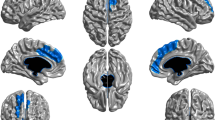
Abstract
The increased frequency of risk taking behavior combined with marked neuromaturation has positioned adolescence as a focal point of research into the neural causes and consequences of substance use. However, little work has provided a summary of the links between adolescent initiated substance use and longer-term brain outcomes. Here we review studies exploring the long-term effects of adolescent-initiated substance use with structural and microstructural neuroimaging. A quarter of all studies reviewed conducted repeated neuroimaging assessments. Long-term alcohol use, as well as tobacco use were consistently associated with smaller frontal cortices and altered white matter microstructure. This association was mostly observed in the ACC, insula and subcortical regions in alcohol users, and for the OFC in tobacco users. Long-term cannabis use was mostly related to altered frontal cortices and hippocampal volumes. Interestingly, cannabis users scanned more years after use initiation tended to show smaller measures of these regions, whereas those with fewer years since initiation showed larger measures. Long-term stimulant use tended to show a similar trend as cannabis in terms of years since initiation in measures of the putamen, insula and frontal cortex. Long-term opioid use was mostly associated with smaller subcortical and insular volumes. Of note, null findings were reported in all substance use categories, most often in cannabis use studies. In the context of the large variety in study designs, substance use assessment, methods, and sample characteristics, we provide recommendations on how to interpret these findings, and considerations for future studies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Not applicable.
References
Sawyer SM, Azzopardi PS, Wickremarathne D, Patton GC. The age of adolescence. Lancet Child Adolesc Health. 2018;2:223–8.
Soliman A, De Sanctis V, Elalaily R, Bedair S. Advances in pubertal growth and factors influencing it: can we increase pubertal growth? Indian J Endocrinol Metab. 2014;18:S53–62.
Yurgelun-Todd D. Emotional and cognitive changes during adolescence. Curr Opin Neurobiol. 2007;17:251–7.
Steinberg L. Cognitive and affective development in adolescence. Trends Cogn Sci. 2005;9:69–74.
Kelley AE, Schochet T, Landry CF. Risk taking and novelty seeking in adolescence: introduction to part I. Ann N Y Acad Sci. 2004;1021:27–32.
Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–63.
Volkow ND, Han B, Einstein EB, Compton WM. Prevalence of substance use disorders by time since first substance use among young people in the US. JAMA Pediatr. 2021;175:640–3.
Halladay J, Woock R, El-Khechen H, Munn C, MacKillop J, Amlung M, et al. Patterns of substance use among adolescents: a systematic review. Drug Alcohol Depend. 2020;216:108222.
Jordan CJ, Andersen SL. Sensitive periods of substance abuse: early risk for the transition to dependence. Dev Cogn Neurosci. 2017;25:29–44.
Sanvisens A, Sanjeevan I, Zuluaga P, Tunez A, de Francisco A, Papaseit E, et al. Five-year incidence of hospital-based emergencies related to acute recreational intoxication in minors. Alcohol Clin Exp Res. 2019;43:2179–86.
Goings TC, Salas-Wright C, Vaughn M. Toward a typology of driving under the influence of alcohol and drugs. Soc Psychiatry Psychiatr Epidemiol. 2023;58:227–38.
Jamt REG, Gjerde H, Romeo G, Bogstrand ST. Association between alcohol and drug use and arrest for driving under the influence after crash involvement in a rural area of Norway: a case-control study. BMJ Open. 2019;9:e023563.
Connery HS, Albright BB, Rodolico JM. Adolescent substance use and unplanned pregnancy: strategies for risk reduction. Obstet Gynecol Clin North Am. 2014;41:191–203.
Collaborators GBDA. Population-level risks of alcohol consumption by amount, geography, age, sex, and year: a systematic analysis for the Global Burden of Disease Study 2020. Lancet. 2022;400:185–235.
Hamidullah S, Thorpe HHA, Frie JA, McCurdy RD, Khokhar JY. Adolescent substance use and the brain: behavioral, cognitive and neuroimaging correlates. Front Hum Neurosci. 2020;14:298.
Scott JC, Slomiak ST, Jones JD, Rosen AFG, Moore TM, Gur RC. Association of cannabis with cognitive functioning in adolescents and young adults: a systematic review and meta-analysis. JAMA Psychiatry. 2018;75:585–95.
Kamal H, Tan GC, Ibrahim SF, Shaikh MF, Mohamed IN, Mohamed RMP, et al. Alcohol use disorder, neurodegeneration, Alzheimers and Parkinson’s disease: interplay between oxidative stress, neuroimmune response and excitotoxicity. Front Cell Neurosci. 2020;14:282.
Visontay R, Rao RT, Mewton L. Alcohol use and dementia: new research directions. Curr Opin Psychiatry. 2021;34:165–70.
Steinberg L. A social neuroscience perspective on adolescent risk-taking. Dev Rev. 2008;28:78–106.
Hernán MA, Hsu J, Healy B. A second chance to get causal inference right: a classification of data science tasks. CHANCE. 2019;32:42–49.
Pasternak O, Kelly S, Sydnor VJ, Shenton ME. Advances in microstructural diffusion neuroimaging for psychiatric disorders. Neuroimage. 2018;182:259–82.
Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30:718–29.
Vijayakumar N, Allen NB, Youssef G, Dennison M, Yücel M, Simmons JG, et al. Brain development during adolescence: a mixed-longitudinal investigation of cortical thickness, surface area, and volume. Hum Brain Mapp. 2016;37:2027–38.
Wierenga LM, Langen M, Oranje B, Durston S. Unique developmental trajectories of cortical thickness and surface area. Neuroimage. 2014;87:120–6.
Stiles J, Jernigan TL. The basics of brain development. Neuropsychol Rev. 2010;20:327–48.
Natu VS, Gomez J, Barnett M, Jeska B, Kirilina E, Jaeger C, et al. Apparent thinning of human visual cortex during childhood is associated with myelination. Proc Natl Acad Sci USA. 2019;116:20750–9.
Schnack HG, van Haren NE, Brouwer RM, Evans A, Durston S, Boomsma DI, et al. Changes in thickness and surface area of the human cortex and their relationship with intelligence. Cereb Cortex. 2015;25:1608–17.
Tamnes CK, Herting MM, Goddings AL, Meuwese R, Blakemore SJ, Dahl RE, et al. Development of the cerebral cortex across adolescence: a multisample study of inter-related longitudinal changes in cortical volume, surface area, and thickness. J Neurosci. 2017;37:3402–12.
Bethlehem RAI, Seidlitz J, White SR, Vogel JW, Anderson KM, Adamson C, et al. Brain charts for the human lifespan. Nature. 2022;604:525–33.
Sydnor VJ, Larsen B, Bassett DS, Alexander-Bloch A, Fair DA, Liston C, et al. Neurodevelopment of the association cortices: patterns, mechanisms, and implications for psychopathology. Neuron. 2021;109:2820–46.
Boer OD, El Marroun H, Franken IHA. Brain morphology predictors of alcohol, tobacco, and cannabis use in adolescence: a systematic review. Brain Res. 2022;1795:148020.
Bedi A, McGlinchey RE, Salat DH, Currao A, Fonda JR, Milberg WP, et al. Age of onset of adolescent binge drinking is differentially associated with cortical thickness in post-9/11 adult Veterans. Alcohol Clin Exp Res. 2021;45:1065–77.
Heikkinen N, Niskanen E, Könönen M, Tolmunen T, Kekkonen V, Kivimäki P, et al. Alcohol consumption during adolescence is associated with reduced grey matter volumes. Addiction. 2017;112:604–13.
Zhao Y, Constable RT, Hien D, Chung T, Potenza MN. Brain anatomical covariation patterns linked to binge drinking and age at first full drink. Neuroimage Clin. 2021;29:102529.
Meda SA, Dager AD, Hawkins KA, Tennen H, Raskin S, Wood RM, et al. Heavy drinking in college students is associated with accelerated gray matter volumetric decline over a 2 year period. Front Behav Neurosci. 2017;11:176.
Luciana M, Collins PF, Muetzel RL, Lim KO. Effects of alcohol use initiation on brain structure in typically developing adolescents. Am J Drug Alcohol Abus. 2013;39:345–55.
Infante MA, Eberson SC, Zhang Y, Brumback T, Brown SA, Colrain IM, et al. Adolescent binge drinking is associated with accelerated decline of gray matter volume. Cereb Cortex. 2022;32:2611–20.
Pfefferbaum A, Kwon D, Brumback T, Thompson WK, Cummins K, Tapert SF, et al. Altered brain developmental trajectories in adolescents after initiating drinking. Am J Psychiatry. 2018;175:370–80.
El Marroun H, Klapwijk ET, Koevoets M, Brouwer RM, Peters S, Van’t Ent D, et al. Alcohol use and brain morphology in adolescence: a longitudinal study in three different cohorts. Eur J Neurosci. 2021;54:6012–26.
Infante MA, Courtney KE, Castro N, Squeglia LM, Jacobus J. Adolescent brain surface area pre- and post-cannabis and alcohol initiation. J Stud Alcohol Drugs. 2018;79:835–43.
Sun D, Adduru VR, Phillips RD, Bouchard HC, Sotiras A, Michael AM, et al. Adolescent alcohol use is linked to disruptions in age-appropriate cortical thinning: an unsupervised machine learning approach. Neuropsychopharmacology. 2023;48:317–26.
Seo S, Beck A, Matthis C, Genauck A, Banaschewski T, Bokde ALW, et al. Risk profiles for heavy drinking in adolescence: differential effects of gender. Addict Biol. 2019;24:787–801.
Mashhoon Y, Czerkawski C, Crowley DJ, Cohen-Gilbert JE, Sneider JT, Silveri MM. Binge alcohol consumption in emerging adults: anterior cingulate cortical “thinness” is associated with alcohol use patterns. Alcohol Clin Exp Res. 2014;38:1955–64.
Doallo S, Cadaveira F, Corral M, Mota N, Lopez-Caneda E, Holguin SR. Larger mid-dorsolateral prefrontal gray matter volume in young binge drinkers revealed by voxel-based morphometry. PloS ONE. 2014;9:e96380.
De Bellis MD, Narasimhan A, Thatcher DL, Keshavan MS, Soloff P, Clark DB. Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcohol Clin Exp Res. 2005;29:1590–1600.
Squeglia LM, Rinker DA, Bartsch H, Castro N, Chung Y, Dale AM, et al. Brain volume reductions in adolescent heavy drinkers. Dev Cogn Neurosci. 2014;9:117–25.
Squeglia LM, Tapert SF, Sullivan EV, Jacobus J, Meloy MJ, Rohlfing T, et al. Brain development in heavy-drinking adolescents. Am J Psychiatry. 2015;172:531–42.
Meda SA, Hawkins KA, Dager AD, Tennen H, Khadka S, Austad CS, et al. Longitudinal effects of alcohol consumption on the hippocampus and parahippocampus in college students. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3:610–7.
De Bellis MD, Clark DB, Beers SR, Soloff PH, Boring AM, Hall J, et al. Hippocampal volume in adolescent-onset alcohol use disorders. Am J Psychiatry. 2000;157:737–44.
Sousa SS, Sampaio A, Lopez-Caneda E, Bec C, Goncalves OF, Crego A. Increased nucleus accumbens volume in college binge drinkers - preliminary evidence from manually segmented MRI analysis. Front Psychiatry. 2019;10:1005.
Shen Q, Heikkinen N, Kärkkäinen O, Gröhn H, Könönen M, Liu Y, et al. Effects of long-term adolescent alcohol consumption on white matter integrity and their correlations with metabolic alterations. Psychiatry Res Neuroimaging. 2019;294:111003.
Smith KW, Gierski F, Andre J, Dowell NG, Cercignani M, Naassila M, et al. Altered white matter integrity in whole brain and segments of corpus callosum, in young social drinkers with binge drinking pattern. Addict Biol. 2017;22:490–501.
Wade NE, Thomas AM, Gruber SA, Tapert SF, Filbey FM, Lisdahl KM. Binge and cannabis co-use episodes in relation to white matter integrity in emerging adults. Cannabis Cannabinoid Res. 2020;5:62–72.
Knodt AR, Meier MH, Ambler A, Gehred MZ, Harrington H, Ireland D, et al. Diminished structural brain integrity in long-term cannabis users reflects a history of polysubstance use. Biol Psychiatry. 2022;92:861–70.
Jacobus J, Squeglia LM, Alejandra Infante M, Bava S, Tapert SF. White matter integrity pre- and post marijuana and alcohol initiation in adolescence. Brain Sci. 2013;3:396–414.
Correas A, Cuesta P, Lopez-Caneda E, Rodriguez Holguin S, Garcia-Moreno LM, Pineda-Pardo JA, et al. Functional and structural brain connectivity of young binge drinkers: a follow-up study. Sci Rep. 2016;6:31293.
Albaugh MD, Ottino-Gonzalez J, Sidwell A, Lepage C, Juliano A, Owens MM, et al. Association of cannabis use during adolescence with neurodevelopment. JAMA Psychiatry. 2021;78:1031–40.
Lisdahl KM, Tamm L, Epstein JN, Jernigan T, Molina BS, Hinshaw SP, et al. The impact of ADHD persistence, recent cannabis use, and age of regular cannabis use onset on subcortical volume and cortical thickness in young adults. Drug Alcohol Depend. 2016;161:135–46.
Churchwell JC, Lopez-Larson M, Yurgelun-Todd DA. Altered frontal cortical volume and decision making in adolescent cannabis users. Front Psychol. 2010;1:225.
Lopez-Larson MP, Bogorodzki P, Rogowska J, McGlade E, King JB, Terry J, et al. Altered prefrontal and insular cortical thickness in adolescent marijuana users. Behav Brain Res. 2011;220:164–72.
Levar N, Francis AN, Smith MJ, Ho WC, Gilman JM. Verbal memory performance and reduced cortical thickness of brain regions along the uncinate fasciculus in young adult cannabis users. Cannabis Cannabinoid Res. 2018;3:56–65.
Filbey FM, Aslan S, Calhoun VD, Spence JS, Damaraju E, Caprihan A, et al. Long-term effects of marijuana use on the brain. Proc Natl Acad Sci USA. 2014;111:16913–8.
Filbey FM, McQueeny T, DeWitt SJ, Mishra V. Preliminary findings demonstrating latent effects of early adolescent marijuana use onset on cortical architecture. Dev Cogn Neurosci. 2015;16:16–22.
Jakabek D, Yucel M, Lorenzetti V, Solowij N. An MRI study of white matter tract integrity in regular cannabis users: effects of cannabis use and age. Psychopharmacology. 2016;233:3627–37.
Gruber SA, Silveri MM, Dahlgren MK, Yurgelun-Todd D. Why so impulsive? White matter alterations are associated with impulsivity in chronic marijuana smokers. Exp Clin Psychopharmacol. 2011;19:231–42.
Shollenbarger SG, Price J, Wieser J, Lisdahl K. Poorer frontolimbic white matter integrity is associated with chronic cannabis use, FAAH genotype, and increased depressive and apathy symptoms in adolescents and young adults. Neuroimage Clin. 2015;8:117–25.
Becker MP, Collins PF, Lim KO, Muetzel RL, Luciana M. Longitudinal changes in white matter microstructure after heavy cannabis use. Dev Cogn Neurosci. 2015;16:23–35.
Burggren AC, Siddarth P, Mahmood Z, London ED, Harrison TM, Merrill DA, et al. Subregional hippocampal thickness abnormalities in older adults with a history of heavy cannabis use. Cannabis Cannabinoid Res. 2018;3:242–51.
Meier MH, Caspi A, R Knodt A, Hall W, Ambler A, Harrington H, et al. Long-term cannabis use and cognitive reserves and hippocampal volume in midlife. Am J Psychiatry. 2022;179:362–74.
Zalesky A, Solowij N, Yucel M, Lubman DI, Takagi M, Harding IH, et al. Effect of long-term cannabis use on axonal fibre connectivity. Brain. 2012;135:2245–55.
Schacht JP, Hutchison KE, Filbey FM. Associations between cannabinoid receptor-1 (CNR1) variation and hippocampus and amygdala volumes in heavy cannabis users. Neuropsychopharmacology. 2012;37:2368–76.
Ashtari M, Avants B, Cyckowski L, Cervellione KL, Roofeh D, Cook P, et al. Medial temporal structures and memory functions in adolescents with heavy cannabis use. J Psychiatr Res. 2011;45:1055–66.
Yucel M, Zalesky A, Takagi MJ, Bora E, Fornito A, Ditchfield M, et al. White-matter abnormalities in adolescents with long-term inhalant and cannabis use: a diffusion magnetic resonance imaging study. J Psychiatry Neurosci. 2010;35:409–12.
Mashhoon Y, Sava S, Sneider JT, Nickerson LD, Silveri MM. Cortical thinness and volume differences associated with marijuana abuse in emerging adults. Drug Alcohol Depend. 2015;155:275–83.
Nurmedov S, Metin B, Ekmen S, Noyan O, Yilmaz O, Darcin A, et al. Thalamic and cerebellar gray matter volume reduction in synthetic cannabinoids users. Eur Addict Res. 2015;21:315–20.
Batalla A, Bhattacharyya S, Yücel M, Fusar-Poli P, Crippa JA, Nogué S, et al. Structural and functional imaging studies in chronic cannabis users: a systematic review of adolescent and adult findings. PloS ONE. 2013;8:e55821.
Battistella G, Fornari E, Annoni JM, Chtioui H, Dao K, Fabritius M, et al. Long-term effects of cannabis on brain structure. Neuropsychopharmacology. 2014;39:2041–8.
Rocchetti M, Crescini A, Borgwardt S, Caverzasi E, Politi P, Atakan Z, et al. Is cannabis neurotoxic for the healthy brain? A meta-analytical review of structural brain alterations in non-psychotic users. Psychiatry Clin Neurosci. 2013;67:483–92.
Cousijn J, Goudriaan AE, Ridderinkhof KR, van den Brink W, Veltman DJ, Wiers RW. Approach-bias predicts development of cannabis problem severity in heavy cannabis users: results from a prospective FMRI study. PloS ONE. 2012;7:e42394.
Orr C, Spechler P, Cao Z, Albaugh M, Chaarani B, Mackey S, et al. Grey matter volume differences associated with extremely low levels of cannabis use in adolescence. J Neurosci. 2019;39:1817–27.
Arnone D, Barrick TR, Chengappa S, Mackay CE, Clark CA, Abou-Saleh MT. Corpus callosum damage in heavy marijuana use: Preliminary evidence from diffusion tensor tractography and tract-based spatial statistics. Neuroimage. 2008;41:1067–74.
Gruber SA, Dahlgren MK, Sagar KA, Gönenç A, Lukas SE. Worth the wait: effects of age of onset of marijuana use on white matter and impulsivity. Psychopharmacology. 2014;231:1455–65.
Ashtari M, Cervellione K, Cottone J, Ardekani BA, Kumra S. Diffusion abnormalities in adolescents and young adults with a history of heavy cannabis use. J Psychiatr Res. 2009;43:189–204.
Matochik JA, Eldreth DA, Cadet JL, Bolla KI. Altered brain tissue composition in heavy marijuana users. Drug Alcohol Depend. 2005;77:23–30.
Orr JM, Paschall CJ, Banich MT. Recreational marijuana use impacts white matter integrity and subcortical (but not cortical) morphometry. Neuroimage Clin. 2016;12:47–56.
Cousijn J, Toenders YJ, van Velzen LS, Kaag AM. The relation between cannabis use, dependence severity and white matter microstructure: a diffusion tensor imaging study. Addict Biol. 2022;27:e13081.
Epstein KA, Kumra S. White matter fractional anisotropy over two time points in early onset schizophrenia and adolescent cannabis use disorder: a naturalistic diffusion tensor imaging study. Psychiatry Res. 2015;232:34–41.
Gillespie NA, Neale MC, Bates TC, Eyler LT, Fennema-Notestine C, Vassileva J, et al. Testing associations between cannabis use and subcortical volumes in two large population-based samples. Addiction. 2018;113:1661–72.
Meier MH, Schriber RA, Beardslee J, Hanson J, Pardini D. Associations between adolescent cannabis use frequency and adult brain structure: A prospective study of boys followed to adulthood. Drug Alcohol Depend. 2019;202:191–9.
Chye Y, Suo C, Lorenzetti V, Batalla A, Cousijn J, Goudriaan AE, et al. Cortical surface morphology in long-term cannabis users: a multi-site MRI study. Eur Neuropsychopharmacol. 2019;29:257–65.
Koenders L, Lorenzetti V, de Haan L, Suo C, Vingerhoets W, van den Brink W, et al. Longitudinal study of hippocampal volumes in heavy cannabis users. J Psychopharmacol. 2017;31:1027–34.
Koenders L, Cousijn J, Vingerhoets WA, van den Brink W, Wiers RW, Meijer CJ, et al. Grey matter changes associated with heavy cannabis use: a longitudinal sMRI study. PloS ONE. 2016;11:e0152482.
Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 2013;14:365–76.
Ioannidis JP. Why most published research findings are false. PLoS Med. 2005;2:e124.
Scheel AM, Schijen MRMJ, Lakens D. An excess of positive results: comparing the standard psychology literature with registered reports. Adv Methods Pract Psychol Sci. 2021;4:25152459211007467.
Luo X, Yang JJ, Buu A, Trucco EM, Li CR. Alcohol and cannabis co-use and longitudinal gray matter volumetric changes in early and late adolescence. Addict Biol. 2022;27:e13208.
Jacobus J, Squeglia LM, Sorg SF, Nguyen-Louie TT, Tapert SF. Cortical thickness and neurocognition in adolescent marijuana and alcohol users following 28 days of monitored abstinence. J Stud Alcohol Drugs. 2014;75:729–43.
Jacobus J, Squeglia LM, Meruelo AD, Castro N, Brumback T, Giedd JN, et al. Cortical thickness in adolescent marijuana and alcohol users: a three-year prospective study from adolescence to young adulthood. Dev Cogn Neurosci. 2015;16:101–9.
Jacobus J, Squeglia LM, Bava S, Tapert SF. White matter characterization of adolescent binge drinking with and without co-occurring marijuana use: a 3-year investigation. Psychiatry Res Neuroimaging. 2013;214:374–81.
Bava S, Frank LR, McQueeny T, Schweinsburg BC, Schweinsburg AD, Tapert SF. Altered white matter microstructure in adolescent substance users. Psychiatry Res Neuroimaging. 2009;173:228–37.
Bava S, Jacobus J, Thayer RE, Tapert SF. Longitudinal changes in white matter integrity among adolescent substance users. Alcohol Clin Exp Res. 2013;37:E181–E189.
Gallinat J, Meisenzahl E, Jacobsen LK, Kalus P, Bierbrauer J, Kienast T, et al. Smoking and structural brain deficits: a volumetric MR investigation. Eur J Neurosci. 2006;24:1744–50.
Conti AA, Baldacchino AM. Neuroanatomical correlates of impulsive choices and risky decision making in young chronic tobacco smokers: a voxel-based morphometry study. Front Psychiatry. 2021;12:708925.
Conti AA, Baldacchino AM. Chronic tobacco smoking, impaired reward-based decision-making, and role of insular cortex: A comparison between early-onset smokers and late-onset smokers. Front Psychiatry. 2022;13:939707.
Akkermans SEA, van Rooij D, Rommelse N, Hartman CA, Hoekstra PJ, Franke B, et al. Effect of tobacco smoking on frontal cortical thickness development: a longitudinal study in a mixed cohort of ADHD-affected and -unaffected youth. Eur Neuropsychopharmacol. 2017;27:1022–31.
Dai HD, Doucet GE, Wang Y, Puga T, Samson K, Xiao P, et al. Longitudinal assessments of neurocognitive performance and brain structure associated with initiation of tobacco use in children, 2016 to 2021. JAMA Netw Open. 2022;5:e2225991.
Morales AM, Ghahremani D, Kohno M, Hellemann GS, London ED. Cigarette exposure, dependence, and craving are related to insula thickness in young adult smokers. Neuropsychopharmacology. 2014;39:1816–22.
Liao Y, Tang J, Deng Q, Deng Y, Luo T, Wang X, et al. Bilateral fronto-parietal integrity in young chronic cigarette smokers: a diffusion tensor imaging study. PloS ONE. 2011;6:e26460.
Van Ewijk H, Groenman AP, Zwiers MP, Heslenfeld DJ, Faraone SV, Hartman CA, et al. Smoking and the developing brain: Altered white matter microstructure in attention-deficit/hyperactivity disorder and healthy controls. Hum Brain Mapp. 2015;36:1180–9.
Yu D, Yuan K, Zhang B, Liu J, Dong M, Jin C, et al. White matter integrity in young smokers: a tract-based spatial statistics study. Addict Biol. 2016;21:679–87.
Jacobsen LK, Picciotto MR, Heath CJ, Frost SJ, Tsou KA, Dwan RA, et al. Prenatal and adolescent exposure to tobacco smoke modulates the development of white matter microstructure. J Neurosci. 2007;27:13491–8.
Thayer RE, Hansen NS, Prashad S, Karoly HC, Filbey FM, Bryan AD, et al. Recent tobacco use has widespread associations with adolescent white matter microstructure. Addict Behav. 2020;101:106152.
de Win MM, Jager G, Booij J, Reneman L, Schilt T, Lavini C, et al. Sustained effects of ecstasy on the human brain: a prospective neuroimaging study in novel users. Brain. 2008;131:2936–45.
Li Y, Zhou W, Dong H, Shen W, Zhang J, Li F, et al. Lower fractional anisotropy in the gray matter of amygdala-hippocampus-nucleus accumbens circuit in methamphetamine users: an in vivo diffusion tensor imaging study. Neurotox Res. 2018;33:801–11.
Narayana PA, Datta S, Tao G, Steinberg JL, Moeller FG. Effect of cocaine on structural changes in brain: MRI volumetry using tensor-based morphometry. Drug Alcohol Depend. 2010;111:191–9.
Sim ME, Lyoo IK, Streeter CC, Covell J, Sarid-Segal O, Ciraulo DA, et al. Cerebellar gray matter volume correlates with duration of cocaine use in cocaine-dependent subjects. Neuropsychopharmacology. 2007;32:2229–37.
Zhuang W, Tang Y, Zhong N, Jiang H, Du J, Wang J, et al. Persistent microstructural deficits of internal capsule in one-year abstinent male methamphetamine users: a longitudinal diffusion tensor imaging study. J Neuroimmune Pharm. 2016;11:523–30.
Liao Y, Tang J, Ma M, Wu Z, Yang M, Wang X, et al. Frontal white matter abnormalities following chronic ketamine use: a diffusion tensor imaging study. Brain. 2010;133:2115–22.
Ma X, Qiu Y, Tian J, Wang J, Li S, Zhan W, et al. Aberrant default-mode functional and structural connectivity in heroin-dependent individuals. PloS ONE. 2015;10:e0120861.
Singla A, Singh P, Panditrao M, Panditrao MM. Is chronic opioid abuse associated with cerebral atrophy? An observational study. Indian J Crit Care Med. 2020;24:276–80.
Alcover KC, Thompson CL. Patterns of mean age at drug use initiation among adolescents and emerging adults, 2004–2017. JAMA Pediatr. 2020;174:725–7.
Mackey S, Stewart JL, Connolly CG, Tapert SF, Paulus MP. A voxel-based morphometry study of young occasional users of amphetamine-type stimulants and cocaine. Drug Alcohol Depend. 2014;135:104–11.
Churchwell JC, Carey PD, Ferrett HL, Stein DJ, Yurgelun-Todd DA. Abnormal striatal circuitry and intensified novelty seeking among adolescents who abuse methamphetamine and cannabis. Dev Neurosci. 2012;34:310–7.
Schwartz DL, Mitchell AD, Lahna DL, Luber HS, Huckans MS, Mitchell SH, et al. Global and local morphometric differences in recently abstinent methamphetamine-dependent individuals. Neuroimage. 2010;50:1392–401.
Nakama H, Chang L, Fein G, Shimotsu R, Jiang CS, Ernst T. Methamphetamine users show greater than normal age-related cortical gray matter loss. Addiction. 2011;106:1474–83.
Uhlmann A, Fouche JP, Koen N, Meintjes EM, Wilson D, Stein DJ. Fronto-temporal alterations and affect regulation in methamphetamine dependence with and without a history of psychosis. Psychiatry Res Neuroimaging. 2016;248:30–38.
Lawyer G, Bjerkan PS, Hammarberg A, Jayaram-Lindstrom N, Franck J, Agartz I. Amphetamine dependence and co-morbid alcohol abuse: associations to brain cortical thickness. BMC Pharm. 2010;10:5.
Alicata D, Chang L, Cloak C, Abe K, Ernst T. Higher diffusion in striatum and lower fractional anisotropy in white matter of methamphetamine users. Psychiatry Res. 2009;174:1–8.
Matuskey D, Bhagwagar Z, Planeta B, Pittman B, Gallezot JD, Chen J, et al. Reductions in brain 5-HT1B receptor availability in primarily cocaine-dependent humans. Biol Psychiatry. 2014;76:816–22.
Ide JS, Zhang S, Hu S, Sinha R, Mazure CM, Li CR. Cerebral gray matter volumes and low-frequency fluctuation of BOLD signals in cocaine dependence: duration of use and gender difference. Drug Alcohol Depend. 2014;134:51–62.
Gardini S, Venneri A. Reduced grey matter in the posterior insula as a structural vulnerability or diathesis to addiction. Brain Res Bull. 2012;87:205–11.
Garza-Villarreal EA, Chakravarty MM, Hansen B, Eskildsen SF, Devenyi GA, Castillo-Padilla D, et al. The effect of crack cocaine addiction and age on the microstructure and morphology of the human striatum and thalamus using shape analysis and fast diffusion kurtosis imaging. Transl Psychiatry. 2017;7:e1122.
Yuan Y, Zhu Z, Shi J, Zou Z, Yuan F, Liu Y, et al. Gray matter density negatively correlates with duration of heroin use in young lifetime heroin-dependent individuals. Brain Cogn. 2009;71:223–8.
Kivisaari R, Rapeli P, Van Leemput K, Kahkonen S, Puuskari V, Jokela O, et al. Cerebral measurements and their correlation with the onset age and the duration of opioid abuse. J Opioid Manag. 2010;6:423–9.
Liang H, Tang WK, Chu WCW, Ernst T, Chen R, Chang L. Striatal and white matter volumes in chronic ketamine users with or without recent regular stimulant use. Drug Alcohol Depend. 2020;213:108063.
Hung CC, Liu YH, Huang CC, Chou CY, Chen CM, Duann JR, et al. Effects of early ketamine exposure on cerebral gray matter volume and functional connectivity. Sci Rep. 2020;10:15488.
Edward Roberts R, Curran HV, Friston KJ, Morgan CJ. Abnormalities in white matter microstructure associated with chronic ketamine use. Neuropsychopharmacology. 2014;39:329–38.
Marek S, Tervo-Clemmens B, Calabro FJ, Montez DF, Kay BP, Hatoum AS, et al. Reproducible brain-wide association studies require thousands of individuals. Nature. 2022;603:654–60.
Makowski C, Brown T, Zhao W, Hagler D, Parekh P, Garavan H, et al. Reports of the death of brain-behavior associations have been greatly exaggerated. bioRxiv. 2023;16:545340.
Brouwer RM, Schutte J, Janssen R, Boomsma DI, Hulshoff Pol HE, Schnack HG. The speed of development of adolescent brain age depends on sex and is genetically determined. Cereb Cortex. 2021;31:1296–306.
McHugh RK, Votaw VR, Sugarman DE, Greenfield SF. Sex and gender differences in substance use disorders. Clin Psychol Rev. 2018;66:12–23.
Whelan R, Watts R, Orr CA, Althoff RR, Artiges E, Banaschewski T, et al. Neuropsychosocial profiles of current and future adolescent alcohol misusers. Nature. 2014;512:185–9.
Malmberg M, Overbeek G, Monshouwer K, Lammers J, Vollebergh WA, Engels RC. Substance use risk profiles and associations with early substance use in adolescence. J Behav Med. 2010;33:474–85.
Kaplow JB, Curran PJ, Dodge KA, Conduct Problems Prevention Research G. Child, parent, and peer predictors of early-onset substance use: a multisite longitudinal study. J Abnorm Child Psychol. 2002;30:199–216.
Van West D, Vermeiren R. Dual disorders in adolescent populations. Co-occurring addictive and psychiatric disorders: a practice-based handbook from a European perspective. Springer 2014, pp 335–47.
Kroll DS, Feldman DE, Wang SA, Zhang R, Manza P, Wiers CE, et al. The associations of comorbid substance use disorders and psychiatric conditions with adolescent brain structure and function: a review. J Neurol Sci. 2020;418:117099.
Folk JB, Hirschtritt ME, McCrary QD, Kalapatapu RK. Agreement between youth self-report and biospecimen-confirmed substance use: a systematic review. Subst Use Misuse. 2022;57:531–8.
Akinci IH, Tarter RE, Kirisci L. Concordance between verbal report and urine screen of recent marijuana use in adolescents. Addict Behav. 2001;26:613–9.
Steinhoff A, Shanahan L, Bechtiger L, Zimmermann J, Ribeaud D, Eisner MP, et al. When substance use is underreported: comparing self-reports and hair toxicology in an urban cohort of young adults. J Am Acad Child Adolesc Psychiatry. 2023;62:791–804.
Jurado C, Kintz P, Menendez M, Repetto M. Influence of the cosmetic treatment of hair on drug testing. Int J Leg Med. 1997;110:159–63.
Skopp G, Potsch L, Moeller MR. On cosmetically treated hair-aspects and pitfalls of interpretation. Forensic Sci Int. 1997;84:43–52.
Miolo G, Tucci M, Menilli L, Stocchero G, Vogliardi S, Scrivano S, et al. A study on photostability of amphetamines and ketamine in hair irradiated under artificial sunlight. Brain Sci. 2018;8:96.
Banks DE, Rowe AT, Mpofu P, Zapolski TCB. Trends in typologies of concurrent alcohol, marijuana, and cigarette use among US adolescents: An ecological examination by sex and race/ethnicity. Drug Alcohol Depend. 2017;179:71–77.
Westreich D, Greenland S. The Table 2 fallacy: presenting and interpreting confounder and modifier coefficients. Am J Epidemiol. 2013;177:292–8.
Singh AK. Alcohol interaction with cocaine, methamphetamine, opioids, nicotine, cannabis, and gamma-hydroxybutyric acid. Biomedicines. 2019;7:16.
Stubbs JL, Taylor JJ, Siddiqi SH, Schaper FLWVJ, Cohen AL, Drew W, et al. Heterogeneous neuroimaging findings across substance use disorders localize to a common brain network. Nat Ment Health. 2023;1:772–81.
Moeller S, Yacoub E, Olman CA, Auerbach E, Strupp J, Harel N, et al. Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magn Reson Med. 2010;63:1144–53.
Ladd ME, Bachert P, Meyerspeer M, Moser E, Nagel AM, Norris DG, et al. Pros and cons of ultra-high-field MRI/MRS for human application. Prog Nucl Magn Reson Spectrosc. 2018;109:1–50.
Abraham A, Pedregosa F, Eickenberg M, Gervais P, Mueller A, Kossaifi J, et al. Machine learning for neuroimaging with scikit-learn. Front Neuroinform. 2014;8:14.
Smith SM, Nichols TE. Statistical challenges in “big data” human neuroimaging. Neuron. 2018;97:263–8.
Casey BJ, Cannonier T, Conley MI, Cohen AO, Barch DM, Heitzeg MM, et al. The Adolescent Brain Cognitive Development (ABCD) study: Imaging acquisition across 21 sites. Dev Cogn Neurosci. 2018;32:43–54.
Miller KL, Alfaro-Almagro F, Bangerter NK, Thomas DL, Yacoub E, Xu J, et al. Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat Neurosci. 2016;19:1523–36.
Chye Y, Mackey S, Gutman BA, Ching CRK, Batalla A, Blaine S, et al. Subcortical surface morphometry in substance dependence: An ENIGMA addiction working group study. Addict Biol. 2020;25:e12830.
Dinga R, Schmaal L, Penninx B, van Tol MJ, Veltman DJ, van Velzen L, et al. Evaluating the evidence for biotypes of depression: Methodological replication and extension of. Neuroimage Clin. 2019;22:101796.
Xu B, Dalla Aglio L, Flournoy J, Bortsova G, Tervo-Clemmens B, Collins P et al. Multivariate brain-based dimensions of child psychiatric problems: degrees of generalizability. medRxiv. 2023;12:23287158.
Luo Y, Peng J, Ma J. When causal inference meets deep learning. Nat Mach Intell. 2020;2:426–7.
van der Velden BHM, Kuijf HJ, Gilhuijs KGA, Viergever MA. Explainable artificial intelligence (XAI) in deep learning-based medical image analysis. Med Image Anal. 2022;79:102470.
Pearl J. Probabilistic reasoning in intelligent systems: networks of plausible inference. Morgan kaufmann1988.
Robert GH, Luo Q, Yu T, Chu C, Ing A, Jia T, et al. Association of gray matter and personality development with increased drunkenness frequency during adolescence. JAMA Psychiatry. 2020;77:409–19.
Owens MM, Albaugh MD, Allgaier N, Yuan D, Robert G, Cupertino RB, et al. Bayesian causal network modeling suggests adolescent cannabis use accelerates prefrontal cortical thinning. Transl Psychiatry. 2022;12:188.
Hernan MA, Robins JM. Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol. 2016;183:758–64.
Labrecque JA, Swanson SA. Target trial emulation: teaching epidemiology and beyond. Eur J Epidemiol. 2017;32:473–5.
Davey Smith G, Ebrahim S. Mendelian randomization: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32:1–22.
O’Connor LJ, Price AL. Distinguishing genetic correlation from causation across 52 diseases and complex traits. Nat Genet. 2018;50:1728–34.
Hatoum AS, Johnson EC, Agrawal A, Bogdan R. Brain structure and problematic alcohol use: a test of plausible causation using latent causal variable analysis. Brain Imaging Behav. 2021;15:2741–5.
Mavromatis LA, Rosoff DB, Cupertino RB, Garavan H, Mackey S, Lohoff FW. Association between brain structure and alcohol use behaviors in adults: a Mendelian randomization and multiomics study. JAMA Psychiatry. 2022;79:869–78.
Lawlor DA, Tilling K, Davey Smith G. Triangulation in aetiological epidemiology. Int J Epidemiol. 2016;45:1866–86.
Miller ML, Chadwick B, Dickstein DL, Purushothaman I, Egervari G, Rahman T, et al. Adolescent exposure to Delta(9)-tetrahydrocannabinol alters the transcriptional trajectory and dendritic architecture of prefrontal pyramidal neurons. Mol Psychiatry. 2019;24:588–600.
Funding
This work was supported by the Stichting Volksbond Rotterdam (OB & HM), the NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation [grant number 27853] (HM); the Netherlands Organization for Health Research and Development [Aspasia grant No.015.016.056] (HM), the Sophia Foundation (S18-20, RM) and the Erasmus MC Fellowship (RM). The study sponsors had no role in the study design, collection, analysis and interpretation of data, writing of the report, or in the decision to submit the paper for publication.
Author information
Authors and Affiliations
Contributions
Olga Boer: Conceptualization, Methodology, Investigation, Data curation, Writing - Original draft, Visualization. Hanan El Marroun: Conceptualization, Data curation, Writing - Original draft, Writing - Review & Editing, Funding acquisition, Supervision. Ryan Muetzel: Conceptualization, Data curation, Writing - Original draft, Writing - Review & Editing, Funding acquisition, Supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Boer, O.D., El Marroun, H. & Muetzel, R.L. Adolescent substance use initiation and long-term neurobiological outcomes: insights, challenges and opportunities. Mol Psychiatry (2024). https://doi.org/10.1038/s41380-024-02471-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41380-024-02471-2