Abstract
Genes restricted to humans may contribute to human-specific traits and provide a different context for diseases. CHRFAM7A is a uniquely human fusion gene and a negative regulator of the α7 nicotinic acetylcholine receptor (α7 nAChR). The α7 nAChR has been a promising target for diseases affecting cognition and higher cortical functions, however, the treatment effect observed in animal models failed to translate into human clinical trials. As CHRFAM7A was not accounted for in preclinical drug screens it may have contributed to the translational gap. Understanding the complex genetic architecture of the locus, deciphering the functional impact of CHRFAM7A on α7 nAChR neurobiology and utilizing human-relevant models may offer novel approaches to explore α7 nAChR as a drug target.
Similar content being viewed by others
Introduction
Genes [1,2,3,4] that emerged since the human chimpanzee divergence have contributed to human-specific traits enriched for brain, immune and metabolic processes [1,2,3,4]. Genetic underpinnings of the differences include gene family duplications, single gene modifications, structural variants, differences in gene transcription levels and alternative splicing. CHRFAM7A is a human restricted fusion gene, a result of multiple rearrangements on chromosome 15 during evolution, including duplication, deletion and inversion events [5]. The region has ongoing instability, and various neurodevelopmental phenotypes have been reported in the context of deletion and duplication syndromes [3]. CHRFAM7A has been implicated in late-onset neuropsychiatric disorders, including schizophrenia, bipolar disorder, dementia with Lewy bodies, Pick’s disease, and Alzheimer’s disease (AD) [5]; all are human-specific and affect association cortices and higher cognitive function.
CHRFAM7A: genetic architecture
CHRFAM7A is a fusion gene between part of CHRNA7 (exons 5-10) and part of FAM7A. The unique sequence is limited to the breakpoint creating detection challenges (Fig. 1A). To add to the complexity, CHRFAM7A can be present in direct or inverted orientation. The inverted allele harbors a 2 bp deletion in exon 6 of the CHRNA7 derived sequence leading to a frameshift mutation. Linkage disequilibrium between the 2 bp deletion and the inversion allows detection of the inverted allele.
A Schematic depicting CHRFAM7A alleles. B Copy number and allele frequency of CHRFAM7A in 657 normal controls by locus specific dual genotyping (TaqMan for dosage and Capillary sequencing for 2 bp deletion). C CHRFAM7A locus characteristics demonstrated in USCS genome browser reference sequence with active tracks including mappability which refers to the fidelity of sequence mapping to the reference genome, existing microarrays probes in frequently used commercial microarrays and the genomic architecture of low copy repeats depicted as orange and grey bars. These LCRs have over 95% sequence homology. These tracks indicate limited mappability (density of red bars); sparse probe coverage of CHRFAM7A in SNP arrays undermining detection of association in GWAS studies and complex genomic architecture with low copy repeats. D PCR mapping of the alleles in 6 samples with known CHRFAM7A genotype (depicted on top): UB068 – 0 copy of CHRFAM7A, null; H9 and UB056 - inverted, I; UB019 and UB134 - heterozygous, HZ; UB052 - direct, D. Primer sets are depicted below. To detect a part of CHRNA7, the forward primer was designed to hybridize with a unique sequence in exon A and the reverse primers - within exons 6, 7, 8, 9, and 10. To decipher the exon composition of FAM7A/ULK4 segment, the forward primers are designed in sequences in exon F, D-C, B, E and the reverse primer - in exon 5 on CHRNA7 segment.
Thus, unambiguous genotyping depends on two independent locus-specific assays: as a first step, TaqMan assay detects the dosage of CHRFAM7A alleles (possible copy number 0-3); as a second step, capillary sequencing detects the 2 bp deletion in exon 6. As exon 6 is present in CHRNA7 (2 copies) and CHRFAM7A (0-3 copies), the ratio of the capillary sequencing peaks (2 bp deletion versus no deletion) deciphers the number of direct and inverted CHRFAM7A structural variants [6, 7]. CHRFAM7A allele frequencies by locus-specific dual genotyping have been studied in various cohorts [6, 8, 9]. The largest cohort of 1174 subjects of European descent revealed that CHRFAM7A is present in 0 (ancestral, 0.1%), 1 (18%), 2 (80%) and 3 (0.02%) copies in the human genome (Fig. 1B). Frequency of the direct allele is 49%, in contrast to 42% of the inverted and 9% of the ancestral allele [6]. Recently, inverted allele frequency was reported from the 1000 genome project revealing diversity in various populations, ranging from 8% in Africa to 66% in East Asia [10]. Of note, detection of the 2 bp deletion allele does not define the genotype, as it can represent hemizygous, heterozygous or homozygous state [10].
To add to the challenges, the genomic architecture is complex; CHRFAM7A and its parent sequences, CHRNA7 and FAM7A/ULK4 are embedded in a complex cluster of low copy repeats (LCR) (Fig. 1C). LCRs undermine genome assembly and lead to gaps and uncertainty in the reference genome, creating barriers to assay CHRFAM7A with genome-wide methods (Fig.1C). The region has limited mappability for single nucleotide polymorphism (SNP), copy number variation (CNV) array probes and next generation sequencing (NGS). All of these factors contribute to sparse SNP coverage and even less probe coverage for microarrays (Fig.1C). PCR mapping of 6 human donors demonstrates variance in the human population (Fig. 1D). In an NGS dataset, the breakpoint sequence is captured in 26% of samples in contrast to an expected frequency of 91% (data not shown).
This multiallelic genetic architecture embedded in LCR with the added complexity of the inversion positions CHRFAM7A in the blind-spot of genome-wide approaches. While algorithms to identify structural variants from whole genome sequencing (WGS) data have evolved over the years, these algorithms are limited to identifying relatively short inversions (<80kbp). These fundamental barriers for genotyping accuracy from whole genome approaches undermine the use of existing datasets. The elusive genotyping from microarray and NGS data underscore that CHRFAM7A has not been fully explored as a risk gene. Emerging long-range sequencing technology (TruSeq Synthetic Long Reads (TSLR), 10X Genomics linked- reads (10XG), Dovetail Genomics (Chicago Method), and Contiguity-Preserving Transposition Sequencing (CPT-Seq) [11] are promising alternatives; however, large datasets typically used in genome-wide association studies (GWAS) are not available. To be able to leverage existing microarray and WGS datasets, long-range sequencing of an adequate sample size of the human population is needed to fully understand diversity and the relationship of the CHRFAM7A alleles to ascertain genetic markers.
Strong selective pressure
Genotype distribution of the 2 copy individuals is 25% direct homozygous, 25% inverted homozygous and 50% heterozygous, reminiscent of Hardy-Weinberg equilibrium. Based on the allele frequencies, the inverted allele underwent similar selective pressure as the direct allele, implicating that the inverted allele is functional. Both direct and inverted CHRFAM7A are transcribed as RNA in various cell types under normal and pathological conditions [12,13,14]. The direct allele is translated and modifies the α7 nAChR [6, 13, 15,16,17] which is a plausible foundation for driving selective pressure, although the mechanism is not known. The inverted allele is controversial; it is translated when overexpressed in SH-EP1 cells, oocytes, and Neuro2a cells-mouse neuroblasts [18, 19]. However, the expression vector was derived from the direct allele with in vitro mutagenesis deleting the 2 bp [18], thus interpretation of these results requires caution regarding translation of the nascent inverted allele. In silico analysis of the nascent inverted allele resulted in predictions including i) the protein is not translated as the distance between the Kozak consensus sequence from initiation makes native translation unlikely [20]; ii) or putative truncated peptide is translated due to the frameshift leading to an early stop codon; iii) or exons 5-10 from CHRNA7 are translated with no unique sequence [14, 15]. More recent human iPSC functional readouts of the inverted allele indicate that the inverted allele is null, at least from the α7 nAChR perspective [6].
Further studies are needed to understand the function of the inverted allele and the underlying genetic mechanism. Small peptides that are not detectable by traditional assays [10, 18], and RNA-based mechanisms are emerging hypotheses in the human genome in general, with recent evidence from mining the “junk genome” [21, 22].
Neuropsychiatric disease association
Despite the clear challenges with the locus, there is a signal that CHRFAM7A is a risk factor for neuropsychiatric diseases. Genetic association studies in neuropsychiatric diseases are summarized in Table 1.
AD and schizophrenia have been studied most, albeit in different ways. In AD, GWAS detected an inverse correlation of CHRFAM7A dosage with AD [7, 23, 24], without deciphering the direct and inverted alleles. In schizophrenia, a candidate gene approach was applied focusing on the 2 bp deletion; in addition, association with endophenotypes such as episodic memory and sensory gating were explored [8, 9, 25,26,27]. In schizophrenia, the presence of the inverted allele inferred the risk. CHRFAM7A expression was increased in post mortem brain tissue in schizophrenia, bipolar disease and major depression. The assay design does not distinguish whether the detected gene expression is from the direct or the inverted allele [14, 28]. The emerging pattern suggests that AD is associated with loss or reduced CHRFAM7A function, albeit agnostic to the orientation of the allele. In psychiatric diseases the inverted allele is associated with the disease state.
Pharmacogenetic studies in acetylcholine esterase inhibitor (AChEI) therapy in AD [6] and varenicline in addiction relapse [29] present the first proof of principle studies that CHRFAM7A is contributing to the translational gap in cholinergic therapies. With the understanding that 99.3% of humans harbor this human-restricted gene and that compound screens have a significant animal validation component without this human context, it is not surprising that treatment for AD and psychiatric disorders remains an unmet need.
CHRFAM7A translated from the direct allele modifies the α7 nAChR
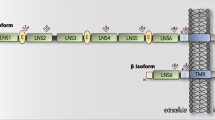
α7 nAChR is a homopentamer receptor with a role in fast synaptic transmission and the highest Ca2+ conductor of the nAChRs (Fig. 2A). Translated CHRFAM7A retains the transmembrane and intracellular domains of CHRNA7, while the extracellular domain is truncated and consists of a unique protein sequence. CHRFAM7A can be incorporated into the α7 nAChR homopentamer as 1-3 copies, resulting in an α7/CHRFAM7A nAChR heteropentamer (Fig. 2B). In mammalian cells, CHRFAM7A subunits are assembled and transported to the cell membrane together with full-length α7 subunits [19, 30]. A physical interaction between CHRNA7 and CHRFAM7A was demonstrated using epitope- and fluorescent protein-tagged CHRNA7 and CHRFAM7A constructs [19, 31].
A Schematic diagram illustrating α7 nAChR-mediated signaling cascades in neuronal cells. Agonist binding to the α7 nAChR causes the receptor activation and an increase in Ca2+ concentration. Ionotropic receptor function is associated with Ca2+ influx from the extracellular space and calcium-induced calcium release (CICR) from the endoplasmic reticulum. The desensitized, inactive receptor is thought to function as a metabotropic receptor activating inositol 1,4,5-trisphosphate (IP3) induced calcium release (IICR) from the ER. Downstream Ca2+ signaling is implicated in 1) neurotransmitter release; 2) structural LTP (depends on sequential activation of Calcium–calmodulin (CaM)-dependent protein kinase II (CaMKII), protein kinase A (PKA), Extracellular signal-regulated kinase (ERK), and cyclic AMP response element binding protein, CREB; 3) activation of Phosphoinositide 3-kinase (PI3K) and Akt that leads to inactivation of glycogen synthase kinase 3 beta (GSK3β), and downregulation of apoptosis through downregulation of BAX and upregulation of Bcl2 that ultimately results in neuroprotection; 4) activation of RhoA that causes a decrease in actin and tubulin polymerization and attenuates neurite outgrowth and microtubule assembly; 5) activation of CDC42 that leads to filopodia membrane specialization in neurite outgrowth, growth cone, and dendritic spine. B CHRFAM7A effect on α7 nAChR-mediated signaling pathways in neurons has been partially elucidated. α7/CHRFAM7A nAChR being a hypomorphic receptor demonstrates decreased activation by electrophysiology and diminished Ca2+ influx. The hypomorphic receptor has decreased agonist (α-BTX) binding and mitigates amyloid beta 1-42 (Aβ1-42) uptake. α7/CHRFAM7A nAChR leads to decreased channel open probability shifting the time spent in CICR to IICR associated with activation of small GTPase Rac1. Downstream, Rac1 switches from CDC42/filopodia to Rac1/lamellipodia membrane structure at all levels of the neuronal unit: neurite outgrowth, growth cone, and dendritic spine. Compared to α7 nAChR (A), α7/CHRFAM7A nAChR associated phenotypes and signaling demonstrate significant gaps in knowledge (B) Dotted lines represent predicted pathways (created with BioRender.com).
The α7/CHRFAM7A nAChR functions as a hypomorphic receptor in various models utilizing transient or stable transfection [18, 19, 30, 32]. While there seems to be a consensus that CHRFAM7A alone cannot produce a functional receptor [18, 30, 32], data on the effect of CHRFAM7A on α7 nAChR specific currents are controversial [18, 30] likely due to differences in model systems and experimental designs. Electrophysiological studies on human iPSC-derived neurons indicate that CHRFAM7A affects PNU 120596-modulated currents of the α7 nAChR [6, 33]. While qualitatively the single-channel current clusters appeared similar in the presence or absence of CHRFAM7A, α7/CHRFAM7A nAChR demonstrated decreased channel open probability and faster run-down [6, 33]. Whole patch-clamp confirmed that the α7/CHRFAM7A nAChR is hypomorphic for the ionotropic effect [19].
Irreversible binding of fluorescent α-bungarotoxin (α-BTX), a selective α7 nAChR antagonist, has been utilized to investigate the effect of CHRFAM7A on α7 nAChR in vitro and in vivo. In rat neuronal PC12 cell line stably expressing CHRFAM7A, α-BTX binding was decreased [34]. α-BTX staining was diminished at the neuromuscular junction and brain tissue in CHRFAM7A transgenic mice compared to wild-type controls [34, 35]. Lower α-BTX binding was interpreted as less α7 nAChR expression on the membrane surface; an alternative interpretation of the data is that the assembled α7/CHRFAM7A nAChR is hypomorphic for α-BTX binding [18, 32]. Since in immune cells overexpression of CHRNA7 and CHRFAM7A results in α7/CHRFAM7A nAChR retention in the endoplasmic reticulum (ER) [31], both mechanisms may play a role depending on cell type.
α7 nAChR regulates intracellular Ca2+ pools [36]. In neurons, ligand-gated activation of α7 nAChR results in high Ca2+ permeability, followed by fast inactivation, extended desensitization and calcium-induced calcium release (CICR) (Fig. 2A). During the channel closed state α7 nAChR can function as a metabotropic receptor, albeit the metabotropic effect has not been fully elucidated due to challenges in the availability of controls [37]. The metabotropic effect is postulated to be through G-proteins (Fig. 2A), supported by structural analysis demonstrating that α7 nAChR possesses a G-protein binding cluster in the M3-M4 intracellular loop [38]. Coupling of α7 nAChR with the α subunit of heterotrimeric Gαq was demonstrated in neurons and with Gαi in microglia [39, 40]. Downstream, the signal transduction involves the activation of phospholipase C (PLC) and inositol triphosphate (IP3)- induced Ca2+ release (IICR) from the endoplasmic reticulum (ER) (Fig. 2A). Recently fluorescent live Ca2+ imaging revealed that CHRFAM7A reduces peak amplitude and area under the curve in single cell Ca2+ dynamics traces [41, 42]. The reduced Ca2+ signal is consistent with a hypomorphic α7/CHRFAM7A nAChR.
CHRFAM7A in the central nervous system
A wealth of information is available on α7nAChR function from studies on cell lines (overwhelmingly from PC12 rat cells), rodent primary neuronal cultures and animal models [43]; in comparison data on how CHRFAM7A alters α7nAChR neurobiology is limited.
CHRFAM7A and CHRNA7 are mostly co-expressed in the central nervous system (CNS) [14, 28, 44]. As both genes contribute to α7 nAChR in humans, CHRFAM7A functional readouts are expected where α7 nAChR is expressed, including neurons [45], astrocytes [46, 47], microglia [48, 49] and brain endothelial cells [50, 51]. Physiological implication of CHRFAM7A are not fully understood, but as a modifier of the α7nAChR it is plausible that CHRFAM7A plays a role in neuronal transmission [36, 52,53,54], neuroinflammation [55,56,57], neuroprotection [58, 59], vascular homeostasis [50] and the blood-brain barrier [50, 60]. In regards of cognitive domains, highest expression of α7nAChR is detected in the hippocampus, prefrontal cortex and amygdala [61,62,63]: areas involved in learning, memory, and attention [64,65,66,67]. How CHRFAM7A may affect these processes has recently gained some insights.
CHRFAM7A function in neurons
In differentiated PC12 cells and animal models, α7 nAChR has been shown to contribute to fundamental neuronal processes (Fig. 2A). Presynaptically, α7 nAChR facilitates long-term potentiation (LTP) and inhibits long-term depression (LTD) [53]. Postsynaptically, it modifies downstream signaling leading to cyclic AMP response element binding protein (CREB) phosphorylation, changes in gene expression and modulation of neuronal activity (Fig. 2A). α7 nAChR is thought to regulate both the microtubule and actin cytoskeleton. In PC12 cells, α7 nAChR co-immunoprecipitates with Gαq and colocalizes with RhoA at the growth cone (GC); α7/ Gαq-dependent IP3 receptor (IP3R) phosphorylation, Phosphatidylinositol 4,5-bisphosphate (PIP2) breakdown, and α7/RhoA-dependent decrease in microtubule capping suggest that activation of α7-Gαq- IP3 pathway negatively affects microtubule dynamics [40, 68] (Fig.2A). α7 nAChR-G protein coupling performed in PC12 cells also leads to a RhoA associated decrease in actin polymerization and inhibition of neurite outgrowth [68]. In human iPSC-derived neurons, activation of α7 nAChR leads to increased CDC42 activity and filopodia membrane specialization at the growth cone and dendritic spine [42] (Fig. 2A). Actin polymerization and microtubule dynamics are the cytoskeletal foundation for neuronal plasticity.
Functional readouts on CHRFAM7A are emerging from CHRFAM7A transgenic mice and from post-mortem human brain (Fig. 2B). Proteomic profiling of CHRFAM7A transgenic mouse brain revealed that the presence of CHRFAM7A upregulates proteins involved in Ca2+ signaling, oxidative phosphorylation, as well as signaling pathways implicated in α7-nAChR-mediated neuropsychiatric disorders: AD, Parkinson’s disease, and Huntington disease [35]. Transcriptomic analysis of post-mortem human brain tissue identified Ca2+ signaling, small GTPases, synaptic structure and actin cytoskeleton being associated with increased CHRFAM7A expression [42].
Mechanistic insights into these omics-based hypotheses on CHRFAM7A-mediated neuronal phenotypes are studied in human isogenic iPSCs [42]. The model validated that CHRFAM7A modifies Ca2+ dynamics leading to the activation of small GTPase Rac1. Downstream, Rac1 creates a dynamic actin cytoskeleton and switches from CDC42/filopodia to Rac1/lamellipodia membrane structure at all levels of the neuronal unit, including neurite outgrowth, growth cone and dendritic spine [42] (Fig. 2B). Lamellipodia also facilitate adaptation to the mechanical properties of the tissue environment [42].
While CHRFAM7A is not a neurodevelopmental gene as null individuals are cognitively normal, brain vulnerability may be related to lower CHRFAM7A levels, explaining why AD is associated with lower CHRFAM7A dosage [7]. Human studies are needed to understand how this actin cytoskeleton gain of function (GOF) affects brain resilience, cognitive reserve and plasticity and to gain mechanistic insights into the neuropsychiatric disease associations.
α7 nAChR neurobiology exhibits other important mechanisms relevant to neurodegeneration. Amyloid beta (Aβ) binds the α7 nAChR with high affinity [69] and Aβ uptake induces neuronal toxicity [69] and apoptosis [70]. The hypomorphic α7/CHRFAM7A nAChR mitigates Aβ1–42 uptake and neurotoxicity in a dose-dependent manner [6, 33]. Intriguingly, mitigated Aβ1–42 uptake is associated with caspase-independent activation of inflammatory cytokines (interleukin 1beta (IL1B) and tumor necrosis factor alpha (TNFA)); suggesting a neuronal cry for help mechanism [33]. Pharmacological treatment with acetylcholine esterase inhibitors, AChEIs (donepezil, rivastigmine) and encenicline, a selective α7 nAChR agonist, revealed decreased efficacy in the presence of CHRFAM7A consistent with the α7/CHRFAM7A nAChR being hypomorphic [6]. In agreement with the in vitro observations, benefit from AChEI therapy was diminished in CHRFAM7A carriers in a human double-blind pharmacogenetic clinical trial [6]. These data resolve the conundrum that CHRFAM7A is associated with reduced risk of AD due to the neuronal structure GOF while response to therapy is mitigated because the α7/CHRFAM7A nAChR is hypomorphic.
Microglia
α7 nAChR is central to the cholinergic anti-inflammatory response by inhibiting nuclear factor kappa B (NFκB) activation and translocation to the nucleus [71], thus downregulates transcription of cytokines interleukin 6 (IL6), IL1B and TNFA [72,73,74]. In microglia, pharmacological modulation of the α7 nAChR with agonist PHA 568487 attenuated neuroinflammation and oxidative stress, while its antagonist methyllycaconitine, MLA, augmented them in the ischemic stroke model [75]. In α7 nAChR knockout (α 7nAChR-/-) mice ischemic stroke was associated with higher levels of proinflammatory cytokines (TNFα, IL6, IL-1β) [76]. α7 nAChR mediated signaling in these experiments involved inhibition of NFκB and an activation of nuclear factor erythroid-2-related factor 2 (Nrf2) leading to upregulation of antioxidant genes [71] (Fig. 3A). Although the signal transduction pathway has not been fully elucidated, there is evidence that α7 nAChR co-immunoprecipitates with Giα protein in microglia [39] consistent with the notion that α7 nAChR signaling in non-neuronal cells involves heterotrimeric G-proteins activation, IICR and effector kinases (Fig. 3A) [77].
A Schematic diagram illustrating α7 nAChR-mediated anti-inflammatory signaling cascades. Activation of α7 nAChR leads to an inhibition of inflammatory cytokines by blocking nuclear factor -κB (NFκB) activity through: 1) Janus kinase 2 (JAK2)-signal transducer and activator-3 (STAT3); STAT3 activates interleukin-1 receptor-associated kinase M (IRAK-M). 2) Gαi-mediated pathway involving activation of phospholipase C (PLC), production of inositol 1,4,5-trisphosphate (IP3), its binding to the receptor (IP3R) in the endoplasmic reticulum (ER), which leads to Ca2+ release from the ER and causes deactivation of c-jun-N-terminal kinase (JNK), p38, and p44/42 mitogen-activated protein kinases. Activation of JAK2 also leads to activation of Phosphoinositide 3-kinase (PI3K) and Akt that phosphorylates and inactivates glycogen synthase kinase 3 beta (GSK3β), which, in turn, leads to activation and nuclear translocation of Nuclear factor erythroid 2-related factor 2 (Nrf2). Nrf2 induces the expression of anti-inflammatory heme oxygenase-1 (HO-1). Activation of α7 nAChR results in decreased inflammation, MMP9 expression and migration, leading to neuroprotection. B CHRFAM7A effect on α7 nAChR-mediated anti-inflammatory signaling. While signaling of the α7/CHRFAM7A nAChR in mononuclear cells are mostly unknown, emerging evidence suggests that the hypomorphic α7/CHRFAM7A nAChR releases NFκB inhibition leading to activation of proinflammatory cytokines (IL6, IL1β, TNFα). CHRFAM7A is associated with additional inflammatory phenotypes, including immune cell mobilization, a decrease in fibrosis and reduction in M2 macrophages and chemotaxis). Dotted lines represent predicted pathways (created with BioRender.com).
In a hiPSC model of neuroinflammation, CHRFAM7A KI isogenic microglia demonstrated increased TNFα, IL6, IL-1β levels at baseline consistent with the release of the cholinergic anti-inflammatory tone due to the hypomorphic α7/CHRFAM7A nAChR [78] (Fig. 3B). When treated with lipopolysaccharide (LPS), a prototype pathogen-associated molecular pattern (PAMP), CHRFAM7A KI microglia demonstrated higher induction of IL-6 compared to null in a cytokine screen [78]. CHRFAM7A prolonged LPS induced NFκB nuclear presence and activation (Fig. 3B). Aβ1–42, a damage-associated molecular pattern (DAMP) that binds to α7 nAChR with high affinity, exhibited decreased Aβ1–42 uptake and increased innate immune response (decreased inhibition) in CHRFAM7A KI microglia [78]. While both the DAMP and PAMP responses are proinflammatory, Aβ1–42 has a direct effect on the α7/CHRFAM7A nAChR; in contrast, the LPS response is modulated by CHRFAM7A.
Similar to neurotoxicity, CHRFAM7A may be protective for microglia survival. In a cellular model of human cerebral ischemia-reperfusion (I/R) [79] overexpression of CHRFAM7A led to inhibition of microglia pyroptosis through NLRP3/ Caspase-1 and resulted in a diminished cerebral I/R injury [79]. In iPSC-derived microglia, the hypomorphic α7/CHRFAM7A nAChR mitigates Aβ1–42 uptake [78], which may serve as the mechanism for microglia protection.
Models are challenging due to the low abundance of the microglia relative to other cell types in the brain [80] and the limitations of immortalized microglia cell lines. Human iPSCs provide a theoretically unlimited resource, however, microglia differentiation protocols are burdensome and have low-yield [78, 81]. The field is evolving and alternative strategies, such as the use of monocytes and macrophages, are being explored [81, 82]. These efforts will facilitate deciphering the role of CHRFAM7A on microglia biology.
CHRFAM7A in the immune system
The cholinergic anti-inflammatory pathway (CAIP) is a neuronal-immune interface, where the nervous system regulates immune function through a neurotransmitter [83]. Our understanding of the CAIP has significantly evolved [55, 84]; and the discovery of α7 nAChR expression in macrophages [57] was a critical step in this process. The neuronal-immune axis utilizes the efferent branches of the vagus and splenic nerves modulating α7 nAChR-expressing macrophages and T-cells [85]. The very nature of the interaction between two separate biological systems historically required in vivo studies in animal models and these experiments represent the foundation of our current understanding. Of note, animal models are agnostic to CHRFAM7A, thus our understanding is incomplete for 75% of the human population, those who carry the direct allele.
α7 nAChR is expressed in most cell types in the immune system [86], including both innate (macrophages, dendritic cells, basophils, and mast cells [87, 88]) and adaptive (T and B lymphocytes [89]) immune cells. In addition to its role in Ca2+ signaling (see microglia section), activation of α7 nAChR leads to signal transduction through Janus kinase 2 (JAK2)-signal transducer and activator-3 (STAT3) and/or Phosphoinositide 3-kinase (PI3K)/Akt signaling pathways [71, 90] (Fig. 3A). Phosphorylation of STAT3 negatively regulates NFκB, preventing its nuclear translocation and binding to DNA, thus decreasing transcriptional activation of proinflammatory cytokines [77] (Fig. 3A). Of note, attempts to target α7 nAChR in order to harness the anti-inflammatory mechanism also identified a translational gap in human clinical trials [91].
CHRFAM7A is abundant in human monocytes, macrophages, and monocytic cell lines [15,16,17, 92]. In macrophages CHRNA7 and CHRFAM7A are independently regulated [92]. Treatment of THP-1 macrophage with LPS substantially decreased CHRFAM7A expression; this effect was prevented by an IκB kinase inhibitor, parthenolide, suggesting NFκB-dependent mechanism [15]. Thus, during inflammation, CHRFAM7A is downregulated which may serve as a negative loop to control the immune response by shifting abundance from α7/CHRFAM7A nAChR to α7 nAChR [15, 17].
CHRFAM7A-associated immune phenotypes were explored [20] using CHRFAM7A transduced cell lines [93] and a transgenic mouse model [13]. Consistent with the hypomorphic receptor, CHRFAM7A reduced cell migration, chemotaxis, and colony formation in THP-1 transduced macrophages [93]. CHRFAM7A KI mice demonstrated an increased hematopoietic stem cell reservoir in the bone marrow, myeloid differentiation, and a shift in cell population from granulocytes to inflammatory monocytes suggesting a role in hematopoiesis [13]. Consistent with the recently elucidated Rac1-actin reorganization [42], immune cells of CHRFAM7A KI mice demonstrated superior immune cell mobilization and invasion of the diseased lung and prevention of secondary bacterial infection [13].
CHRFAM7A is protective against inflammation-associated fibrosis. Overexpression of CHRFAM7A led to decreased fibrosis in the hypertrophic scar mouse model [94]. In this experimental paradigm, a reduction of M2 macrophages and activation of Notch signaling was observed in mice transfected with CHRFAM7A. In an obstructive nephropathy model overexpression of CHRFAM7A decreased the release of inflammatory cytokines in the kidneys and had a protective effect against renal fibrosis [95].
In human studies of sepsis and inflammatory bowel disease (IBD) CHRFAM7A expression is associated with augmented cytokine response consistent with the release of NFκB inhibition [12, 31, 96]. On the other hand, CHRFAM7A expression inversely correlates with HIV-associated neurocognitive disorders [44] and critical Covid-19 [97] suggesting a more efficient immune response. As inflammation plays a role in a broad spectrum of disease pathology, elucidating the full spectrum of the CHRFAM7A inflammatory phenotype may suggest novel treatment approaches [98].
CHRFAM7A in cancer
Cigarette smoking is one of the most studied risk factors for cancer and has been associated with malignant neoplasms along the respiratory system (oral cavity, lung, pharyngeal) and remote sites [99, 100]. Nicotine, the prototypical nAChR agonist, and nicotine’s metabolic intermediates, nitrosamines 4-(methylnitrosamino)-1-(3-pyrydyl)-1-butanone (NNK) and N-nitrosonornicotine, emerged as the cause of this association [101]. α7 nAChR is expressed in several types of human cancer, including head and neck squamous cell carcinoma, bladder, squamous cell lung cancer cells, lung adenocarcinoma and small cell lung cancer [102, 103].
Features of the tumor environment, such as physical constraints, hypoxia, inflammation and metabolic stress activate complex signaling pathways known to play critical roles in both embryogenesis and tumor development. Experimental data on α7 nAChR from various cancer cell lines converge on phenotypes including cell proliferation, apoptosis, angiogenesis and metastatic potential; and inflammation in the tumor microenvironment [102].
There is a growing body of evidence that nicotine-mediated tumor progression is associated with the α7 nAChRs [104] (Fig. 4A). Although clinicopathological studies are sparse, increased α7 nAChR expression in cholangiosarcoma specimens is associated with higher histological grade (p < 0.01), tumor stage (p < 0.05), lymphatic (p < 0.01), and distant metastasis (p < 0.01). α7 nAChR expression also correlated with shorter survival (p < 0.0001) [105].
A Schematic diagram illustrating α7 nAChR-mediated signaling pathways regulating cell proliferation, angiogenesis, and metastasis (created with BioRender.com). Activation of α7 nAChR by nicotine leads to the activation of Ca2+/calmodulin-dependent signaling pathways increasing: (1) proliferation (Phosphoinositide 3-kinase (PI3K)/Akt; Mitogen-activated protein kinase/ERK kinase (MEK)/ Extracellular signal-regulated kinase (ERK); RAF1/Rb, and Sp1/GATA1); (2) angiogenesis (PI3K)/Akt/NFκB, FGF2); (3) metastasis, (4) epithelial-mesenchymal transition (EMT), and (5) migration (PI3K, MEK/ERK, focal adhesion kinase (FAK), and SOX2). B CHRFAM7A effect on α7 nAChR-mediated signaling pathways in cancer. Activation of α7/CHRFAM7A nAChR receptor by nicotine results in opposite phenotypes consistent with the hypomorphic response to an agonist: a decrease in proliferation, metastasis, EMT, and migration. Signal transduction is unknown (A) (created with BioRender.com).
While CHRFAM7A in cancer has limited information (Fig. 4B), nicotine-associated tumor biology in the presence of CHRFAM7A is consistent with the hypomorphic receptor [106, 107]. In lung cancer, squamous cell carcinoma (SQC) specimens, the more nicotine-dependent type, had lower gene expression levels of CHRFAM7A in the peri-tumoral normal tissue compared to normal tissue in the less nicotine-dependent adenocarcinoma specimens (ADC). This suggests that in SQC the predominance of wild-type α7 nAChR may drive the nicotine association, while in ADC the hypomorphic α7/CHRFAM7A nAChR mitigates the role of nicotine. Of note, compared to normal tissue, CHRFAM7A was downregulated in both SQC and ADC [106] which suggests an interaction between CHRFAM7A and tumor biology.
The hypomorphic receptor decreases α7 nAChR mediated effects leading to decreased proliferation when exposed to nicotine, abrogated nicotine-induced epithelial-mesenchymal-transition (EMT), decreased migration and decreased nicotine-associated tumor growth of CHRFAM7A overexpressing xenografts [107]. However, invasion and inflammation, the two CHRFAM7A GOF phenotypes [42, 78] have not been studied in this report [107]. These emerging mechanistic insights into CHRFAM7A cell biology creates an opportunity to expand hypotheses regarding the role of CHRFAM7A in human cancer.
Beyond the mitigation of nicotine effect, CHRFAM7A has been shown to affect three fundamental biological processes that may be relevant to cancer: cellular response to the mechanical properties of the tissue microenvironment [42]; actin cytoskeletal changes leading to lamellipodia formation and invasion [42] and inflammation through NFκB activation, increasing IL-6 levels in particular [78]. All these pathways have been established in human cancer biology; the emerging experimental data positions CHRFAM7A as an upstream modulator of these processes.
CHRFAM7A may play a role in tumor metastasis
Cellular motility is the foundation of the metastatic process, which includes invasion of tumor cells into the surrounding tissues and penetration of vessels and migration toward distant sites of the body away from the primary sites [108, 109]. Epithelial cancers lose cell polarity or undergo reprogramming leading to metastatic states dependent on microenvironmental signals and completion of the transdifferentiation process to promote cell motility. Actin restructuring drives the final outcome associated with invasion and migration phenotypes. During invasion cells rearrange their actin cytoskeleton, which creates special membrane structures called lamellipodia or invadopodia. In migration, cytoskeletal rearrangement results in cell elongation and directional motility [110]. The mode of motility is associated with distinct small GTPase activation [111, 112]
Human-restricted CHRFAM7A has been recently implicated in actin cytoskeleton dynamics and organization [42]. Mechanistically, the hypomorphic α7/CHRFAM7A nAChR leads to Ca2+ dynamic changes, resulting in Rac1 small GTPase activation [42]. Rac1 organizes the actin cytoskeleton to support lamellipodia and invasion [113]. In addition, the actin cytoskeletal reinforcement leads to adaptation to the mechanical properties of the tissue environments [42, 114, 115]; an important pillar of tumor biology [116]. As Rac1 is central to the metastatic process, CHRFAM7A likely has a significant role, especially in the invasion of the surrounding tissue and local spread for cancer. As Rac1 is an active drug target in metastatic cancer [117] understanding the role of CHRFAM7A has important translational significance [6].
CHRFAM7A may alter the tumor environment through inflammation
As part of the complex process of metastatic behavior, tumor cells secrete small soluble proteins, such as cytokines, to stimulate neoplastic cells (autocrine effect) and prepare tumoral microenvironment (paracrine effect). Dysregulation of IL-6, a pleiotropic cytokine that plays an important role in multiple physiological processes, is associated with poor outcomes in cancer [118]. IL-6 pathway dysregulation contributes to cell proliferation through IL-6/JAK/STAT3 signaling and to cancer cell invasion [119,120,121,122]. CHRFAM7A has been shown to increase NFκB activation leading to cytokine release with highest effect on IL-6 in microglia [78]. An increased level of IL-6 was also detected in transgenic CHRFAM7A mice compared to wild type in an osteoarthritis animal model [123]. It is plausible that CHRFAM7A contributes to IL-6-associated cancer metastasis, implicating genotype-specific therapeutic potential.
Further studies are needed to understand the role of CHRFAM7A in tumor biology. Of importance, CHRFAM7A is not a cancer gene itself, rather gives a contextual human biology to cancer. As CHRFAM7A carrier status splits the human population 75% to 25% [6, 42], we expect profound translational significance for metastatic cancer treatment and immunotherapy.
CHRFAM7A in other diseases
Although CHRFAM7A biology has not been studied in other organs and cell types, CHRFAM7A as a dominant negative modulator of the α7 nAChR is expected to modify α7 nAChR biology. Human data is available for α7 nAChR ionotropic effects on the cardiac conduction system from randomized clinical trials using α7 agonists and allosteric modulators. Of note, major effects on cardiac conduction has not been established [124, 125].
α7 nAChR effect on the heart is complex, involving both a direct ionotropic effect on heart rate and an immune-mediated effect connected to the vagus nerve and measured by heart rate variability. In clinical conditions associated with systemic inflammation (e.g. endotoxemia and sepsis), reduced heart rate variability and increased cardiac cycle regularity are observed. These effects can be triggered by LPS injection and IL-6 administration indicating a role of inflammation [126]. α7 nAChR is implicated in myocardial fibrosis, including right ventricular (RV) fibrosis, a maladaptive RV hypertrophy associated with poor outcomes in pulmonary hypertension [127].
In atherosclerosis, α7 nAChR mediates the immune response to cholesterol deposits, triggers the proliferation of vascular smooth muscle cells, and leads to oxidative stress and apoptosis [128,129,130]. In the pathomechanism of fatty liver and subsequent liver fibrosis, α7 nAChR was demonstrated to alter energy expenditure and O2 consumption, increase ECM expression and activate IL-6 [131]. In COPD, chronic bronchitis and lung fibrosis, α7 nAChR mediates increased mucin production and ECM expression [132, 133].
α7 nAChR attenuated experimental skin fibrosis in bleomycin-induced inflammation in mouse and human fibroblasts and the non-inflammation driven Transforming growth factor-beta (TGFβ) receptor Iact mouse model. α7 nAChR agonists reduced TGFβ1-mediated expression of collagen and myofibroblast. These actions were linked to modulation of the redox-sensitive transcription factor JunB and impairment of the mitochondrial respiratory system [134]. Anti-glomerular basement membrane antibody-induced glomerulonephritis in α7 nAChR KO mouse model exacerbated the glomerulosclerosis by increasing expression of fibrin, collagen TGFβ and TIMP-2 [135]. In CHRFAM7A transgenic mice with obstructive nephropathy, overexpression of CHRFAM7A decreased the release of inflammatory cytokines and had a protective effect against renal fibrosis [95]. Upregulation of CHRFAM7A gene expression and associated downregulation of CHRNA7 expression was detected in patients with IBD [12].
α7 nAChR effect on several organs reveals a common theme of fibrosis and inflammation as a disease mechanism. As CHRFAM7A is a human-specific additional layer of immune regulation, further studies are needed in models incorporating CHRFAM7A to develop rational therapeutic approaches.
Conclusion
CHRFAM7A remains an understudied area of neurobiology and for clear reasons. The genetic architecture is complex and the inversion event markedly diminishes the fidelity of the reference sequence and mappability, which resulted in the avoidance of this region in whole genome assay development. α7 nAChR biology accumulated substantial experimental data over the years on its role in brain function and disease, immunology and cancer. Clinical trials of drugs targeting the α7 nAChR have failed. Recent work on CHRFAM7A is starting to shed light on its biological function, suggesting that the biology of the hypomorphic α7/CHRFAM7A nAChR is distinctly different from the α7 nAChR readouts, which may contribute to the translational gap. Functional insights into the two alleles will inform genetic association and pharmacogenetic studies by refining the genetic model.
To fully understand the impact of CHRFAM7A in humans, there is a need to develop tools. Long-range sequencing is needed to build a reliable reference sequence of the locus and to understand human diversity. Human relevant models, that provide the human context have just started to emerge. The function of the inverted allele needs systematic exploration from human datasets. Once all of these are achieved, we can reiterate existing large datasets and re-analyze clinical trials with pharmacogenetic design. These are reasonable short-term goals which may lead to therapies for diseases where the unmet need is palpable.
References
Bitar M, Kuiper S, O’Brien EA, Barry G. Genes with human-specific features are primarily involved with brain, immune and metabolic evolution. BMC Bioinforma. 2019;20:406.
Chen Z, Zhang D, Reynolds RH, Gustavsson EK, Garcia-Ruiz S, D’Sa K, et al. Human-lineage-specific genomic elements are associated with neurodegenerative disease and APOE transcript usage. Nat Commun. 2021;12:2076.
Florio M, Heide M, Pinson A, Brandl H, Albert M, Winkler S, et al. Evolution and cell-type specificity of human-specific genes preferentially expressed in progenitors of fetal neocortex. Elife. 2018;7:e32332.
Song JHT, Grant RL, Behrens VC, Kucka M, Roberts Kingman GA, Soltys V, et al. Genetic studies of human-chimpanzee divergence using stem cell fusions. Proc Natl Acad Sci USA. 2021;118:e2117557118.
Sinkus ML, Graw S, Freedman R, Ross RG, Lester HA, Leonard S. The human CHRNA7 and CHRFAM7A genes: A review of the genetics, regulation, and function. Neuropharmacology. 2015;96:274–88.
Szigeti K, Ihnatovych I, Birkaya B, Chen Z, Ouf A, Indurthi DC, et al. CHRFAM7A: A human specific fusion gene, accounts for the translational gap for cholinergic strategies in Alzheimer’s disease. EBioMedicine. 2020;59:102892.
Szigeti K, Kellermayer B, Lentini JM, Trummer B, Lal D, Doody RS, et al. Ordered subset analysis of copy number variation association with age at onset of Alzheimer’s disease. J Alzheimers Dis. 2014;41:1063–71.
Flomen RH, Collier DA, Osborne S, Munro J, Breen G, St Clair D, et al. Association study of CHRFAM7A copy number and 2 bp deletion polymorphisms with schizophrenia and bipolar affective disorder. Am J Med Genet B Neuropsychiatr Genet. 2006;141b:571–5.
Flomen RH, Shaikh M, Walshe M, Schulze K, Hall MH, Picchioni M, et al. Association between the 2-bp deletion polymorphism in the duplicated version of the alpha7 nicotinic receptor gene and P50 sensory gating. Eur J Hum Genet. 2013;21:76–81.
Di Lascio S, Fornasari D, Benfante R. The Human-restricted Isoform of the α7 nAChR, CHRFAM7A: A double-edged sword in neurological and inflammatory disorders. Int J Mol Sci. 2022;23:3463.
Eslami Rasekh M, Chiatante G, Miroballo M, Tang J, Ventura M, Amemiya CT, et al. Discovery of large genomic inversions using long-range information. BMC Genomics. 2017;18:65.
Baird A, Coimbra R, Dang X, Eliceiri BP, Costantini TW. Up-regulation of the human-specific CHRFAM7A gene in inflammatory bowel disease. BBA Clin. 2016;5:66–71.
Costantini TW, Chan TW, Cohen O, Langness S, Treadwell S, Williams E, et al. Uniquely human CHRFAM7A gene increases the hematopoietic stem cell reservoir in mice and amplifies their inflammatory response. Proc Natl Acad Sci USA. 2019;116:7932–40.
Kunii Y, Zhang W, Xu Q, Hyde TM, McFadden W, Shin JH, et al. CHRNA7 and CHRFAM7A mRNAs: co-localized and their expression levels altered in the postmortem dorsolateral prefrontal cortex in major psychiatric disorders. Am J Psychiatry. 2015;172:1122–30.
Benfante R, Antonini RA, De Pizzol M, Gotti C, Clementi F, Locati M, et al. Expression of the α7 nAChR subunit duplicate form (CHRFAM7A) is down-regulated in the monocytic cell line THP-1 on treatment with LPS. J Neuroimmunol. 2011;230:74–84.
Villiger Y, Szanto I, Jaconi S, Blanchet C, Buisson B, Krause KH, et al. Expression of an alpha7 duplicate nicotinic acetylcholine receptor-related protein in human leukocytes. J Neuroimmunol. 2002;126:86–98.
Maroli A, Di Lascio S, Drufuca L, Cardani S, Setten E, Locati M, et al. Effect of donepezil on the expression and responsiveness to LPS of CHRNA7 and CHRFAM7A in macrophages: A possible link to the cholinergic anti-inflammatory pathway. J Neuroimmunol. 2019;332:155–66.
Araud T, Graw S, Berger R, Lee M, Neveu E, Bertrand D, et al. The chimeric gene CHRFAM7A, a partial duplication of the CHRNA7 gene, is a dominant negative regulator of α7*nAChR function. Biochem Pharm. 2011;82:904–14.
Wang Y, Xiao C, Indersmitten T, Freedman R, Leonard S, Lester HA. The duplicated α7 subunits assemble and form functional nicotinic receptors with the full-length α7. J Biol Chem. 2014;289:26451–63.
Costantini TW, Dang X, Coimbra R, Eliceiri BP, Baird A. CHRFAM7A, a human-specific and partially duplicated α7-nicotinic acetylcholine receptor gene with the potential to specify a human-specific inflammatory response to injury. J Leukoc Biol. 2015;97:247–57.
Decoding noncoding RNAs. Nat Methods. 2022;19:1147-8.
Leslie M. New universe of miniproteins is upending cell biology and genetics. Science. 2019. https://doi.org/10.1126/science.aaz8818.
Swaminathan S, Huentelman MJ, Corneveaux JJ, Myers AJ, Faber KM, Foroud T, et al. Analysis of copy number variation in Alzheimer’s disease in a cohort of clinically characterized and neuropathologically verified individuals. PLoS One. 2012;7:e50640.
Swaminathan S, Kim S, Shen L, Risacher SL, Foroud T, Pankratz N, et al. Genomic copy number analysis in Alzheimer’s disease and mild cognitive impairment: An ADNI study. Int J Alzheimers Dis. 2011;2011:729478.
Dempster EL, Toulopoulou T, McDonald C, Bramon E, Walshe M, Wickham H, et al. Episodic memory performance predicted by the 2bp deletion in exon 6 of the “alpha 7-like” nicotinic receptor subunit gene. Am J Psychiatry. 2006;163:1832–4.
Petrovsky N, Schmechtig A, Flomen RH, Kumari V, Collier D, Makoff A, et al. CHRFAM7A copy number and 2-bp deletion polymorphisms and antisaccade performance. Int J Neuropsychopharmacol. 2009;12:267–73.
Sinkus ML, Lee MJ, Gault J, Logel J, Short M, Freedman R, et al. A 2-base pair deletion polymorphism in the partial duplication of the alpha7 nicotinic acetylcholine gene (CHRFAM7A) on chromosome 15q14 is associated with schizophrenia. Brain Res. 2009;1291:1–11.
De Luca V, Likhodi O, Van Tol HHM, Kennedy JL, Wong AHC. Regulation of α7-nicotinic receptor subunit and α7-like gene expression in the prefrontal cortex of patients with bipolar disorder and schizophrenia. Acta Psychiatr Scand. 2006;114:211–5.
Cameli C, Bacchelli E, De Paola M, Giucastro G, Cifiello S, Collo G, et al. Genetic variation in CHRNA7 and CHRFAM7A is associated with nicotine dependence and response to varenicline treatment. Eur J Hum Genet. 2018;26:1824–31.
Lasala M, Corradi J, Bruzzone A, Esandi MDC, Bouzat C. A human-specific, truncated α7 nicotinic receptor subunit assembles with full-length α7 and forms functional receptors with different stoichiometries. J Biol Chem. 2018;293:10707–17.
Maldifassi MC, Martín-Sánchez C, Atienza G, Cedillo JL, Arnalich F, Bordas A, et al. Interaction of the α7-nicotinic subunit with its human-specific duplicated dupα7 isoform in mammalian cells: Relevance in human inflammatory responses. J Biol Chem. 2018;293:13874–88.
de Lucas-Cerrillo AM, Maldifassi MC, Arnalich F, Renart J, Atienza G, Serantes R, et al. Function of partially duplicated human α77 nicotinic receptor subunit CHRFAM7A gene: potential implications for the cholinergic anti-inflammatory response. J Biol Chem. 2011;286:594–606.
Ihnatovych I, Nayak TK, Ouf A, Sule N, Birkaya B, Chaves L, et al. iPSC model of CHRFAM7A effect on α7 nicotinic acetylcholine receptor function in the human context. Transl Psychiatry. 2019;9:59.
Chan T, Williams E, Cohen O, Eliceiri BP, Baird A, Costantini TW. CHRFAM7A alters binding to the neuronal alpha-7 nicotinic acetylcholine receptor. Neurosci Lett. 2019;690:126–31.
Jiang Y, Yuan H, Huang L, Hou X, Zhou R, Dang X. Global proteomic profiling of the uniquely human CHRFAM7A gene in transgenic mouse brain. Gene. 2019;714:143996.
Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev. 2009;89:73–120.
Kabbani N, Nichols RA. Beyond the channel: metabotropic signaling by nicotinic receptors. Trends Pharm Sci. 2018;39:354–66.
King JR, Nordman JC, Bridges SP, Lin MK, Kabbani N. Identification and characterization of a G protein-binding cluster in α7 Nicotinic Acetylcholine Receptors. J Biol Chem. 2015;290:20060–70.
King JR, Gillevet TC, Kabbani N. A G protein-coupled α7 nicotinic receptor regulates signaling and TNF-α release in microglia. FEBS Open Biol. 2017;7:1350–61.
Nordman JC, Kabbani N. Microtubule dynamics at the growth cone are mediated by α7 nicotinic receptor activation of a Gαq and IP3 receptor pathway. FASEB j. 2014;28:2995–3006.
Martín-Sánchez C, Alés E, Balseiro-Gómez S, Atienza G, Arnalich F, Bordas A, et al. The human-specific duplicated α7 gene inhibits the ancestral α7, negatively regulating nicotinic acetylcholine receptor-mediated transmitter release. J Biol Chem. 2021;296:100341.
Szigeti K, Ihnatovych I, Rosas N, Dorn RP, Notari E, Cortes Gomez E, et al. Neuronal actin cytoskeleton gain of function in the human brain. EBioMedicine. 2023;95:104725.
Xu ZQ, Zhang WJ, Su DF, Zhang GQ, Miao CY. Cellular responses and functions of α7 nicotinic acetylcholine receptor activation in the brain: a narrative review. Ann Transl Med. 2021;9:509.
Ramos FM, Delgado-Vélez M, Ortiz ÁL, Báez-Pagán CA, Quesada O, Lasalde-Dominicci JA. Expression of CHRFAM7A and CHRNA7 in neuronal cells and postmortem brain of HIV-infected patients: considerations for HIV-associated neurocognitive disorder. J Neurovirol. 2016;22:327–35.
Alkondon M, Albuquerque EX. The nicotinic acetylcholine receptor subtypes and their function in the hippocampus and cerebral cortex. Prog Brain Res. 2004;145:109–20.
Sharma G, Vijayaraghavan S. Nicotinic cholinergic signaling in hippocampal astrocytes involves calcium-induced calcium release from intracellular stores. Proc Natl Acad Sci. 2001;98:4148–53.
Shen JX, Yakel JL. Functional α7 nicotinic ACh receptors on astrocytes in rat hippocampal CA1 slices. J Mol Neurosci. 2012;48:14–21.
Shytle RD, Mori T, Townsend K, Vendrame M, Sun N, Zeng J, et al. Cholinergic modulation of microglial activation by α7 nicotinic receptors. J Neurochem. 2004;89:337–43.
Suzuki T, Hide I, Matsubara A, Hama C, Harada K, Miyano K, et al. Microglial alpha7 nicotinic acetylcholine receptors drive a phospholipase C/IP3 pathway and modulate the cell activation toward a neuroprotective role. J Neurosci Res. 2006;83:1461–70.
Kimura I, Dohgu S, Takata F, Matsumoto J, Kawahara Y, Nishihira M, et al. Activation of the α7 nicotinic acetylcholine receptor upregulates blood-brain barrier function through increased claudin-5 and occludin expression in rat brain endothelial cells. Neurosci Lett. 2019;694:9–13.
Krafft PR, Caner B, Klebe D, Rolland WB, Tang J, Zhang JH. PHA-543613 Preserves Blood–Brain barrier integrity after intracerebral hemorrhage in mice. Stroke. 2013;44:1743–7.
Cheng Q, Yakel JL. The effect of alpha7 nicotinic receptor activation on glutamatergic transmission in the hippocampus. Biochem Pharm. 2015;97:439–44.
Borroni V, Barrantes FJ. Homomeric and Heteromeric alpha7 Nicotinic Acetylcholine receptors in health and some central nervous system diseases. Membranes. 2021;11:664.
Larsen HM, Hansen SK, Mikkelsen JD, Hyttel P, Stummann TC. Alpha7 nicotinic acetylcholine receptors and neural network synaptic transmission in human induced pluripotent stem cell-derived neurons. Stem Cell Res. 2019;41:101642.
Pavlov VA, Wang H, Czura CJ, Friedman SG, Tracey KJ. The cholinergic anti-inflammatory pathway: a missing link in neuroimmunomodulation. Mol Med. 2003;9:125–34.
Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007;117:289–96.
Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, et al. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–8.
Ito T, Inden M, Ueda T, Asaka Y, Kurita H, Hozumi I. The neuroprotective effects of activated alpha7 nicotinic acetylcholine receptor against mutant copper-zinc superoxide dismutase 1-mediated toxicity. Sci Rep. 2020;10:22157.
Liu Y, Zeng X, Hui Y, Zhu C, Wu J, Taylor DH, et al. Activation of alpha7 nicotinic acetylcholine receptors protects astrocytes against oxidative stress-induced apoptosis: implications for Parkinson’s disease. Neuropharmacology. 2015;91:87–96.
Zhang B, Yu JY, Liu LQ, Peng L, Chi F, Wu CH, et al. Alpha7 nicotinic acetylcholine receptor is required for blood-brain barrier injury-related CNS disorders caused by Cryptococcus neoformans and HIV-1 associated comorbidity factors. BMC Infect Dis. 2015;15:352.
Feduccia AA, Chatterjee S, Bartlett SE. Neuronal nicotinic acetylcholine receptors: neuroplastic changes underlying alcohol and nicotine addictions. Front Mol Neurosci. 2012;5:83.
Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharm Sci. 2006;27:482–91.
Zoli M, Pistillo F, Gotti C. Diversity of native nicotinic receptor subtypes in mammalian brain. Neuropharmacology. 2015;96:302–11.
Bloem B, Poorthuis RB, Mansvelder HD. Cholinergic modulation of the medial prefrontal cortex: the role of nicotinic receptors in attention and regulation of neuronal activity. Front Neural Circuits. 2014;8:17.
Pastor V, Medina JH. alpha7 nicotinic acetylcholine receptor in memory processing. Eur J Neurosci. 2023:1–17.
Wallace TL, Porter RH. Targeting the nicotinic alpha7 acetylcholine receptor to enhance cognition in disease. Biochem Pharm. 2011;82:891–903.
Young JW, Finlayson K, Spratt C, Marston HM, Crawford N, Kelly JS, et al. Nicotine improves sustained attention in mice: evidence for involvement of the α7 Nicotinic Acetylcholine Receptor. Neuropsychopharmacology. 2004;29:891–900.
King JR, Kabbani N. Alpha 7 nicotinic receptor coupling to heterotrimeric G proteins modulates RhoA activation, cytoskeletal motility, and structural growth. J Neurochem. 2016;138:532–45.
Wang HY, Lee DH, D’Andrea MR, Peterson PA, Shank RP, Reitz AB. beta-Amyloid(1-42) binds to alpha7 nicotinic acetylcholine receptor with high affinity. Implications for Alzheimer’s disease pathology. J Biol Chem. 2000;275:5626–32.
Wang XL, Deng YX, Gao YM, Dong YT, Wang F, Guan ZZ, et al. Activation of α7 nAChR by PNU-282987 improves synaptic and cognitive functions through restoring the expression of synaptic-associated proteins and the CaM-CaMKII-CREB signaling pathway. Aging. 2020;12:543–70.
Egea J, Buendia I, Parada E, Navarro E, Leon R, Lopez MG. Anti-inflammatory role of microglial alpha7 nAChRs and its role in neuroprotection. Biochem Pharm. 2015;97:463–72.
De Simone R, Ajmone-Cat MA, Carnevale D, Minghetti L. Activation of α7 nicotinic acetylcholine receptor by nicotine selectively up-regulates cyclooxygenase-2 and prostaglandin E2 in rat microglial cultures. J Neuroinflamm. 2005;2:4.
Parada E, Egea J, Buendia I, Negredo P, Cunha AC, Cardoso S, et al. The microglial α7-acetylcholine nicotinic receptor is a key element in promoting neuroprotection by inducing heme oxygenase-1 via nuclear factor erythroid-2-related factor 2. Antioxid Redox Signal. 2013;19:1135–48.
Thomsen MS, Mikkelsen JD. The α7 nicotinic acetylcholine receptor ligands methyllycaconitine, NS6740 and GTS-21 reduce lipopolysaccharide-induced TNF-α release from microglia. J Neuroimmunol. 2012;251:65–72.
Han Z, Li L, Wang L, Degos V, Maze M, Su H. Alpha-7 nicotinic acetylcholine receptor agonist treatment reduces neuroinflammation, oxidative stress, and brain injury in mice with ischemic stroke and bone fracture. J Neurochem. 2014;131:498–508.
Hoogland ICM, Yik J, Westhoff D, Engelen-Lee JY, Valls Seron M, Man WK, et al. Microglial cell response in α7 nicotinic acetylcholine receptor-deficient mice after systemic infection with Escherichia coli. J Neuroinflamm. 2022;19:94.
Piovesana R, Salazar Intriago MS, Dini L, Tata AM. Cholinergic Modulation of Neuroinflammation: Focus on α7 Nicotinic Receptor. Int J Mol Sci. 2021;22:4912.
Ihnatovych I, Birkaya B, Notari E, Szigeti K. iPSC-Derived Microglia for modeling human-specific DAMP and PAMP responses in the context of Alzheimer’s disease. Int J Mol Sci. 2020;21:9668.
Cao X, Wang Y, Gao L. CHRFAM7A overexpression attenuates cerebral ischemia-reperfusion injury via inhibiting microglia pyroptosis mediated by the NLRP3/Caspase-1 pathway. Inflammation. 2021;44:1023–34.
Ochocka N, Kaminska B Microglia Diversity in Healthy and Diseased Brain: Insights from Single-Cell Omics. Int J Mol Sci. 2021;22:3027.
Speicher AM, Wiendl H, Meuth SG, Pawlowski M. Generating microglia from human pluripotent stem cells: novel in vitro models for the study of neurodegeneration. Mol Neurodegener. 2019;14:46.
Warden AS, Han C, Hansen E, Trescott S, Nguyen C, Kim R, et al. Tools for studying human microglia: In vitro and in vivo strategies. Brain, Behav, Immun. 2023;107:369–82.
Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–62.
Martelli D, McKinley MJ, McAllen RM. The cholinergic anti-inflammatory pathway: a critical review. Auton Neurosci. 2014;182:65–9.
Kelly MJ, Breathnach C, Tracey KJ, Donnelly SC. Manipulation of the inflammatory reflex as a therapeutic strategy. Cell Rep. Med. 2022;3:100696.
Mizrachi T, Vaknin-Dembinsky A, Brenner T, Treinin M. Neuroinflammation modulation via alpha7 Nicotinic Acetylcholine receptor and its Chaperone, RIC-3. Molecules. 2021;26:6139.
Kageyama-Yahara N, Suehiro Y, Yamamoto T, Kadowaki M. IgE-induced degranulation of mucosal mast cells is negatively regulated via nicotinic acetylcholine receptors. Biochem Biophys Res Commun. 2008;377:321–5.
Kawashima K, Yoshikawa K, Fujii YX, Moriwaki Y, Misawa H. Expression and function of genes encoding cholinergic components in murine immune cells. Life Sci. 2007;80:2314–9.
Fujii T, Mashimo M, Moriwaki Y, Misawa H, Ono S, Horiguchi K, et al. Expression and function of the Cholinergic system in immune cells. Front Immunol. 2017;8:1085.
Marrero MB, Bencherif M. Convergence of alpha 7 nicotinic acetylcholine receptor-activated pathways for anti-apoptosis and anti-inflammation: central role for JAK2 activation of STAT3 and NF-kappaB. Brain Res. 2009;1256:1–7.
Yang T, Xiao T, Sun Q, Wang K. The current agonists and positive allosteric modulators of alpha7 nAChR for CNS indications in clinical trials. Acta Pharm Sin B. 2017;7:611–22.
Costantini TW, Dang X, Yurchyshyna MV, Coimbra R, Eliceiri BP, Baird A. A human-specific α7-Nicotinic Acetylcholine receptor gene in human leukocytes: identification, regulation and the consequences of CHRFAM7A Expression. Mol Med. 2015;21:323–36.
Chan TW, Langness S, Cohen O, Eliceiri BP, Baird A, Costantini TW. CHRFAM7A reduces monocyte/macrophage migration and colony formation in vitro. Inflamm Res. 2020;69:631–3.
Li T, Chen W, Zhang Q, Deng C. Human-specific gene CHRFAM7A mediates M2 macrophage polarization via the Notch pathway to ameliorate hypertrophic scar formation. Biomed Pharmacother. 2020;131:110611.
Zhou B, Zhang Y, Dang X, Li B, Wang H, Gong S, et al. Up-regulation of the human-specific CHRFAM7A gene protects against renal fibrosis in mice with obstructive nephropathy. J Cell Mol Med. 2023;27:52–65.
Pattanaik B, Hammarlund M, Mjörnstedt F, Ulleryd MA, Zhong W, Uhlén M, et al. Polymorphisms in alpha 7 nicotinic acetylcholine receptor gene, CHRNA7, and its partially duplicated gene, CHRFAM7A, associate with increased inflammatory response in human peripheral mononuclear cells. FASEB j. 2022;36:e22271.
Courties A, Boussier J, Hadjadj J, Yatim N, Barnabei L, Péré H, et al. Regulation of the acetylcholine/α7nAChR anti-inflammatory pathway in COVID-19 patients. Sci Rep. 2021;11:11886.
Hoover DB. Cholinergic modulation of the immune system presents new approaches for treating inflammation. Pharmacol Ther. 2017;179:1–16.
Jacob L, Freyn M, Kalder M, Dinas K, Kostev K. Impact of tobacco smoking on the risk of developing 25 different cancers in the UK: a retrospective study of 422,010 patients followed for up to 30 years. Oncotarget. 2018;9:17420–9.
Malhotra J, Borron C, Freedman ND, Abnet CC, van den Brandt PA, White E, et al. Association between cigar or pipe smoking and cancer risk in men: a pooled analysis of five cohort studies. Cancer Prev Res. 2017;10:704–9.
Grando SA. Connections of nicotine to cancer. Nat Rev Cancer. 2014;14:419–29.
Wang S, Hu Y. α7 nicotinic acetylcholine receptors in lung cancer. Oncol Lett. 2018;16:1375–82.
Zhao Y. The oncogenic functions of Nicotinic Acetylcholine Receptors. J Oncol. 2016;2016:9650481.
Arredondo J, Chernyavsky AI, Grando SA. Nicotinic receptors mediate tumorigenic action of tobacco-derived nitrosamines on immortalized oral epithelial cells. Cancer Biol Ther. 2006;5:511–7.
Chen S, Kang X, Liu G, Zhang B, Hu X, Feng Y. α7-Nicotinic Acetylcholine receptor promotes Cholangiocarcinoma progression and Epithelial-Mesenchymal transition process. Dig Dis Sci. 2019;64:2843–53.
Bordas A, Cedillo JL, Arnalich F, Esteban-Rodriguez I, Guerra-Pastrián L, de Castro J, et al. Expression patterns for nicotinic acetylcholine receptor subunit genes in smoking-related lung cancers. Oncotarget. 2017;8:67878–90.
Cedillo JL, Bordas A, Arnalich F, Esteban-Rodríguez I, Martín-Sánchez C, Extremera M, et al. Anti-tumoral activity of the human-specific duplicated form of α7-nicotinic receptor subunit in tobacco-induced lung cancer progression. Lung Cancer. 2019;128:134–44.
Clark AG, Vignjevic DM. Modes of cancer cell invasion and the role of the microenvironment. Curr Opin Cell Biol. 2015;36:13–22.
Puisieux A, Brabletz T, Caramel J. Oncogenic roles of EMT-inducing transcription factors. Nat Cell Biol. 2014;16:488–94.
Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–96.
Yamao M, Naoki H, Kunida K, Aoki K, Matsuda M, Ishii S. Distinct predictive performance of Rac1 and Cdc42 in cell migration. Sci Rep. 2015;5:17527.
Ridley AJ. Rho GTPase signalling in cell migration. Curr Opin Cell Biol. 2015;36:103–12.
Ma N, Xu E, Luo Q, Song G. Rac1: A regulator of cell migration and a potential target for cancer therapy. Molecules. 2023;28:2976.
Fletcher DA, Mullins RD. Cell mechanics and the cytoskeleton. Nature. 2010;463:485–92.
Luo T, Robinson D. The role of the Actin Cytoskeleton in Mechanosensation. Mechanosensitivity and Mechanotransduction. 2011;4:25–65.
Tang K, Xin Y, Li K, Chen X, Tan Y. Cell cytoskeleton and stiffness are mechanical indicators of organotropism in breast cancer. Biology. 2021;10:259.
Bid HK, Roberts RD, Manchanda PK, Houghton PJ. RAC1: an emerging therapeutic option for targeting cancer angiogenesis and metastasis. Mol Cancer Ther. 2013;12:1925–34.
Tawara K, Scott H, Emathinger J, Wolf C, LaJoie D, Hedeen D, et al. HIGH expression of OSM and IL-6 are associated with decreased breast cancer survival: synergistic induction of IL-6 secretion by OSM and IL-1β. Oncotarget. 2019;10:2068–85.
Sanguinete MMM, Oliveira PH, Martins-Filho A, Micheli DC, Tavares-Murta BM, Murta EFC, et al. Serum IL-6 and IL-8 correlate with prognostic factors in ovarian cancer. Immunol Invest. 2017;46:677–88.
Yeh KY, Li YY, Hsieh LL, Lu CH, Chou WC, Liaw CC, et al. Analysis of the effect of serum interleukin-6 (IL-6) and soluble IL-6 receptor levels on survival of patients with colorectal cancer. Jpn J Clin Oncol. 2010;40:580–7.
An J, Gu Q, Cao L, Yang H, Deng P, Hu C, et al. Serum IL-6 as a vital predictor of severe lung cancer. Ann Palliat Med. 2021;10:202–9.
Riedel F, Zaiss I, Herzog D, Götte K, Naim R, Hörmann K. Serum levels of interleukin-6 in patients with primary head and neck squamous cell carcinoma. Anticancer Res. 2005;25:2761–5.
Courties A, Olmer M, Myers K, Berenbaum F, Sellam J, Lotz MK. Human specific duplicate Chrfam7a gene is associated with more severe Osteoarthritis And modulates general baseline for pain behaviors. Osteoarthr Cartil. 2022;30:S77.
Gault LM, Ritchie CW, Robieson WZ, Pritchett Y, Othman AA, Lenz RA. A phase 2 randomized, controlled trial of the α7 agonist ABT-126 in mild-to-moderate Alzheimer’s dementia. Alzheimer’s Dement: Transl Res Clin Interventions. 2015;1:81–90.
Terry AV Jr., Callahan PM. α7 nicotinic acetylcholine receptors as therapeutic targets in schizophrenia: Update on animal and clinical studies and strategies for the future. Neuropharmacology. 2020;170:108053.
Mazloom R, Eftekhari G, Rahimi-Balaei M, Khori V, Hajizadeh S, Dehpour AR, et al. The role of α7 nicotinic acetylcholine receptor in modulation of heart rate dynamics in endotoxemic rats. PLoS One. 2013;8:e82251.
Vang A, da Silva Gonçalves Bos D, Fernandez-Nicolas A, Zhang P, Morrison AR, Mancini TJ, et al. α7 Nicotinic acetylcholine receptor mediates right ventricular fibrosis and diastolic dysfunction in pulmonary hypertension. JCI Insight. 2021;6:e142945.
Vieira-Alves I, Coimbra-Campos LMC, Sancho M, da Silva RF, Cortes SF, Lemos VS. Role of the α7 Nicotinic Acetylcholine receptor in the pathophysiology of Atherosclerosis. Front Physiol. 2020;11:621769.
Johansson ME, Ulleryd MA, Bernardi A, Lundberg AM, Andersson A, Folkersen L, et al. α7 Nicotinic Acetylcholine receptor is expressed in human Atherosclerosis and inhibits disease in mice—brief report. Arterioscler Thromb Vasc Biol. 2014;34:2632–6.
Li DJ, Zhao T, Xin RJ, Wang YY, Fei YB, Shen FM. Activation of α7 Nicotinic Acetylcholine receptor protects against oxidant stress damage through reducing vascular Peroxidase-1 in a JNK signaling-dependent manner in endothelial cells. Cell Physiol Biochem. 2014;33:468–78.
Li DJ, Liu J, Hua X, Fu H, Huang F, Fei YB, et al. Nicotinic acetylcholine receptor α7 subunit improves energy homeostasis and inhibits inflammation in nonalcoholic fatty liver disease. Metabolism. 2018;79:52–63.
Rasmussen L, Tang L, Patel K, Mazur M, Fortinberry H, Kem W, et al. Pharmacologic Targeting of Alpha7 Nicotinic Receptors to Treat COPD-Related Chronic Bronchitis. American Journal of Respiratory and Critical Care Medicine 2017;195:A2426.
Sun P, Li L, Zhao C, Pan M, Qian Z, Su X. Deficiency of α7 nicotinic acetylcholine receptor attenuates bleomycin-induced lung fibrosis in mice. Mol Med. 2017;23:34–9.
Stegemann A, Flis D, Ziolkowski W, Distler JHW, Steinbrink K, Böhm M. The α7 Nicotinic Acetylcholine Receptor: A promising target for the treatment of fibrotic skin disorders. J Invest Dermatol. 2020;140:2371–9.
Truong LD, Trostel J, Garcia GE. Absence of nicotinic acetylcholine receptor α7 subunit amplifies inflammation and accelerates onset of fibrosis: an inflammatory kidney model. FASEB J. 2015;29:3558–70.
Fehér A, Juhász A, Rimanóczy A, Csibri E, Kálmán J, Janka Z. Association between a Genetic Variant of the Alpha-7 Nicotinic Acetylcholine Receptor Subunit and Four Types of Dementia Dementia and Geriatric Cognitive Disorders 2009;28:56–62. https://doi.org/10.1159/000230036.
Lin M, Huang W, Kabbani N, Theiss MM, Hamilton JF, Ecklund JM, et al. Effect of CHRFAM7A Δ2bp gene variant on secondary inflammation after spinal cord injury. PLoS One. 2021;16:e0251110.
Huang W, Kabbani N, Brannan TK, Lin MK, Theiss MM, Hamilton JF, et al. Association of a Functional Polymorphism in the CHRFAM7A Gene with Inflammatory Response Mediators and Neuropathic Pain after Spinal Cord Injury. J Neurotrauma. 2019;36:3026–33.
Gillentine MA, Lozoya R, Yin J, Grochowski CM, White JJ, Schaaf CP, et al. CHRNA7 copy number gains are enriched in adolescents with major depressive and anxiety disorders. J Affect Disord. 2018;239:247–52.
Soler-Alfonso C, Carvalho CM, Ge J, Roney EK, Bader PI, Kolodziejska KE, et al. CHRNA7 triplication associated with cognitive impairment and neuropsychiatric phenotypes in a three-generation pedigree. Eur J Hum Genet. 2014;22:1071–6.
Miller DT, Shen Y, Weiss LA, Korn J, Anselm I, Bridgemohan C, et al. Microdeletion/duplication at 15q13.2q13.3 among individuals with features of autism and other neuropsychiatric disorders. J Med Genet. 2009;46:242–8.
Acknowledgements
This work is supported in part by the Community Foundation for Greater Buffalo.
We would like to thank Ryu P. Dorn for his help with figures preparation.
Author information
Authors and Affiliations
Contributions
II worked on and wrote the first draft of sections 5–6, contributed to the design and preparation of the figures, and worked on the final draft of the manuscript. R-AS performed the literature search, contributed to section 3 of the manuscript, and preparation of figures. NS worked on and wrote the first draft of section 7, contributed to Fig. 4 design, and worked on the final draft of the manuscript. KS conceptualized the review paper, contributed to the figure design, worked on and wrote the first draft of sections 1–4 and 8, and wrote the final draft of the whole manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ihnatovych, I., Saddler, RA., Sule, N. et al. Translational implications of CHRFAM7A, an elusive human-restricted fusion gene. Mol Psychiatry (2024). https://doi.org/10.1038/s41380-023-02389-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41380-023-02389-1