry. 2019;24:588–600.
Rubino T, Realini N, Braida D, Guidi S, Capurro V, Viganò D, et al. Changes in hippocampal morphology and neuroplasticity induced by adolescent THC treatment are associated with cognitive impairment in adulthood. Hippocampus. 2009;19:763–72.
Spencer S, Neuhofer D, Chioma VC, Garcia-Keller C, Schwartz DJ, Allen N, et al. A Model of Δ9-Tetrahydrocannabinol Self-administration and Reinstatement That Alters Synaptic Plasticity in Nucleus Accumbens. Biol. Psychiatry. 2018;84:601–10.
Freels TG, Westbrook SR, Wright HR, Kuyat JR, Zamberletti E, Malena AM, et al. Sex differences in adolescent cannabis vapor self-administration mediate enduring effects on behavioral flexibility and prefrontal microglia activation in rats. BioRxiv. 2023. https://doi.org/10.1101/2023.01.21.524468
Spiga S, Lintas A, Diana M. Altered Mesolimbic Dopamine System in THC Dependence. Curr. Neuropharmacol. 2011;9:200–4.
Bonilla‐Del Río I, Puente N, Mimenza A, Ramos A, Serrano M, Lekunberri L, et al. Acute Δ9-tetrahydrocannabinol prompts rapid changes in cannabinoid CB1 receptor immunolabeling and subcellular structure in CA1 hippocampus of young adult male mice. J. Comp. Neurol. 2021;529:2332–46.
Avalos MP, Guzman AS, Garcia-Keller C, Mongi-Bragato B, Esparza MA, Rigoni D, et al. Impairment of glutamate homeostasis in the nucleus accumbens core underpins cross-sensitization to cocaine following chronic restraint stress. Front. Physiol. 2022;13:896268.
Blazquez-Llorca L, Miguéns M, Montero-Crespo M, Selvas A, Gonzalez-Soriano J, Ambrosio E, et al. 3D Synaptic Organization of the Rat CA1 and Alterations Induced by Cocaine Self-Administration. Cereb. Cortex. 2021;31:1927–52.
Caffino L, Messa G, Fumagalli F. A single cocaine administration alters dendritic spine morphology and impairs glutamate receptor synaptic retention in the medial prefrontal cortex of adolescent rats. Neuropharmacology. 2018;140:209–16.
Christian DT, Wang X, Chen EL, Sehgal LK, Ghassemlou MN, Miao JJ, et al. Dynamic Alterations of Rat Nucleus Accumbens Dendritic Spines over 2 Months of Abstinence from Extended-Access Cocaine Self-Administration. Neuropsychopharmacology. 2017;42:748–56.
Dos Santos M, Salery M, Forget B, Garcia Perez MA, Betuing S, Boudier T, et al. Rapid Synaptogenesis in the Nucleus Accumbens Is Induced by a Single Cocaine Administration and Stabilized by Mitogen-Activated Protein Kinase Interacting Kinase-1 Activity. Biol. Psychiatry. 2017;82:806–18.
Ferrario CR, Gorny G, Crombag HS, Li Y, Kolb B, Robinson TE. Neural and Behavioral Plasticity Associated with the Transition from Controlled to Escalated Cocaine Use. Biol. Psychiatry. 2005;58:751–9.
Ka M, Kook Y-H, Liao K, Buch S, Kim W-Y. Transactivation of TrkB by Sigma-1 receptor mediates cocaine-induced changes in dendritic spine density and morphology in hippocampal and cortical neurons. Cell Death amp; Dis. 2016;7:e2414.
Kolb B, Gorny G, Li Y, Samaha A-N, Robinson TE. Amphetamine or cocaine limits the ability of later experience to promote structural plasticity in the neocortex and nucleus accumbens. Proc. Natl Acad. Sci. 2003;100:10523–8.
Li H, Chen R, Zhou Y, Wang H, Sun L, Yang Z, et al. Endocannabinoids regulate cocaine-associated memory through brain AEA–CB1R signalling activation. Mol. Metab. 2022;65:101597.
Radley JJ, Anderson RM, Cosme CV, Glanz RM, Miller MC, Romig-Martin SA, et al. The Contingency of Cocaine Administration Accounts for Structural and Functional Medial Prefrontal Deficits and Increased Adrenocortical Activation. J. Neurosci. 2015;35:11897–910.
Rasakham K, Schmidt HD, Kay K, Huizenga MN, Calcagno N, Pierce RC, et al. Synapse Density and Dendritic Complexity Are Reduced in the Prefrontal Cortex following Seven Days of Forced Abstinence from Cocaine Self-Administration. PLoS ONE. 2014;9:e102524.
Robinson TE, Gorny G, Mitton E, Kolb B. Cocaine self-administration alters the morphology of dendrites and dendritic spines in the nucleus accumbens and neocortex. Synapse. 2001;39:257–66.
Siemsen BM, Barry SM, Vollmer KM, Green LM, Brock AG, Westphal AM, et al. A Subset of Nucleus Accumbens Neurons Receiving Dense and Functional Prelimbic Cortical Input Are Required for Cocaine Seeking. Front. Cell Neurosci. 2022;16:844243.
Spencer S, Garcia-Keller C, Roberts-Wolfe D, Heinsbroek JA, Mulvaney M, Sorrell A, et al. Cocaine Use Reverses Striatal Plasticity Produced During Cocaine Seeking. Biol. Psychiatry. 2017;81:616–24.
Stankeviciute NM, Scofield MD, Kalivas PW, Gipson CD. Rapid, transient potentiation of dendritic spines in context-induced relapse to cocaine seeking. Addiction Biol. 2014;19:972–4.
Zhu W, Ge X, Gao P, Li M, Guan Y, Guan X. Adolescent cocaine exposure induces prolonged synaptic modifications in medial prefrontal cortex of adult rats. Brain Struct. Funct. 2018;223:1829–38.
Chen H, Chen L, Yuan Z, Yuan J, Li Y, Xu Y, et al. Glutamate receptor-interacting protein 1 in D1- and D2-dopamine receptor-expressing medium spiny neurons differentially regulates cocaine acquisition, reinstatement, and associated spine plasticity. Front. Cell Neurosci. 2022;16:979078.
Lissek T, Andrianarivelo A, Saint‐Jour E, Allichon M, Bauersachs HG, Nassar M, et al. Npas4 regulates medium spiny neuron physiology and gates cocaine‐induced hyperlocomotion. EMBO Rep. 2021;22.
Brown RW, Kolb B. Nicotine sensitization increases dendritic length and spine density in the nucleus accumbens and cingulate cortex. Brain Res. 2001;899:94–100.
Ehlinger DG, Bergstrom HC, Burke JC, Fernandez GM, McDonald CG, Smith RF. Adolescent nicotine-induced dendrite remodeling in the nucleus accumbens is rapid, persistent, and D1-dopamine receptor dependent. Brain Structure Funct. 2016;221:133–45.
Gipson CD, Reissner KJ, Kupchik YM, Smith ACW, Stankeviciute N, Hensley-Simon ME, et al. Reinstatement of nicotine seeking is mediated by glutamatergic plasticity. Proc. Natl Acad. Sci. 2013;110:9124–9.
Cai Y, Yang L, Hu G, Chen X, Niu F, Yuan L, et al. Regulation of morphine-induced synaptic alterations: Role of oxidative stress, ER stress, and autophagy. J. Cell Biol. 2016;215:245–58.
Fakira AK, Massaly N, Cohensedgh O, Berman A, Morón JA. Morphine-Associated Contextual Cues Induce Structural Plasticity in Hippocampal CA1 Pyramidal Neurons. Neuropsychopharmacology. 2016;41:2668–78.
Jia M, Wang X, Zhang H, Wang X, Ma H, Yang M, et al. MicroRNA‐132 is involved in morphine dependence via modifying the structural plasticity of the dentate gyrus neurons in rats. Addict. Biol. 2022;27:e13086.
Kobrin KL, Moody O, Arena DT, Moore CF, Heinrichs SC, Kaplan GB. Acquisition of morphine conditioned place preference increases the dendritic complexity of nucleus accumbens core neurons. Addiction Biol. 2016;21:1086–96.
Arroyo-García LE, Tendilla-Beltrán H, Vázquez-Roque RA, Jurado-Tapia EE, Díaz A, Aguilar-Alonso P, et al. Amphetamine sensitization alters hippocampal neuronal morphology and memory and learning behaviors. Mol. Psychiatry. 2021;26:4784–94.
Diaz Heijtz R, Kolb B, Forssberg H. Can a therapeutic dose of amphetamine during pre-adolescence modify the pattern of synaptic organization in the brain? Eur. J. Neurosci. 2003;18:3394–9.
Li Y, Kolb B, Robinson TE. The Location of Persistent Amphetamine-Induced Changes in the Density of Dendritic Spines on Medium Spiny Neurons in the Nucleus Accumbens and Caudate-Putamen. Neuropsychopharmacology. 2003;28:1082–5.
Morshedi MM, Rademacher DJ, Meredith GE. Increased synapses in the medial prefrontal cortex are associated with repeated amphetamine administration. Synapse. 2008;63:126–35.
Acknowledgements
The work described in this manuscript was funded in part by the State of Connecticut, Department of Mental Health and Addiction Services, but this publication does not express the views of the Department of Mental Health and Addiction Services or the State of Connecticut. The views and opinions expressed are those of the authors.
Funding
This study was supported in part by the NIDA grants R01 DA052454-03 (GAA, MNP), R01 AT010508-01A1 (MNP, GAA), R21 DA046030 (GAA) and P30 DA046345 (GAA, HNPO). During manuscript preparation HNPO and GAA received helpful feedback at the Critical Research Issues in Latinx Mental Health annual meeting, which is sponsored in parts by NIMH and NIDA 1R13MH132238 (PI Gallego).
Author information
Authors and Affiliations
Contributions
All authors (HNPO, TPP, EJN, KPC, RR, MNP, GAA) conceptualized the scope and content of this review. HNPO, TPP, EJN and GAA drafted the manuscript. KPC, RR, MNP and GAA critically revised the manuscript and approved the final version to be published.
Corresponding author
Ethics declarations
Competing interests
MNP discloses that he has consulted for Opiant Therapeutics, Game Day Data, Baria-Tek, the Addiction Policy Forum, AXA and Idorsia Pharmaceuticals; been involved in a patent application with Yale University and Novartis; received research support from the Mohegan Sun Casino, Children and Screens and the Connecticut Council on Problem Gambling; and consulted for legal and gambling entities on issues related to impulse control, internet use and addictions. The other authors report no disclosures.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Oliva, H.N.P., Prudente, T.P., Nunes, E.J. et al. Substance use and spine density: a systematic review and meta-analysis of preclinical studies. Mol Psychiatry (2024). https://doi.org/10.1038/s41380-024-02519-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41380-024-02519-3