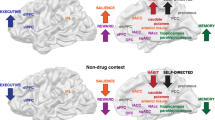
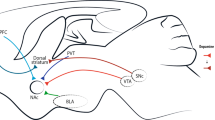
Abstract
In 1986, Gawin and Kleber reported a progressive increase in cue-induced drug craving in individuals with cocaine use disorders during prolonged abstinence. After years of controversy, as of 2001, this phenomenon was confirmed in rodent studies using self-administration model, and defined as the incubation of drug craving. The intensification of cue-induced drug craving after withdrawal exposes abstinent individuals to a high risk of relapse, which urged us to develop effective interventions to prevent incubated craving. Substantial achievements have been made in deciphering the neural mechanisms, with potential implications for reducing drug craving and preventing the relapse. The present review discusses promising drug targets that have been well investigated in animal studies, including some neurotransmitters, neuropeptides, neurotrophic factors, and epigenetic markers. We also discuss translational exploitation and challenges in the field of the incubation of drug craving, providing insights into future investigations and highlighting the potential of pharmacological interventions, environment-based interventions, and neuromodulation techniques.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Brandon TH, Vidrine JI, Litvin EB. Relapse and relapse prevention. Annu Rev Clin Psychol. 2007;3:257–84.
Volkow ND, Boyle M. Neuroscience of addiction: Relevance to prevention and treatment. Am J Psychiatry. 2018;175:729–40.
Ahmed SH, Badiani A, Miczek KA, Muller CP. Non-pharmacological factors that determine drug use and addiction. Neurosci Biobehav Rev. 2020;110:3–27.
Ruisoto P, Contador I. The role of stress in drug addiction. An integrative review. Physiol Behav. 2019;202:62–8.
O’Brien CP, Childress AR, McLellan AT, Ehrman R. Classical conditioning in drug-dependent humans. Ann N. Y Acad Sci. 1992;654:400–15.
George O, Koob GF. Control of craving by the prefrontal cortex. Proc Natl Acad Sci USA. 2013;110:4165–6.
Sayette MA. The role of craving in substance use disorders: theoretical and methodological issues. Annu Rev Clin Psychol. 2016;12:407–33.
Gawin FH, Kleber HD. Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Arch Gen Psychiatry. 1986;43:107–13.
Grimm JW, Hope BT, Wise RA, Shaham Y. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–2.
Shalev U, Morales M, Hope B, Yap J, Shaham Y. Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacology. 2001;156:98–107.
Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20:798–805.
Parvaz MA, Moeller SJ, Goldstein RZ. Incubation of cue-induced craving in adults addicted to cocaine measured by electroencephalography. JAMA Psychiatry. 2016;73:1127–34.
Nicolas C, Russell TI, Pierce AF, Maldera S, Holley A, You ZB, et al. Incubation of cocaine craving after intermittent-access self-administration: sex differences and estrous cycle. Biol Psychiatry. 2019;85:915–24.
Nugent AL, Anderson EM, Larson EB, Self DW. Incubation of cue-induced reinstatement of cocaine, but not sucrose, seeking in C57BL/6J mice. Pharm Biochem Behav. 2017;159:12–7.
Lubbers BR, Matos MR, Horn A, Visser E, Van der Loo RC, Gouwenberg Y, et al. The extracellular matrix protein brevican limits time-dependent enhancement of cocaine conditioned place preference. Neuropsychopharmacology. 2016;41:1907–16.
Bedi G, Preston KL, Epstein DH, Heishman SJ, Marrone GF, Shaham Y, et al. Incubation of cue-induced cigarette craving during abstinence in human smokers. Biol Psychiatry. 2011;69:708–11.
Li P, Wu P, Xin X, Fan YL, Wang GB, Wang F, et al. Incubation of alcohol craving during abstinence in patients with alcohol dependence. Addict Biol. 2015;20:513–22.
Koskela M, Piepponen TP, Andressoo JO, Võikar V, Airavaara M. Towards developing a model to study alcohol drinking and craving in female mice housed in automated cages. Behav Brain Res. 2018;352:116–24.
Treloar Padovano H, Miranda R Jr. Incubation of alcohol craving as it naturally occurs in a developmentally diverse sample of dependent and nondependent drinkers. Addict Biol. 2021;26:e12934.
Venniro M, Russell TI, Zhang M, Shaham Y. Operant social reward decreases incubation of heroin craving in male and female rats. Biol Psychiatry. 2019;86:848–56.
Airavaara M, Pickens CL, Stern AL, Wihbey KA, Harvey BK, Bossert JM, et al. Endogenous GDNF in ventral tegmental area and nucleus accumbens does not play a role in the incubation of heroin craving. Addict Biol. 2011;16:261–72.
Li YQ, Li FQ, Wang XY, Wu P, Zhao M, Xu CM, et al. Central amygdala extracellular signal-regulated kinase signaling pathway is critical to incubation of opiate craving. J Neurosci. 2008;28:13248–57.
Wang G, Shi J, Chen N, Xu L, Li J, Li P, et al. Effects of length of abstinence on decision-making and craving in methamphetamine abusers. PLoS One. 2013;8:68791–6.
Zhao D, Zhang M, Tian W, Cao X, Yin L, Liu Y, et al. Neurophysiological correlate of incubation of craving in individuals with methamphetamine use disorder. Mol Psychiatry. 2021;26:6198–208.
Fredriksson I, Tsai PJ, Shekara A, Duan Y, Applebey SV, Lu H, et al. Orbitofrontal cortex and dorsal striatum functional connectivity predicts incubation of opioid craving after voluntary abstinence. Proc Natl Acad Sci USA. 2021;118:e2106624118.
Gyawali U, Martin DA, Sulima A, Rice KC, Calu DJ. Role of BNST CRFR1 receptors in incubation of fentanyl seeking. Front Behav Neurosci. 2020;14:153.
Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of the incubation of drug craving. Trends Neurosci. 2011;34:411–20.
Li X, Caprioli D, Marchant NJ. Recent updates on incubation of drug craving: a mini-review. Addict Biol. 2015;20:872–6.
Altshuler RD, Lin H, Li X. Neural mechanisms underlying incubation of methamphetamine craving: a mini-review. Pharm Biochem Behav. 2020;199:173058–71.
Venniro M, Reverte I, Ramsey LA, Papastrat KM, D’Ottavio G, Milella MS, et al. Factors modulating the incubation of drug and non-drug craving and their clinical implications. Neurosci Biobehav Rev. 2021;131:847–64.
Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–72.
Shin CB, Serchia MM, Shahin JR, Ruppert-Majer MA, Kippin TE, Szumlinski KK. Incubation of cocaine-craving relates to glutamate over-flow within ventromedial prefrontal cortex. Neuropharmacology. 2016;102:103–10.
Shin CB, Templeton TJ, Chiu AS, Kim J, Gable ES, Vieira PA, et al. Endogenous glutamate within the prelimbic and infralimbic cortices regulates the incubation of cocaine-seeking in rats. Neuropharmacology. 2018;128:293–300.
Lu L, Uejima JL, Gray SM, Bossert JM, Shaham Y. Systemic and central amygdala injections of the mGluR(2/3) agonist LY379268 attenuate the expression of incubation of cocaine craving. Biol Psychiatry. 2007;61:591–8.
Wolf ME, Tseng KY. Calcium-permeable AMPA receptors in the VTA and nucleus accumbens after cocaine exposure: When, how, and why? Front Mol Neurosci. 2012;5:72–100.
Dong Y, Taylor JR, Wolf ME, Shaham Y. Circuit and synaptic plasticity mechanisms of drug relapse. J Neurosci. 2017;37:10867–76.
Pomierny-Chamiolo L, Rup K, Pomierny B, Niedzielska E, Kalivas PW, Filip M. Metabotropic glutamatergic receptors and their ligands in drug addiction. Pharm Ther. 2014;142:281–305.
Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, et al. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–21.
Ferrario CR, Loweth JA, Milovanovic M, Ford KA, Galiñanes GL, Heng LJ, et al. Alterations in AMPA receptor subunits and TARPs in the rat nucleus accumbens related to the formation of Ca2+-permeable AMPA receptors during the incubation of cocaine craving. Neuropharmacology. 2011;61:1141–51.
Lee BR, Ma YY, Huang YH, Wang X, Otaka M, Ishikawa M, et al. Maturation of silent synapses in amygdala-accumbens projection contributes to incubation of cocaine craving. Nat Neurosci. 2013;16:1644–51.
Ma YY, Lee BR, Wang X, Guo C, Liu L, Cui R, et al. Bidirectional modulation of incubation of cocaine craving by silent synapse-based remodeling of prefrontal cortex to accumbens projections. Neuron. 2014;83:1453–67.
Scheyer AF, Loweth JA, Christian DT, Uejima J, Rabei R, Le T, et al. AMPA receptor plasticity in accumbens core contributes to incubation of methamphetamine craving. Biol Psychiatry. 2016;80:661–70.
Faccidomo S, Cogan ES, Hon OJ, Hoffman JL, Saunders BL, Eastman VR, et al. Calcium-permeable AMPA receptor activity and GluA1 trafficking in the basolateral amygdala regulate operant alcohol self-administration. Addict Biol. 2021;26:e13049.
Liechti ME, Lhuillier L, Kaupmann K, Markou A. Metabotropic glutamate 2/3 receptors in the ventral tegmental area and the nucleus accumbens shell are involved in behaviors relating to nicotine dependence. J Neurosci. 2007;27:9077–85.
Schoepp DD. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J Pharm Exp Ther. 2001;299:12–20.
Caprioli D, Justinova Z, Venniro M, Shaham Y. Effect of novel allosteric modulators of metabotropic glutamate receptors on drug self-administration and relapse: a review of preclinical studies and their clinical implications. Biol Psychiatry. 2018;84:180–92.
Conn PJ, Christopoulos A, Lindsley CW. Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat Rev Drug Disco. 2009;8:41–54.
Conn PJ, Lindsley CW, Meiler J, Niswender CM. Opportunities and challenges in the discovery of allosteric modulators of GPCRs for treating CNS disorders. Nat Rev Drug Disco. 2014;13:692–708.
Loweth JA, Scheyer AF, Milovanovic M, LaCrosse AL, Flores-Barrera E, Werner CT, et al. Synaptic depression via mGluR1 positive allosteric modulation suppresses cue-induced cocaine craving. Nat Neurosci. 2014;17:73–80.
Caprioli D, Venniro M, Zeric T, Li X, Adhikary S, Madangopal R, et al. Effect of the novel positive allosteric modulator of metabotropic glutamate receptor 2 AZD8529 on incubation of methamphetamine craving after prolonged voluntary abstinence in a rat model. Biol Psychiatry. 2015;78:463–73.
Volkow ND, Wise RA, Baler R. The dopamine motive system: Implications for drug and food addiction. Nat Rev Neurosci. 2017;18:741–52.
Grimm JW, Shaham Y, Hope BT. Effect of cocaine and sucrose withdrawal period on extinction behavior, cue-induced reinstatement, and protein levels of the dopamine transporter and tyrosine hydroxylase in limbic and cortical areas in rats. Behav Pharm. 2002;13:379–88.
Alonso IP, O’Connor BM, Bryant KG, Mandalaywala RK, Espana RA. Incubation of cocaine craving coincides with changes in dopamine terminal neurotransmission. Addict Neurosci. 2022;3:100029.
Rossi LM, Reverte I, Ragozzino D, Badiani A, Venniro M, Caprioli D. Role of nucleus accumbens core but not shell in incubation of methamphetamine craving after voluntary abstinence. Neuropsychopharmacology. 2020;45:256–65.
Xi ZX, Li X, Li J, Peng XQ, Song R, Gaál J, et al. Blockade of dopamine D3 receptors in the nucleus accumbens and central amygdala inhibits incubation of cocaine craving in rats. Addict Biol. 2013;18:665–77.
Grimm JW, Harkness JH, Ratliff C, Barnes J, North K, Collins S. Effects of systemic or nucleus accumbens-directed dopamine D1 receptor antagonism on sucrose seeking in rats. Psychopharmacol (Berl). 2011;216:219–33.
Le Merrer J, Becker JA, Befort K, Kieffer BL. Reward processing by the opioid system in the brain. Physiol Rev. 2009;89:1379–412.
Dikshtein Y, Barnea R, Kronfeld N, Lax E, Roth-Deri I, Friedman A, et al. β-endorphin via the delta opioid receptor is a major factor in the incubation of cocaine craving. Neuropsychopharmacology. 2013;38:2508–14.
Bailey CP, Husbands SM. Novel approaches for the treatment of psychostimulant and opioid abuse - focus on opioid receptor-based therapies. Expert Opin Drug Disco. 2014;9:1333–44.
Theberge FR, Li X, Kambhampati S, Pickens CL, St Laurent R, Bossert JM, et al. Effect of chronic delivery of the Toll-like receptor 4 antagonist (+)-naltrexone on incubation of heroin craving. Biol Psychiatry. 2013;73:729–37.
Theberge FR, Pickens CL, Goldart E, Fanous S, Hope BT, Liu QR, et al. Association of time-dependent changes in mu opioid receptor mRNA, but not BDNF, TrkB, or MeCP2 mRNA and protein expression in the rat nucleus accumbens with incubation of heroin craving. Psychopharmacol (Berl). 2012;224:559–71.
Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–9.
DiLeone RJ, Georgescu D, Nestler EJ. Lateral hypothalamic neuropeptides in reward and drug addiction. Life Sci. 2003;73:759–68.
James MH, Stopper CM, Zimmer BA, Koll NE, Bowrey HE, Aston-Jones G. Increased number and activity of a lateral subpopulation of hypothalamic orexin/hypocretin neurons underlies the expression of an addicted state in rats. Biol Psychiatry. 2019;85:925–35.
Steiner N, Rossetti C, Sakurai T, Yanagisawa M, de Lecea L, Magistretti PJ, et al. Hypocretin/orexin deficiency decreases cocaine abuse liability. Neuropharmacology. 2018;133:395–403.
Nagahara AH, Tuszynski MH. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat Rev Drug Disco. 2011;10:209–19.
Wang CS, Kavalali ET, Monteggia LM. BDNF signaling in context: From synaptic regulation to psychiatric disorders. Cell. 2022;185:62–76.
Schmidt HD, Sangrey GR, Darnell SB, Schassburger RL, Cha JH, Pierce RC, et al. Increased brain-derived neurotrophic factor (BDNF) expression in the ventral tegmental area during cocaine abstinence is associated with increased histone acetylation at BDNF exon I-containing promoters. J Neurochem. 2012;120:202–9.
Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci. 2003;23:742–7.
Li X, DeJoseph MR, Urban JH, Bahi A, Dreyer JL, Meredith GE, et al. Different roles of BDNF in nucleus accumbens core versus shell during the incubation of cue-induced cocaine craving and its long-term maintenance. J Neurosci. 2013;33:1130–42.
Kuntz-Melcavage KL, Brucklacher RM, Grigson PS, Freeman WM, Vrana KE. Gene expression changes following extinction testing in a heroin behavioral incubation model. BMC Neurosci. 2009;10:95.
Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y. Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat Neurosci. 2005;8:212–9.
Lu L, Wang X, Wu P, Xu C, Zhao M, Morales M, et al. Role of ventral tegmental area glial cell line-derived neurotrophic factor in incubation of cocaine craving. Biol Psychiatry. 2009;66:137–45.
Szumlinski KK, Ary AW, Shin CB, Wroten MG, Courson J, Miller BW, et al. PI3K activation within ventromedial prefrontal cortex regulates the expression of drug-seeking in two rodent species. Addict Biol. 2019;24:1216–26.
Chiu AS, Kang MC, Huerta Sanchez LL, Fabella AM, Holder KN, Barger BD, et al. Preclinical evidence to support repurposing everolimus for craving reduction during protracted drug withdrawal. Neuropsychopharmacology. 2021;46:2090–100.
Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci. 2011;12:623–37.
Nielsen DA, Huang W, Hamon SC, Maili L, Witkin BM, Fox RG, et al. Forced abstinence from cocaine self-administration is associated with DNA methylation changes in myelin genes in the corpus callosum: a preliminary study. Front Psychiatry. 2012;3:60–8.
Massart R, Barnea R, Dikshtein Y, Suderman M, Meir O, Hallett M, et al. Role of DNA methylation in the nucleus accumbens in incubation of cocaine craving. J Neurosci. 2015;35:8042–58.
Borrelli E, Nestler EJ, Allis CD, Sassone-Corsi P. Decoding the epigenetic language of neuronal plasticity. Neuron. 2008;60:961–74.
Li X, Carreria MB, Witonsky KR, Zeric T, Lofaro OM, Bossert JM, et al. Role of dorsal striatum histone deacetylase 5 in incubation of methamphetamine craving. Biol Psychiatry. 2018;84:213–22.
Wang GB, Zhang XL, Zhao LY, Sun LL, Wu P, Lu L, et al. Drug-related cues exacerbate decision making and increase craving in heroin addicts at different abstinence times. Psychopharmacol (Berl). 2012;221:701–8.
Moeller SJ, Goldstein RZ. Impaired self-awareness in human addiction: deficient attribution of personal relevance. Trends Cogn Sci. 2014;18:635–41.
Crowne DP, Marlowe D. A new scale of social desirability independent of psychopathology. J Consult Psychol. 1960;24:349–54.
Hajcak G, MacNamara A, Foti D, Ferri J, Keil A. The dynamic allocation of attention to emotion: Simultaneous and independent evidence from the late positive potential and steady state visual evoked potentials. Biol Psychol. 2013;92:447–55.
Hajcak G, Olvet DM. The persistence of attention to emotion: Brain potentials during and after picture presentation. Emotion 2008;8:250–5.
Franken IH, Dietvorst RC, Hesselmans M, Franzek EJ, van de Wetering BJ, Van Strien JW. Cocaine craving is associated with electrophysiological brain responses to cocaine-related stimuli. Addict Biol. 2008;13:386–92.
Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–13.
Chen B, Wang Y, Liu X, Liu Z, Dong Y, Huang YH. Sleep regulates incubation of cocaine craving. J Neurosci. 2015;35:13300–10.
Chauvet C, Goldberg SR, Jaber M, Solinas M. Effects of environmental enrichment on the incubation of cocaine craving. Neuropharmacology. 2012;63:635–41.
Zlebnik NE, Carroll ME. Prevention of the incubation of cocaine seeking by aerobic exercise in female rats. Psychopharmacol (Berl). 2015;232:3507–13.
Sanchez V, Bakhti-Suroosh A, Chen A, Brunzell DH, Erisir A, Lynch WJ. Exercise during abstinence normalizes ultrastructural synaptic plasticity associated with nicotine-seeking following extended access self-administration. Eur J Neurosci. 2019;50:2707–21.
Venniro M, Zhang M, Shaham Y, Caprioli D. Incubation of methamphetamine but not heroin craving after voluntary abstinence in male and female rats. Neuropsychopharmacology. 2017;42:1126–35.
D’Ottavio G, Reverte I, Ragozzino D, Meringolo M, Milella MS, Boix F, et al. Increased heroin intake and relapse vulnerability in intermittent relative to continuous self-administration: Sex differences in rats. Br J Pharmacol. 2022; https://doi.org/10.1111/bph.15791.
Krasnova IN, Marchant NJ, Ladenheim B, McCoy MT, Panlilio LV, Bossert JM, et al. Incubation of methamphetamine and palatable food craving after punishment-induced abstinence. Neuropsychopharmacology. 2014;39:2008–16.
Madsen HB, Zbukvic IC, Luikinga SJ, Lawrence AJ, Kim JH. Extinction of conditioned cues attenuates incubation of cocaine craving in adolescent and adult rats. Neurobiol Learn Mem. 2017;143:88–93.
Heilig M, Epstein DH, Nader MA, Shaham Y. Time to connect: Bringing social context into addiction neuroscience. Nat Rev Neurosci. 2016;17:592–9.
Bach P, Weil G, Pompili E, Hoffmann S, Hermann D, Vollstädt-Klein S, et al. Incubation of neural alcohol cue reactivity after withdrawal and its blockade by naltrexone. Addict Biol. 2020;25:12717–27.
Loeber S, Kiefer F, Wagner F, Mann K, Croissant B. Treatment outcome after inpatient alcohol withdrawal: Impact of motivational interventions: a comparative study. Nervenarzt. 2009;80:1085–92.
Narayanaswami V, Dwoskin LP. Obesity: current and potential pharmacotherapeutics and targets. Pharm Ther. 2017;170:116–47.
Epstein AM, King AC. Naltrexone attenuates acute cigarette smoking behavior. Pharm Biochem Behav. 2004;77:29–37.
Volpicelli JR, Alterman AI, Hayashida M, O’Brien CP. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49:876–80.
Croop RS, Faulkner EB, Labriola DF. The safety profile of naltrexone in the treatment of alcoholism. Results from a multicenter usage study. The naltrexone usage study group. Arch Gen Psychiatry. 1997;54:1130–5.
Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003–17.
Chauvet C, Lardeux V, Goldberg SR, Jaber M, Solinas M. Environmental enrichment reduces cocaine seeking and reinstatement induced by cues and stress but not by cocaine. Neuropsychopharmacology. 2009;34:2767–78.
Solinas M, Chauvet C, Thiriet N, El Rawas R, Jaber M. Reversal of cocaine addiction by environmental enrichment. Proc Natl Acad Sci USA. 2008;105:17145–50.
Fredriksson I, Applebey SV, Minier-Toribio A, Shekara A, Bossert JM, Shaham Y. Effect of the dopamine stabilizer (-)-OSU6162 on potentiated incubation of opioid craving after electric barrier-induced voluntary abstinence. Neuropsychopharmacology. 2020;45:770–9.
Caprioli D, Venniro M, Zhang M, Bossert JM, Warren BL, Hope BT, et al. Role of dorsomedial striatum neuronal wnsembles in incubation of methamphetamine craving after voluntary abstinence. J Neurosci. 2017;37:1014–27.
Weiss G. Food fantasies of incarcerated drug users. Int J Addict. 1982;17:905–12.
Zador D, Lyons Wall PM, Webster I. High sugar intake in a group of women on methadone maintenance in south western Sydney, Australia. Addiction. 1996;91:1053–61.
Neale J, Nettleton S, Pickering L, Fischer J. Eating patterns among heroin users: a qualitative study with implications for nutritional interventions. Addiction. 2012;107:635–41.
Venniro M, Shaham Y. An operant social self-administration and choice model in rats. Nat Protoc. 2020;15:1542–59.
Venniro M, Zhang M, Caprioli D, Hoots JK, Golden SA, Heins C, et al. Volitional social interaction prevents drug addiction in rat models. Nat Neurosci. 2018;21:1520–9.
Venniro M, Russell TI, Ramsey LA, Richie CT, Lesscher HMB, Giovanetti SM, et al. Abstinence-dependent dissociable central amygdala microcircuits control drug craving. Proc Natl Acad Sci USA. 2020;117:8126–34.
Smith JE, Meyers RJ, Miller WR. The community reinforcement approach to the treatment of substance use disorders. Am J Addictions. 2001;10:51–9.
Meyers RJ, Roozen HG, Smith JE. The community reinforcement approach: an update of the evidence. Alcohol Res Health. 2011;33:380–8.
De Jong CA, Roozen HG, van Rossum LG, Krabbe PF, Kerkhof AJ. High abstinence rates in heroin addicts by a new comprehensive treatment approach. Am J Addict. 2007;16:124–30.
Won SM, Song E, Reeder JT, Rogers JA. Emerging modalities and implantable technologies for neuromodulation. Cell. 2020;181:115–35.
Chase HW, Boudewyn MA, Carter CS, Phillips ML. Transcranial direct current stimulation: a roadmap for research, from mechanism of action to clinical implementation. Mol Psychiatry. 2020;25:397–407.
Diana M, Raij T, Melis M, Nummenmaa A, Leggio L, Bonci A. Rehabilitating the addicted brain with transcranial magnetic stimulation. Nat Rev Neurosci. 2017;18:685–93.
Krauss JK, Lipsman N, Aziz T, Boutet A, Brown P, Chang JW, et al. Technology of deep brain stimulation: current status and future directions. Nat Rev Neurol. 2021;17:75–87.
Habelt B, Arvaneh M, Bernhardt N, Minev I. Biomarkers and neuromodulation techniques in substance use disorders. Bioelectron Med. 2020;6:4–20.
Spagnolo PA, Goldman D. Neuromodulation interventions for addictive disorders: Challenges, promise, and roadmap for future research. Brain. 2017;140:1183–203.
Alizadehgoradel J, Nejati V, Sadeghi Movahed F, Imani S, Taherifard M, Mosayebi-Samani M, et al. Repeated stimulation of the dorsolateral-prefrontal cortex improves executive dysfunctions and craving in drug addiction: a randomized, double-blind, parallel-group study. Brain Stimul. 2020;13:582–93.
Su H, Zhong N, Gan H, Wang JJ, Han H, Chen TZ, et al. High frequency repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex for methamphetamine use disorders: a randomised clinical trial. Drug Alcohol Depend. 2017;175:84–91.
Zangen A, Moshe H, Martinez D, Barnea-Ygael N, Vapnik T, Bystritsky A, et al. Repetitive transcranial magnetic stimulation for smoking cessation: a pivotal multicenter double-blind randomized controlled trial. World Psychiatry. 2021;20:397–404.
Xue YX, Luo YX, Wu P, Shi HS, Xue LF, Chen C, et al. A memory retrieval-extinction procedure to prevent drug craving and relapse. Science. 2012;336:241–5.
Xue YX, Deng JH, Chen YY, Zhang LB, Wu P, Huang GD, et al. Effect of selective inhibition of reactivated nicotine-associated memories with propranolol on nicotine craving. JAMA Psychiatry. 2017;74:224–32.
Beisteiner R, Matt E, Fan C, Baldysiak H, Schonfeld M, Philippi Novak T, et al. Transcranial pulse stimulation with ultrasound in Alzheimer’s disease-A new navigated focal brain therapy. Adv Sci (Weinh). 2020;7:1902583.
Provenza NR, Sheth SA, Dastin-van Rijn EM, Mathura RK, Ding Y, Vogt GS, et al. Long-term ecological assessment of intracranial electrophysiology synchronized to behavioral markers in obsessive-compulsive disorder. Nat Med. 2021;27:2154–64.
Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: The role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–98.
Sinha R. New findings on biological factors predicting addiction relapse vulnerability. Curr Psychiatry Rep. 2011;13:398–405.
Nicolas C, Zlebnik NE, Farokhnia M, Leggio L, Ikemoto S, Shaham Y. Sex differences in opioid and psychostimulant craving and relapse: a critical review. Pharmacol Rev. 2022;74:119–40.
Kerstetter KA, Aguilar VR, Parrish AB, Kippin TE. Protracted time-dependent increases in cocaine-seeking behavior during cocaine withdrawal in female relative to male rats. Psychopharmacol (Berl). 2008;198:63–75.
Corbett CM, Dunn E, Loweth JA. Effects of sex and estrous cycle on the time course of incubation of cue-induced craving following extended-access cocaine self-administration. eNeuro. 2021;8:ENEURO.0054–21.2021.
Johnson AR, Thibeault KC, Lopez AJ, Peck EG, Sands LP, Sanders CM, et al. Cues play a critical role in estrous cycle-dependent enhancement of cocaine reinforcement. Neuropsychopharmacology. 2019;44:1189–97.
Creed M. Current and emerging neuromodulation therapies for addiction: Insight from pre-clinical studies. Curr Opin Neurobiol. 2018;49:168–74.
Liu X, Zhao X, Liu T, Liu Q, Tang L, Zhang H, et al. The effects of repetitive transcranial magnetic stimulation on cue-induced craving in male patients with heroin use disorder. EBioMedicine. 2020;56:102809–15.
Ceccanti M, Inghilleri M, Attilia ML, Raccah R, Fiore M, Zangen A, et al. Deep TMS on alcoholics: effects on cortisolemia and dopamine pathway modulation. A pilot study. Can J Physiol Pharm. 2015;93:283–90.
Li X, Hartwell KJ, Henderson S, Badran BW, Brady KT, George MS. Two weeks of image-guided left dorsolateral prefrontal cortex repetitive transcranial magnetic stimulation improves smoking cessation: a double-blind, sham-controlled, randomized clinical trial. Brain Stimul. 2020;13:1271–9.
Yuan J, Liu W, Liang Q, Cao X, Lucas MV, Yuan TF. Effect of low-frequency repetitive transcranial magnetic stimulation on impulse inhibition in abstinent patients with methamphetamine addiction: a randomized clinical trial. JAMA Netw Open. 2020;3:e200910.
Garza-Villarreal EA, Alcala-Lozano R, Fernandez-Lozano S, Morelos-Santana E, Dávalos A, Villicaña V, et al. Clinical and functional connectivity outcomes of 5-Hz repetitive transcranial magnetic stimulation as an add-on treatment in cocaine use disorder: a double-blind randomized controlled trial. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021;6:745–57.
Xu X, Ding X, Chen L, Chen T, Su H, Li X, et al. The transcranial direct current stimulation over prefrontal cortex combined with the cognitive training reduced the cue-induced craving in female individuals with methamphetamine use disorder: a randomized controlled trial. J Psychiatr Res. 2021;134:102–10.
Fasano A, Aquino CC, Krauss JK, Honey CR, Bloem BR. Axial disability and deep brain stimulation in patients with Parkinson disease. Nat Rev Neurol. 2015;11:98–110.
Oliveria SF, Rodriguez RL, Bowers D, Kantor D, Hilliard JD, Monari EH, et al. Safety and efficacy of dual-lead thalamic deep brain stimulation for patients with treatment-refractory multiple sclerosis tremor: a single-centre, randomised, single-blind, pilot trial. Lancet Neurol. 2017;16:691–700.
Vidailhet M, Yelnik J, Lagrange C, Fraix V, Grabli D, Thobois S, et al. Bilateral pallidal deep brain stimulation for the treatment of patients with dystonia-choreoathetosis cerebral palsy: A prospective pilot study. Lancet Neurol. 2009;8:709–17.
Zhu R, Zhang Y, Wang T, Wei H, Zhang C, Li D, et al. Deep brain stimulation of nucleus accumbens with anterior capsulotomy for drug addiction: a case report. Stereotact Funct Neurosurg. 2020;98:345–9.
Author information
Authors and Affiliations
Contributions
XXL wrote the draft manuscript. XXL, KY, and TSL constructed the figures and prepared the tables. XL, WZ, YXX, and JS revised the manuscript. LL and YH supervised this review and revised the manuscript. All authors contributed to the article and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, X., Yuan, K., Lu, T. et al. Preventing incubation of drug craving to treat drug relapse: from bench to bedside. Mol Psychiatry 28, 1415–1429 (2023). https://doi.org/10.1038/s41380-023-01942-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-023-01942-2