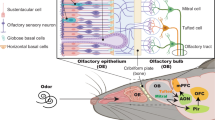
Abstract
Smell deficits and neurobiological changes in the olfactory bulb (OB) and olfactory epithelium (OE) have been observed in schizophrenia and related disorders. The OE is the most peripheral olfactory system located outside the cranium, and is connected with the brain via direct neuronal projections to the OB. Nevertheless, it is unknown whether and how a disturbance of the OE affects the OB in schizophrenia and related disorders. Addressing this gap would be the first step in studying the impact of OE pathology in the disease pathophysiology in the brain. In this cross-species study, we observed that chronic, local OE inflammation with a set of upregulated genes in an inducible olfactory inflammation (IOI) mouse model led to a volume reduction, layer structure changes, and alterations of neuron functionality in the OB. Furthermore, IOI model also displayed behavioral deficits relevant to negative symptoms (avolition) in parallel to smell deficits. In first episode psychosis (FEP) patients, we observed a significant alteration in immune/inflammation-related molecular signatures in olfactory neuronal cells (ONCs) enriched from biopsied OE and a significant reduction in the OB volume, compared with those of healthy controls (HC). The increased expression of immune/inflammation-related molecules in ONCs was significantly correlated to the OB volume reduction in FEP patients, but no correlation was found in HCs. Moreover, the increased expression of human orthologues of the IOI genes in ONCs was significantly correlated with the OB volume reduction in FEP, but not in HCs. Together, our study implies a potential mechanism of the OE-OB pathology in patients with psychotic disorders (schizophrenia and related disorders). We hope that this mechanism may have a cross-disease implication, including COVID-19-elicited mental conditions that include smell deficits.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The RNA-seq data and analysis scripts have been made publicly accessible on GitHub: https://github.com/KunYang99/Inflammation_ONC.
References
Cohen AS, Brown LA, Auster TL. Olfaction, ‘olfiction,’ and the schizophrenia-spectrum: an updated meta-analysis on identification and acuity. Schizophr Res. 2012;135:152–7.
Ishizuka K, Tajinda K, Colantuoni C, Morita M, Winicki J, Le C, et al. Negative symptoms of schizophrenia correlate with impairment on the University of Pennsylvania smell identification test. Neurosci Res. 2010;66:106–10.
Kamath V, Lasutschinkow P, Ishizuka K, Sawa A. Olfactory Functioning in First-Episode Psychosis. Schizophr Bull. 2018;44:672–80.
Kiparizoska S, Ikuta T. Disrupted Olfactory Integration in Schizophrenia: Functional Connectivity Study. Int J Neuropsychopharmacol. 2017;20:740–6.
Kopala LC, Good K, Honer WG. Olfactory identification ability in pre- and postmenopausal women with schizophrenia. Biol Psychiatry. 1995;38:57–63.
Malaspina D, Wray AD, Friedman JH, Amador X, Yale S, Hasan A, et al. Odor discrimination deficits in schizophrenia: association with eye movement dysfunction. J Neuropsychiatry Clin Neurosci. 1994;6:273–8.
Moberg PJ, Kamath V, Marchetto DM, Calkins ME, Doty RL, Hahn C-G, et al. Meta-analysis of olfactory function in schizophrenia, first-degree family members, and youths at-risk for psychosis. Schizophr Bull. 2014;40:50–59.
Turetsky BI, Hahn C-G, Borgmann-Winter K, Moberg PJ. Scents and nonsense: olfactory dysfunction in schizophrenia. Schizophr Bull. 2009;35:1117–31.
Chen X, Xu J, Li B, Guo W, Zhang J, Hu J. Olfactory impairment in first-episode schizophrenia: a case-control study, and sex dimorphism in the relationship between olfactory impairment and psychotic symptoms. BMC Psychiatry. 2018;18:199.
Kamath V, Crawford J, DuBois S, Nucifora FC, Nestadt G, Sawa A, et al. Contributions of olfactory and neuropsychological assessment to the diagnosis of first-episode schizophrenia. Neuropsychology. 2019;33:203–11.
Kamath V, Turetsky BI, Calkins ME, Kohler CG, Conroy CG, Borgmann-Winter K, et al. Olfactory processing in schizophrenia, non-ill first-degree family members, and young people at-risk for psychosis. World J Biol Psychiatry J World Fed Soc Biol Psychiatry. 2014;15:209–18.
Good KP, Sullivan RL. Olfactory function in psychotic disorders: Insights from neuroimaging studies. World J Psychiatry. 2015;5:210–21.
Cumming AG, Matthews NL, Park S. Olfactory identification and preference in bipolar disorder and schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2011;261:251–9.
Brewer WJ, Pantelis C, Anderson V, Velakoulis D, Singh B, Copolov DL, et al. Stability of olfactory identification deficits in neuroleptic-naive patients with first-episode psychosis. Am J Psychiatry. 2001;158:107–15.
Good KP, Tibbo P, Milliken H, Whitehorn D, Alexiadis M, Robertson N, et al. An investigation of a possible relationship between olfactory identification deficits at first episode and four-year outcomes in patients with psychosis. Schizophr Res. 2010;124:60–65.
Turetsky BI, Moberg PJ, Quarmley M, Dress E, Calkins ME, Ruparel K, et al. Structural anomalies of the peripheral olfactory system in psychosis high-risk subjects. Schizophr Res. 2018;195:197–205.
Corcoran C, Whitaker A, Coleman E, Fried J, Feldman J, Goudsmit N, et al. Olfactory deficits, cognition and negative symptoms in early onset psychosis. Schizophr Res. 2005;80:283–93.
Takahashi T, Nakamura M, Sasabayashi D, Komori Y, Higuchi Y, Nishikawa Y, et al. Olfactory deficits in individuals at risk for psychosis and patients with schizophrenia: relationship with socio-cognitive functions and symptom severity. Eur Arch Psychiatry Clin Neurosci. 2018;268:689–98.
Good KP, Whitehorn D, Rui Q, Milliken H, Kopala LC. Olfactory identification deficits in first-episode psychosis may predict patients at risk for persistent negative and disorganized or cognitive symptoms. Am J Psychiatry. 2006;163:932–3.
Lin A, Brewer WJ, Yung AR, Nelson B, Pantelis C, Wood SJ. Olfactory identification deficits at identification as ultra-high risk for psychosis are associated with poor functional outcome. Schizophr Res. 2015;161:156–62.
Mori K, Sakano H. How is the olfactory map formed and interpreted in the mammalian brain? Annu Rev Neurosci. 2011;34:467–99.
Mori K, Sakano H. Olfactory Circuitry and Behavioral Decisions. Annu Rev Physiol. 2021;83:231–56.
Hasegawa Y, Ma M, Sawa A, Lane AP, Kamiya A. Olfactory impairment in psychiatric disorders: Does nasal inflammation impact disease psychophysiology? Transl Psychiatry. 2022;12:314.
Bhattarai JP, Etyemez S, Jaaro-Peled H, Janke E, Leon Tolosa UD, Kamiya A, et al. Olfactory modulation of the medial prefrontal cortex circuitry: Implications for social cognition. Semin Cell Dev Biol. 2022;129:31–39.
Kabbani N, Olds JL. Does COVID19 Infect the Brain? If So, Smokers Might Be at a Higher Risk. Mol Pharm. 2020;97:351–3.
Zubair AS, McAlpine LS, Gardin T, Farhadian S, Kuruvilla DE, Spudich S. Neuropathogenesis and Neurologic Manifestations of the Coronaviruses in the Age of Coronavirus Disease 2019: A Review. JAMA Neurol. 2020;77:1018–27.
Gao Q, Xu Q, Guo X, Fan H, Zhu H. Particulate matter air pollution associated with hospital admissions for mental disorders: A time-series study in Beijing, China. Eur Psychiatry J Assoc Eur Psychiatr. 2017;44:68–75.
Newbury JB, Arseneault L, Beevers S, Kitwiroon N, Roberts S, Pariante CM, et al. Association of Air Pollution Exposure With Psychotic Experiences During Adolescence. JAMA Psychiatry. 2019;76:614–23.
Pedersen CB, Raaschou-Nielsen O, Hertel O, Mortensen PB. Air pollution from traffic and schizophrenia risk. Schizophr Res. 2004;66:83–85.
Evgrafov OV, Armoskus C, Wrobel BB, Spitsyna VN, Souaiaia T, Herstein JS, et al. Gene Expression in Patient-Derived Neural Progenitors Implicates WNT5A Signaling in the Etiology of Schizophrenia. Biol Psychiatry. 2020;88:236–47.
Rhie SK, Schreiner S, Witt H, Armoskus C, Lay FD, Camarena A, et al. Using 3D epigenomic maps of primary olfactory neuronal cells from living individuals to understand gene regulation. Sci Adv. 2018;4:eaav8550.
Fan Y, Abrahamsen G, McGrath JJ, Mackay-Sim A. Altered cell cycle dynamics in schizophrenia. Biol Psychiatry. 2012;71:129–35.
Féron F, Perry C, Hirning MH, McGrath J, Mackay-Sim A. Altered adhesion, proliferation and death in neural cultures from adults with schizophrenia. Schizophr Res. 1999;40:211–8.
English JA, Fan Y, Föcking M, Lopez LM, Hryniewiecka M, Wynne K, et al. Reduced protein synthesis in schizophrenia patient-derived olfactory cells. Transl Psychiatry. 2015;5:e663.
Namkung H, Yukitake H, Fukudome D, Lee BJ, Tian M, Ursini G, et al. The miR-124-AMPAR pathway connects polygenic risks with behavioral changes shared between schizophrenia and bipolar disorder. Neuron. 2023;111:220–235.e9.
Jaaro-Peled H, Landek-Salgado MA, Cascella NG, Nucifora FC, Coughlin JM, Nestadt G, et al. Sex-specific involvement of the Notch-JAG pathway in social recognition. Transl Psychiatry. 2022;12:99.
Mihaljevic M, Lam M, Ayala-Grosso C, Davis-Batt F, Schretlen DJ, Ishizuka K, et al. Olfactory neuronal cells as a promising tool to realize the ‘druggable genome’ approach for drug discovery in neuropsychiatric disorders. Front Neurosci. 2022;16:1081124.
Takayanagi Y, Ishizuka K, Laursen TM, Yukitake H, Yang K, Cascella NG, et al. From population to neuron: exploring common mediators for metabolic problems and mental illnesses. Mol Psychiatry. 2021;26:3931–42.
Kano S, Colantuoni C, Han F, Zhou Z, Yuan Q, Wilson A, et al. Genome-wide profiling of multiple histone methylations in olfactory cells: further implications for cellular susceptibility to oxidative stress in schizophrenia. Mol Psychiatry. 2013;18:740–2.
Hasegawa Y, Namkung H, Smith A, Sakamoto S, Zhu X, Ishizuka K, et al. Causal impact of local inflammation in the nasal cavity on higher brain function and cognition. Neurosci Res. 2021;172:110–5.
Chen M, Reed RR, Lane AP. Chronic Inflammation Directs an Olfactory Stem Cell Functional Switch from Neuroregeneration to Immune Defense. Cell Stem Cell. 2019;25:501–513.e5.
Lane AP, Turner J, May L, Reed R. A genetic model of chronic rhinosinusitis-associated olfactory inflammation reveals reversible functional impairment and dramatic neuroepithelial reorganization. J Neurosci J Soc Neurosci. 2010;30:2324–9.
Saito A, Taniguchi Y, Rannals MD, Merfeld EB, Ballinger MD, Koga M, et al. Early postnatal GABAA receptor modulation reverses deficits in neuronal maturation in a conditional neurodevelopmental mouse model of DISC1. Mol Psychiatry. 2016;21:1449–59.
Dieterich A, Liu T, Samuels BA. Chronic non-discriminatory social defeat stress reduces effort-related motivated behaviors in male and female mice. Transl Psychiatry. 2021;11:125.
Mihaljevic M, Lam M, Ayala-Grosso C, Davis-Batt F, Schretlen DJ, Ishizuka K, et al. Olfactory neuronal cells as a promising tool to realize the “druggable genome” approach for drug discovery in neuropsychiatric disorders. Front Neurosci. 2023;16:108112.
Turetsky BI, Moberg PJ, Yousem DM, Doty RL, Arnold SE, Gur RE. Reduced olfactory bulb volume in patients with schizophrenia. Am J Psychiatry. 2000;157:828–30.
Leucht S, Samara M, Heres S, Davis JM. Dose Equivalents for Antipsychotic Drugs: The DDD Method. Schizophr Bull. 2016;42:S90–94.
Andrews S. FastQC: a quality control tool for high throughput sequence data. 2010. http://www.bioinformatics.babraham.ac.uk/projects/fastqc.
Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011;17:10–12.
Pertea M, Kim D, Pertea GM, Leek JT, Salzberg SL. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat Protoc. 2016;11:1650–67.
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550.
Leek JT, Johnson WE, Parker HS, Jaffe AE, Storey JD. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics. 2012;28:882–3.
Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinforma. 2008;9:559.
Reimand J, Arak T, Adler P, Kolberg L, Reisberg S, Peterson H, et al. g:Profiler-a web server for functional interpretation of gene lists (2016 update). Nucleic Acids Res. 2016;44:W83–89.
Bhattarai JP, Schreck M, Moberly AH, Luo W, Ma M. Aversive Learning Increases Release Probability of Olfactory Sensory Neurons. Curr Biol CB. 2020;30:31–41.e3.
Bressel OC, Khan M, Mombaerts P. Linear correlation between the number of olfactory sensory neurons expressing a given mouse odorant receptor gene and the total volume of the corresponding glomeruli in the olfactory bulb. J Comp Neurol. 2016;524:199–209.
Yang K, Hua J, Etyemez S, Paez A, Prasad N, Ishizuka K, et al. Volumetric alteration of olfactory bulb and immune-related molecular changes in olfactory epithelium in first episode psychosis patients. Schizophr Res. 2021;235:9–11.
Asal N, Bayar Muluk N, Inal M, Şahan MH, Doğan A, Buturak SV. Olfactory bulbus volume and olfactory sulcus depth in psychotic patients and patients with anxiety disorder/depression. Eur Arch Otorhinolaryngol. 2018;275:3017–24.
Nguyen AD, Pelavin PE, Shenton ME, Chilakamarri P, McCarley RW, Nestor PG, et al. Olfactory sulcal depth and olfactory bulb volume in patients with schizophrenia: an MRI study. Brain Imaging Behav. 2011;5:252–61.
Turetsky BI, Moberg PJ, Arnold SE, Doty RL, Gur RE. Low olfactory bulb volume in first-degree relatives of patients with schizophrenia. Am J Psychiatry. 2003;160:703–8.
Rantanen LM, Bitar M, Lampinen R, Stewart R, Quek H, Oikari LE, et al. An Alzheimer’s Disease Patient-Derived Olfactory Stem Cell Model Identifies Gene Expression Changes Associated with Cognition. Cells. 2022;11:3258.
Lavoie J, Gassó Astorga P, Segal-Gavish H, Wu YC, Chung Y, Cascella NG, et al. The Olfactory Neural Epithelium As a Tool in Neuroscience. Trends Mol Med. 2017;23:100–3.
Mor E, Kano S-I, Colantuoni C, Sawa A, Navon R, Shomron N. MicroRNA-382 expression is elevated in the olfactory neuroepithelium of schizophrenia patients. Neurobiol Dis. 2013;55:1–10.
Jafari A, de Lima Xavier L, Bernstein JD, Simonyan K, Bleier BS. Association of Sinonasal Inflammation With Functional Brain Connectivity. JAMA Otolaryngol Head Neck Surg. 2021;147:534–43.
DeConde AS, Soler ZM. Chronic rhinosinusitis: Epidemiology and burden of disease. Am J Rhinol Allergy. 2016;30:134–9.
Arslan F, Tasdemir S, Durmaz A, Tosun F. The effect of nasal polyposis related nasal obstruction on cognitive functions. Cogn Neurodyn. 2018;12:385–90.
Arnold SE, Han LY, Moberg PJ, Turetsky BI, Gur RE, Trojanowski JQ, et al. Dysregulation of olfactory receptor neuron lineage in schizophrenia. Arch Gen Psychiatry. 2001;58:829–35.
Tan BKJ, Han R, Zhao JJ, Tan NKW, Quah ESH, Tan CJ-W, et al. Prognosis and persistence of smell and taste dysfunction in patients with covid-19: meta-analysis with parametric cure modelling of recovery curves. BMJ. 2022;378:e069503.
Liu N, Yang D, Zhang T, Sun J, Fu J, Li H. Systematic review and meta-analysis of olfactory and gustatory dysfunction in COVID-19. Int J Infect Dis. 2022;117:155–61.
Thaweethai T, Jolley SE, Karlson EW, Levitan EB, Levy B, McComsey GA, et al. Development of a Definition of Postacute Sequelae of SARS-CoV-2 Infection. JAMA. 2023;329:1934–46.
Nakamura ZM, Nash RP, Laughon SL, Rosenstein DL. Neuropsychiatric Complications of COVID-19. Curr Psychiatry Rep. 2021;23:25.
Premraj L, Kannapadi NV, Briggs J, Seal SM, Battaglini D, Fanning J, et al. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: A meta-analysis. J Neurol Sci. 2022;434:120162.
Acknowledgements
This study is supported by the National Institutes of Mental Health Grants MH-092443 (to AS), MH-094268 (to AS, AK), MH-105660 (to AS), MH-107730 (to AS), and MH-128765 (to AK); foundation grants from Stanley (to AS), RUSK/S-R (to AS), a NARSAD young investigator award from the Brain and Behavior Research Foundation (to AS, KY), NS-108452 (to JH, VK), AG-064093 (to JH, VK), DA-041208 (to AK), AG-065168 (to AK), DC-016106 (to AL), AI-132590 (to AL), DC-006213 (to MM), Kanae (YH), and Mitsui Sumitomo Insurance Welfare Foundation (YH). Figures 1A and 2B were created with a graphical software provided by Biorender.com. The authors wish to extend their gratitude to the participants in the current study. The authors thank Dr. Yu Miyahara and Ms. Vesna Tran for assisting mouse study, thank Ms. Yukiko Lema for research management and manuscript organization, and thank Dr. Melissa A Landek-Salgado for scientific and English editions.
Author information
Authors and Affiliations
Contributions
The current research was designed by AS (Sawa). The analytic pipeline was designed by KY. The preclinical study regarding anatomical assessment and mouse behavioral test was designed by AK and carried out by YH. The preclinical study regarding patch clamp recording was designed by MM and carried out by JPB with the assistance of YW and YFZ. Both clinical and preclinical data were analyzed by KY, with the assistance of YH, JPB, MD, and SE. The anatomical assessment of the human olfactory bulb was supervised by JH and carried out by NP, LD, and AP. Nasal biopsy was designed and conducted by APL, with the assistance of AS (Smith). The olfactory neuronal cells were enriched from biopsied olfactory epithelium by KI. The clinical data interpretation was guided by AS (Sawa), JH, and VK. The manuscript was drafted by KY, YH, JPB, KI, MM, AK, and AS (Sawa). All authors contributed to the discussion of the results and have approved the final manuscript to be published.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, K., Hasegawa, Y., Bhattarai, J.P. et al. Inflammation-related pathology in the olfactory epithelium: its impact on the olfactory system in psychotic disorders. Mol Psychiatry (2024). https://doi.org/10.1038/s41380-024-02425-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41380-024-02425-8