Abstract
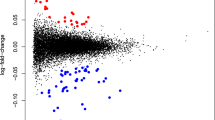
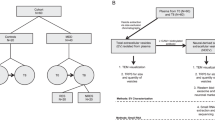
The absence of non-invasive tests that can monitor the status of the brain is a major obstacle for psychiatric care. In order to address this need, we assessed the feasibility of using tissue-specific gene expression to determine the origin of extracellular vesicle (EV) mRNAs in peripheral blood. Using the placenta as a model, we discovered that 26 messenger RNAs that are specifically expressed in the placenta are present in EVs circulating in maternal blood. Twenty-three of these transcripts were either exclusively or highly expressed in maternal blood during pregnancy only and not in the postpartum period, verifying the feasibility of using tissue-specific gene expression to infer the tissue of origin for EV mRNAs. Using the same bioinformatic approach, which provides better specificity than isolating L1 cell-adhesion molecule containing EVs, we discovered that 181 mRNAs that are specifically expressed in the female brain are also present in EVs circulating in maternal blood. Gene set enrichment analysis revealed that these transcripts, which are involved in synaptic functions and myelination, are enriched for genes implicated in mood disorders, schizophrenia, and substance use disorders. The EV mRNA levels of 13 of these female brain-specific transcripts are associated with postpartum depression (adjusted p-vals = 3 × 10−5 to 0.08), raising the possibility that they can be used to infer the state of the brain. In order to determine the extent to which EV mRNAs reflect transcription in the brain, we compared mRNAs isolated from cells and EVs in an iPSC-derived brain microphysiological system differentiated for 3 and 9 weeks. We discovered that, although cellular and extracellular mRNA levels are not identical, they do correlate, and it is possible to extrapolate cellular RNA expression changes in the brain via EV mRNA levels. Our findings bring EV mRNAs to the forefront of peripheral biomarker development efforts in psychiatric diseases by demonstrating the feasibility of inferring transcriptional changes in the brain via blood EV mRNA levels.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Code availability
The R code used for the analysis in this manuscript is available upon request.
Data availability
The sequencing data is available from the NCBI Sequence Read Archive under Bioproject PRJNA1006349.
References
Fiandaca MS, Kapogiannis D, Mapstone M, Boxer A, Eitan E, Schwartz JB, et al. Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: a case-control study. Alzheimers Dement. 2015;11:600.e601.
Jia L, Qiu Q, Zhang H, Chu L, Du Y, Zhang J, et al. Concordance between the assessment of Aβ42, T-tau, and P-T181-tau in peripheral blood neuronal-derived exosomes and cerebrospinal fluid. Alzheimers Dement. 2019;15:1071–80.
Norman M, Ter-Ovanesyan D, Trieu W, Lazarovits R, Kowal EJK, Lee JH, et al. L1CAM is not associated with extracellular vesicles in human cerebrospinal fluid or plasma. Nat Methods. 2021;18:631–4.
Osborne LM, Payne JL, Sherer ML, Sabunciyan S. Altered extracellular mRNA communication in postpartum depression is associated with decreased autophagy. Mol Psychiatry. 2022;27:4526–35.
Vorperian SK, Moufarrej MN, Tabula Sapiens C, Quake SR. Cell types of origin of the cell-free transcriptome. Nat Biotechnol. 2022;40:855–61.
Osborne LM, Voegtline K, Standeven LR, Sundel B, Pangtey M, Hantsoo L, et al. High worry in pregnancy predicts postpartum depression. J Affect Disord. 2021;294:701–6.
Pontén F, Schwenk JM, Asplund A, Edqvist PH. The Human Protein Atlas as a proteomic resource for biomarker discovery. J Intern Med. 2011;270:428–46.
GTEx Consortium. The Genotype-Tissue Expression (GTEx) project. Nat Genet. 2013;45:580–5.
Tran MN, Maynard KR, Spangler A, Huuki LA, Montgomery KD, Sadashivaiah V, et al. Single-nucleus transcriptome analysis reveals cell-type-specific molecular signatures across reward circuitry in the human brain. Neuron. 2021;109:3088–3103.e5.
Subhash S, Kanduri C. GeneSCF: a real-time based functional enrichment tool with support for multiple organisms. BMC Bioinforma. 2016;17:365.
Bullen CK, Hogberg HT, Bahadirli-Talbott A, Bishai WR, Hartung T, Keuthan C, et al. Infectability of human BrainSphere neurons suggests neurotropism of SARS-CoV-2. Altex. 2020;37:665–71.
Smirnova L, Harris G, Delp J, Valadares M, Pamies D, Hogberg HT, et al. A LUHMES 3D dopaminergic neuronal model for neurotoxicity testing allowing long-term exposure and cellular resilience analysis. Arch Toxicol. 2016;90:2725–43.
Harris G, Hogberg H, Hartung T, Smirnova L. 3D differentiation of LUHMES cell line to study recovery and delayed neurotoxic effects. Curr Protoc Toxicol. 2017;73:11.23.11–11.23.28.
Roehr JT, Dieterich C, Reinert K. Flexbar 3.0 - SIMD and multicore parallelization. Bioinformatics. 2017;33:2941–2.
Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015;12:357–60.
Darby MM, Leek JT, Langmead B, Yolken RH, Sabunciyan S. Widespread splicing of repetitive element loci into coding regions of gene transcripts. Hum Mol Genet. 2016;25:4962–82.
Hicks SC, Okrah K, Paulson JN, Quackenbush J, Irizarry RA, Bravo HC. Smooth quantile normalization. Biostatistics. 2018;19:185–98.
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47.
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550.
Pamies D, Barreras P, Block K, Makri G, Kumar A, Wiersma D, et al. A human brain microphysiological system derived from induced pluripotent stem cells to study neurological diseases and toxicity. Altex. 2017;34:362–76.
Huang Q, Tang B, Romero JC, Yang Y, Elsayed SK, Pahapale G, et al. Shell microelectrode arrays (MEAs) for brain organoids. Sci Adv. 2022;8:eabq5031.
Romero JC, Berlinicke C, Chow S, Duan Y, Wang Y, Chamling X, et al. Oligodendrogenesis and myelination tracing in a CRISPR/Cas9-engineered brain microphysiological system. Front Cell Neurosci. 2022;16:1094291.
Leite PEC, Pereira MR, Harris G, Pamies D, Dos Santos LMG, Granjeiro JM, et al. Suitability of 3D human brain spheroid models to distinguish toxic effects of gold and poly-lactic acid nanoparticles to assess biocompatibility for brain drug delivery. Part Fibre Toxicol. 2019;16:22.
Zhong X, Harris G, Smirnova L, Zufferey V, Sá R, Baldino Russo F, et al. Antidepressant paroxetine exerts developmental neurotoxicity in an iPSC-derived 3D human brain model. Front Cell Neurosci. 2020;14:25.
Modafferi S, Zhong X, Kleensang A, Murata Y, Fagiani F, Pamies D, et al. Gene environment interactions in developmental neurotoxicity - a case study of synergy between chlorpyrifos and CHD8 knockout in human BrainSpheres. EHP. 2021;129:77001.
Pulliam L, Sun B, Mustapic M, Chawla S, Kapogiannis D. Plasma neuronal exosomes serve as biomarkers of cognitive impairment in HIV infection and Alzheimer’s disease. J Neurovirol. 2019;25:702–9.
Shi M, Kovac A, Korff A, Cook TJ, Ginghina C, Bullock KM, et al. CNS tau efflux via exosomes is likely increased in Parkinson’s disease but not in Alzheimer’s disease. Alzheimers Dement. 2016;12:1125–31.
Jones RC, Karkanias J, Krasnow MA, Pisco AO, Quake SR, Salzman J, et al. The Tabula Sapiens: a multiple-organ, single-cell transcriptomic atlas of humans. Science. 2022;376:eabl4896.
Hasselmann DO, Rappl G, Tilgen W, Reinhold U. Extracellular tyrosinase mRNA within apoptotic bodies is protected from degradation in human serum. Clin Chem. 2001;47:1488–9.
Gupta AK, Holzgreve W, Huppertz B, Malek A, Schneider H, Hahn S. Detection of fetal DNA and RNA in placenta-derived syncytiotrophoblast microparticles generated in vitro. Clin Chem. 2004;50:2187–90.
Drag MH, Kilpeläinen TO. Cell-free DNA and RNA-measurement and applications in clinical diagnostics with focus on metabolic disorders. Physiol Genomics. 2021;53:33–46.
Gilazieva Z, Ponomarev A, Rutland C, Rizvanov A, Solovyeva V. Promising applications of tumor spheroids and organoids for personalized medicine. Cancers. 2020;12:2727.
Modabbernia A, Velthorst E, Reichenberg A. Environmental risk factors for autism: an evidence-based review of systematic reviews and meta-analyses. Mol Autism. 2017;8:13.
Maleki M, Noorimotlagh Z, Mirzaee SA, Jaafarzadeh N, Martinez SS, Rahim F, et al. An updated systematic review on the maternal exposure to environmental pesticides and involved mechanisms of autism spectrum disorder (ASD) progression risk in children. Rev Environ Health. 2022;38:727–740.
Lin C-K, Chang Y-T, Lee F-S, Chen S-T, Christiani D. Association between exposure to ambient particulate matters and risks of autism spectrum disorder in children: a systematic review and exposure-response meta-analysis. Environ Res Lett. 2021;16:063003.
Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993;29:85–96.
Wapeesittipan P, Joshi A. Integrated analysis of robust sex-biased gene signatures in human brain. Biol Sex Differ. 2023;14:36.
Rollins B, Martin MV, Morgan L, Vawter MP. Analysis of whole genome biomarker expression in blood and brain. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:919–36.
Tylee DS, Kawaguchi DM, Glatt SJ. On the outside, looking in: a review and evaluation of the comparability of blood and brain “-omes”. Am J Med Genet B Neuropsychiatr Genet. 2013;162B:595–603.
Hess JL, Quinn TP, Zhang C, Hearn GC, Chen S, Neuropsychiatric Consortium for A. et al. BrainGENIE: the brain gene expression and network imputation engine. Transl Psychiatry. 2023;13:98.
Nishitani S, Isozaki M, Yao A, Higashino Y, Yamauchi T, Kidoguchi M, et al. Cross-tissue correlations of genome-wide DNA methylation in Japanese live human brain and blood, saliva, and buccal epithelial tissues. Transl Psychiatry. 2023;13:72.
Braun PR, Han S, Hing B, Nagahama Y, Gaul LN, Heinzman JT, et al. Genome-wide DNA methylation comparison between live human brain and peripheral tissues within individuals. Transl Psychiatry. 2019;9:47.
Hannon E, Lunnon K, Schalkwyk L, Mill J. Interindividual methylomic variation across blood, cortex, and cerebellum: implications for epigenetic studies of neurological and neuropsychiatric phenotypes. Epigenetics. 2015;10:1024–32.
Edgar RD, Jones MJ, Meaney MJ, Turecki G, Kobor MS. BECon: a tool for interpreting DNA methylation findings from blood in the context of brain. Transl Psychiatry. 2017;7:e1187.
Sommerer Y, Ohlei O, Dobricic V, Oakley DH, Wesse T, Sedghpour Sabet S, et al. A correlation map of genome-wide DNA methylation patterns between paired human brain and buccal samples. Clin Epigenetics. 2022;14:139.
Acknowledgements
The authors would like to thank Ms. Ou Chen for her technical help. This study was funded by the Stanley Medical Research Institute and the following NIH grants NIH-NIMH R01 MH112704, NIH-NIMH 1K23 MH110607 R01ES034554. The authors acknowledge the Integrated Imaging Center and the Advanced Research Computing at Hopkins (ARCH) core facility at Johns Hopkins University. This publication was partially developed under Assistance Agreement No RD83950501 awarded by the U.S. EPA to LS. It had not been formally reviewed by EPA. The views expressed in the publication are solely those of LS and co-authors and do not necessarily reflect those of the Agency. EPA does not endorse any products or commercial services mentioned in this publication.
Author information
Authors and Affiliations
Contributions
LS, LMO, JLP and SS conceived the idea and designed the initial experiments. LS, SM, CS performed the bMPS experiments. LMO and JLP collected the postpartum depression samples, characterized the patients and selected the cohorts used in the study. SS performed the EV assays, constructed NGS libraries and performed bioinformatic analysis. Everyone was involved in data interpretation and writing of the paper.
Corresponding author
Ethics declarations
Competing interests
JLP has served as a consultant for SAGE Therapeutics, Brii Biosciences, and Pure Tech Health. JLP has received an honorarium from Karuna Therapeutics for speaking to the company. JLP owns a patent entitled “Epigenetic Biomarkers of Postpartum Depression.” The other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Smirnova, L., Modafferi, S., Schlett, C. et al. Blood extracellular vesicles carrying brain-specific mRNAs are potential biomarkers for detecting gene expression changes in the female brain. Mol Psychiatry (2024). https://doi.org/10.1038/s41380-023-02384-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41380-023-02384-6