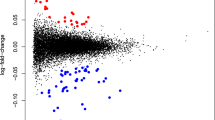
Abstract
We investigated whether extracellular RNA communication, which is a recently discovered mode of intercellular communication that is involved in a variety of important biological processes including pregnancy, is associated with postpartum depression (PPD). Extracellular RNA communication is increased during pregnancy and is involved in embryo implantation, uterine spiral artery remodeling, parturition, preterm birth, immunity, and the inflammatory response. Since immune anomalies are associated with PPD, we characterized the mRNA content of extracellular vesicles (EV) in a cohort of prospectively collected blood plasma samples at six time-points throughout pregnancy and the postpartum (2nd trimester, 3rd trimester, 2 weeks postpartum, 6 weeks postpartum, 3 months postpartum, and 6 months postpartum) in an academic medical setting from women who went on to develop PPD (N = 7, defined as euthymic in pregnancy with postpartum-onset depressive symptoms assessed by Edinburgh Postnatal Depression Scale ≥13 at any postpartum time point) and matched unaffected controls (N = 7, defined as euthymic throughout pregnancy and postpartum). Blood samples were available for all participants at the T2 and W6 timepoints, with fewer samples available at other time points. This analysis revealed that EV mRNA levels during pregnancy and the postpartum period were extensively altered in women who went on to develop PPD. Gene set enrichment analysis revealed that mRNAs associated with autophagy were decreased in PPD cases. In contrast, EV mRNAs from ribosomes and mitochondria, two organelles that are selectively targeted by autophagy, were elevated in PPD cases. Cellular deconvolution analysis discovered that EV mRNAs associated with PPD originated from monocytes and macrophages. Quantitative PCR analysis for four relevant genes in another cohort replicated these findings and confirmed that extracellular RNA levels are altered in PPD. We demonstrate that EV mRNA communication is robustly altered during pregnancy and the postpartum period in women who go on to develop PPD. Our work also establishes a direct link between reduced autophagy and PPD in patient samples. These data warrant investigating the feasibility of developing EV mRNA based biomarkers and therapeutic agents for PPD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The raw data will be made available via the NIMH Data Archive. In the meantime, the raw data is available upon request.
Code availability
R code for the analysis is available upon request.
References
Lindahl V, Pearson JL, Colpe L. Prevalence of suicidality during pregnancy and the postpartum. Arch Women’s Ment Health. 2005;8:77–87.
Righetti-Veltema M, Conne-Perréard E, Bousquet A, Manzano J. Postpartum depression and mother–infant relationship at 3 months old. J Affect Disord. 2002;70:291–306.
Righetti-Veltema M, Bousquet A, Manzano J. Impact of postpartum depressive symptoms on mother and her 18-month-old infant. Eur Child Adolesc Psychiatry. 2003;12:75–83.
Bernard-Bonnin AC. Maternal depression and child development. Paediatrics Child Health. 2004;9:575–83.
Murray L. The impact of postnatal depression on infant development. J Child Psychol Psychiatry. 1992;33:543–61.
Lyons-Ruth K, Zoll D, Connell D, Grunebaum HU. The depressed mother and her one-year-old infant: Environment, interaction, attachment, and infant development. New Directions for Child and Adolescent. Development. 1986;1986:61–82.
Halligan SL, Murray L, Martins C, Cooper PJ. Maternal depression and psychiatric outcomes in adolescent offspring: A 13-year longitudinal study. J Affect Disord. 2007;97:145–54.
Ruth Feldman PD, Adi Granat PD, Clara Pariente MA, Hannah Kanety PD, Jacob Kuint MD, Eva, et al. Maternal depression and anxiety across the postpartum year and infant social engagement, fear regulation, and stress reactivity. J Am Acad Child Adolesc Psychiatry. 2009;48:919–27.
McEvoy K, Osborne LM, Nanavati J, Payne JL. Reproductive affective disorders: a review of the genetic evidence for premenstrual dysphoric disorder and postpartum depression. Curr Psychiatry Rep. 2017;19:94.
Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR. Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry. 2000;157:924–30.
Guintivano J, Brown T, Newcomer A, Jones M, Cox O, Maher BS, et al. Identification and replication of a combined epigenetic and genetic biomarker predicting suicide and suicidal behaviors. Am J Psychiatry. 2014;171:1287–96.
Kimmel M, Clive M, Gispen F, Guintivano J, Brown T, Cox O, et al. Oxytocin receptor DNA methylation in postpartum depression. Psychoneuroendocrinology. 2016;69:150–60.
McEvoy K, Payne JL, Osborne LM. Neuroactive steroids and perinatal depression: a review of recent literature. Curr Psychiatry Rep. 2018;20:78.
Jairaj C, O’Leary N, Doolin K, Farrell C, McCarthy A, McAuliffe FM, et al. The hypothalamic-pituitary-adrenal axis in the perinatal period: Its relationship with major depressive disorder and early life adversity. World J Biol Psychiatry: Off J World Federation Societies Biol Psychiatry. 2020;21:552–63.
Minaldi E, D’Andrea S, Castellini C, Martorella A, Francavilla F, Francavilla S, et al. Thyroid autoimmunity and risk of post-partum depression: a systematic review and meta-analysis of longitudinal studies. J Endocrinol Invest. 2020;43:271–7.
Osborne LM, Monk C. Perinatal depression-the fourth inflammatory morbidity of pregnancy?: Theory and literature review. Psychoneuroendocrinology. 2013;38:1929–52.
Osborne LM, Gilden J, Kamperman AM, Hoogendijk WJG, Spicer J, Drexhage HA, et al. T-cell defects and postpartum depression. Brain Behav Immun. 2020;87:397–403.
Sherer ML, Posillico CK, Schwarz JM. The psychoneuroimmunology of pregnancy. Front Neuroendocrinol. 2018;51:25–35.
Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14:195–208.
Andaloussi SEL, Mäger I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Disco. 2013;12:347–57.
Salomon C, Torres MJ, Kobayashi M, Scholz-Romero K, Sobrevia L, Dobierzewska A, et al. A gestational profile of placental exosomes in maternal plasma and their effects on endothelial cell migration. PloS One. 2014;9:e98667.
Radu CM, Campello E, Spiezia L, Dhima S, Visentin S, Gavasso S, et al. Origin and levels of circulating microparticles in normal pregnancy: A longitudinal observation in healthy women. Scand J Clin Lab Invest. 2015;75:487–95.
Ng YH, Rome S, Jalabert A, Forterre A, Singh H, Hincks CL, et al. Endometrial exosomes/microvesicles in the uterine microenvironment: a new paradigm for embryo-endometrial cross talk at implantation. PloS One. 2013;8:e58502.
Miura K, Higashijima A, Mishima H, Miura S, Kitajima M, Kaneuchi M, et al. Pregnancy-associated microRNAs in plasma as potential molecular markers of ectopic pregnancy. Fertil Steril. 2015;103:1202–8.e1.
Vilella F, Moreno-Moya JM, Balaguer N, Grasso A, Herrero M, Martínez S, et al. Hsa-miR-30d, secreted by the human endometrium, is taken up by the pre-implantation embryo and might modify its transcriptome. Development. 2015;142:3210–21.
Salomon C, Yee S, Scholz-Romero K, Kobayashi M, Vaswani K, Kvaskoff D, et al. Extravillous trophoblast cells-derived exosomes promote vascular smooth muscle cell migration. Front Pharmacol. 2014;5:175.
Delorme-Axford E, Donker RB, Mouillet JF, Chu T, Bayer A, Ouyang Y, et al. Human placental trophoblasts confer viral resistance to recipient cells. Proc Natl Acad Sci USA. 2013;110:12048–53. https://doi.org/10.1073/pnas.1304718110.
Fallen S, Baxter D, Wu X, Kim TK, Shynlova O, Lee MY, et al. Extracellular vesicle RNAs reflect placenta dysfunction and are a biomarker source for preterm labour. J Cell Mol Med. 2018;22:2760–73.
Richardson LS, Taylor RN, Menon R. Reversible EMT and MET mediate amnion remodeling during pregnancy and labor. Sci Signal. 2020;13:eaay1486.
Nation GK, Saffold CE, Pua HH. Secret messengers: Extracellular RNA communication in the immune system. Immunol Rev. 2021;304:62–76.
Salomon C, Scholz-Romero K, Sarker S, Sweeney E, Kobayashi M, Correa P, et al. Gestational diabetes mellitus is associated with changes in the concentration and bioactivity of placenta-derived exosomes in maternal circulation across gestation. Diabetes. 2016;65:598–609.
Jayabalan N, Nair S, Nuzhat Z, Rice GE, Zuñiga FA, Sobrevia L, et al. Cross talk between adipose tissue and placenta in obese and gestational diabetes mellitus pregnancies via exosomes. Front Endocrinol (Lausanne). 2017;8:239.
Salomon C, Guanzon D, Scholz-Romero K, Longo S, Correa P, Illanes SE, et al. Placental exosomes as early biomarker of preeclampsia: potential role of exosomal MicroRNAs across gestation. J Clin Endocrinol Metab. 2017;102:3182–94.
Knight M, Redman CW, Linton EA, Sargent IL. Shedding of syncytiotrophoblast microvilli into the maternal circulation in pre-eclamptic pregnancies. Br J Obstet Gynaecol. 1998;105:632–40.
Germain SJ, Sacks GP, Sooranna SR, Sargent IL, Redman CW. Systemic inflammatory priming in normal pregnancy and preeclampsia: the role of circulating syncytiotrophoblast microparticles. J Immunol. 2007;178:5949–56.
Pillay P, Moodley K, Moodley J, Mackraj I. Placenta-derived exosomes: potential biomarkers of preeclampsia. Int J Nanomed. 2017;12:8009–23.
Benichou G, Wang M, Ahrens K, Madsen JC. Extracellular vesicles in allograft rejection and tolerance. Cell Immunol. 2020;349:104063.
Nasca C, Dobbin J, Bigio B, Watson K, de Angelis P, Kautz M, et al. Insulin receptor substrate in brain-enriched exosomes in subjects with major depression: on the path of creation of biosignatures of central insulin resistance. Mol Psychiatry. 2021;26:5140–9.
Saeedi S, Nagy C, Ibrahim P, Théroux JF, Wakid M, Fiori LM, et al. Neuron-derived extracellular vesicles enriched from plasma show altered size and miRNA cargo as a function of antidepressant drug response. Mol Psychiatry. 2021;26:7417–24.
Goetzl EJ, Wolkowitz OM, Srihari VH, Reus VI, Goetzl L, Kapogiannis D, et al. Abnormal levels of mitochondrial proteins in plasma neuronal extracellular vesicles in major depressive disorder. Mol Psychiatry. 2021;26:7355–62.
Osborne LM, Voegtline K, Standeven LR, Sundel B, Pangtey M, Hantsoo L, et al. High worry in pregnancy predicts postpartum depression. J Affect Disord. 2021;294:701–6.
Therneau T, Hart S, Kocher J-P. Calculating samplesSize estimates for RNA Seq studies. R package version 1320. 2021.
Roehr JT, Dieterich C, Reinert K. Flexbar 3.0 - SIMD and multicore parallelization. Bioinforma (Oxf, Engl). 2017;33:2941–2.
Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015;12:357–60. https://doi.org/10.1038/nmeth.3317.
Darby MM, Leek JT, Langmead B, Yolken RH, Sabunciyan S. Widespread splicing of repetitive element loci into coding regions of gene transcripts. Hum Mol Genet. 2016;25:4962–82.
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550.
Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinforma (Oxf, Engl). 2010;26:139–40.
Newman AM, Steen CB, Liu CL, Gentles AJ, Chaudhuri AA, Scherer F, et al. Determining cell type abundance and expression from bulk tissues with digital cytometry. Nat Biotechnol. 2019;37:773–82.
Krishnaswami SR, Grindberg RV, Novotny M, Venepally P, Lacar B, Bhutani K, et al. Using single nuclei for RNA-seq to capture the transcriptome of postmortem neurons. Nat Protoc. 2016;11:499–524.
Huang DW, Sherman BT, Tan Q, Kir J, Liu D, Bryant D, et al. DAVID Bioinformatics Resources: expanded annotation database and novel algorithms to better extract biology from large gene lists. Nucleic Acids Res. 2007;35:W169–75.
Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469:323–35.
Nakamura S, Yoshimori T. New insights into autophagosome-lysosome fusion. J Cell Sci. 2017;130:1209–16.
Yu L, Chen Y, Tooze SA. Autophagy pathway: Cellular and molecular mechanisms. Autophagy. 2018;14:207–15.
Ao X, Zou L, Wu Y. Regulation of autophagy by the Rab GTPase network. Cell Death Differ. 2014;21:348–58.
Webster CP, Smith EF, Grierson AJ, De Vos KJ. C9orf72 plays a central role in Rab GTPase-dependent regulation of autophagy. Small GTPases. 2018;9:399–408.
Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. Embo J. 2000;19:5720–8.
He M, Ding Y, Chu C, Tang J, Xiao Q, Luo ZG. Autophagy induction stabilizes microtubules and promotes axon regeneration after spinal cord injury. Proc Natl Acad Sci USA. 2016;113:11324–9.
Barve G, Sanyal P, Manjithaya R. Septin localization and function during autophagy. Curr Genet. 2018;64:1037–41.
Sotthibundhu A, McDonagh K, von Kriegsheim A, Garcia-Munoz A, Klawiter A, Thompson K, et al. Rapamycin regulates autophagy and cell adhesion in induced pluripotent stem cells. Stem Cell Res Ther. 2016;7:166.
Fu R, Jiang X, Li G, Zhu Y, Zhang H. Junctional complexes in epithelial cells: sentinels for extracellular insults and intracellular homeostasis. Febs J. 2021. Online ahead of print.
Fung C, Lock R, Gao S, Salas E, Debnath J. Induction of autophagy during extracellular matrix detachment promotes cell survival. Mol Biol Cell. 2008;19:797–806.
Zhang J, Wu K, Xiao X, Liao J, Hu Q, Chen H, et al. Autophagy as a regulatory component of erythropoiesis. Int J Mol Sci. 2015;16:4083–94.
Martinez-Martin N, Maldonado P, Gasparrini F, Frederico B, Aggarwal S, Gaya M, et al. A switch from canonical to noncanonical autophagy shapes B cell responses. Science 2017;355:641–7.
Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–64.
Sabunciyan S. Gene expression profiles associated with brain aging are altered in schizophrenia. Sci Rep. 2019;9:5896.
Darby MM, Yolken RH, Sabunciyan S. Consistently altered expression of gene sets in postmortem brains of individuals with major psychiatric disorders. Transl Psychiatry. 2016;6:e890. https://doi.org/10.1038/tp.2016.173.
Gandal MJ, Haney JR, Parikshak NN, Leppa V, Ramaswami G, Hartl C, et al. Shared molecular neuropathology across major psychiatric disorders parallels polygenic overlap. Science. 2018;359:693–7.
Fromer M, Roussos P, Sieberts SK, Johnson JS, Kavanagh DH, Perumal TM. et al. Gene expression elucidates functional impact of polygenic risk for schizophrenia. Nat Neurosci. 2016;19:1442–53.
Mizushima N, Levine B. Autophagy in mammalian development and differentiation. Nat Cell Biol. 2010;12:823–30.
Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–4.
Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–9.
Nixon RA, Wegiel J, Kumar A, Yu WH, Peterhoff C, Cataldo A, et al. Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J Neuropathol Exp Neurol. 2005;64:113–22.
Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, et al. The role of autophagy during the early neonatal starvation period. Nature. 2004;432:1032–6.
Tomoda T, Yang K, Sawa A. Neuronal autophagy in synaptic functions and psychiatric disorders. Biol Psychiatry. 2020;87:787–96.
Gassen NC, Rein T. Is there a role of autophagy in depression and antidepressant action? Front Psychiatry. 2019;10:337.
Po WW, Thein W, Khin PP, Khing TM, Han KWW, Park CH, et al. Fluoxetine simultaneously induces both apoptosis and autophagy in human gastric adenocarcinoma cells. Biomol Ther (Seoul). 2020;28:202–10.
Sun D, Zhu L, Zhao Y, Jiang Y, Chen L, Yu Y, et al. Fluoxetine induces autophagic cell death via eEF2K-AMPK-mTOR-ULK complex axis in triple negative breast cancer. Cell Prolif. 2018;51:e12402.
Bowie M, Pilie P, Wulfkuhle J, Lem S, Hoffman A, Desai S, et al. Fluoxetine induces cytotoxic endoplasmic reticulum stress and autophagy in triple negative breast cancer. World J Clin Oncol. 2015;6:299–311.
Gulbins A, Schumacher F, Becker KA, Wilker B, Soddemann M, Boldrin F, et al. Antidepressants act by inducing autophagy controlled by sphingomyelin-ceramide. Mol Psychiatry. 2018;23:2324–46.
Shu X, Sun Y, Sun X, Zhou Y, Bian Y, Shu Z, et al. The effect of fluoxetine on astrocyte autophagy flux and injured mitochondria clearance in a mouse model of depression. Cell Death Dis. 2019;10:577.
Yan J, Porch MW, Court-Vazquez B, Bennett MVL, Zukin RS. Activation of autophagy rescues synaptic and cognitive deficits in fragile X mice. Proc Natl Acad Sci USA. 2018;115:E9707–e16.
Tang G, Gudsnuk K, Kuo SH, Cotrina ML, Rosoklija G, Sosunov A, et al. Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron 2014;83:1131–43.
Gonçalves VF, Cappi C, Hagen CM, Sequeira A, Vawter MP, Derkach A, et al. A comprehensive analysis of nuclear-encoded mitochondrial genes in schizophrenia. Biol Psychiatry. 2018;83:780–9.
Kim Y, Vadodaria KC, Lenkei Z, Kato T, Gage FH, Marchetto MC, et al. Mitochondria, metabolism, and redox mechanisms in psychiatric disorders. Antioxid Redox Signal. 2019;31:275–317.
Rezin GT, Amboni G, Zugno AI, Quevedo J, Streck EL. Mitochondrial dysfunction and psychiatric disorders. Neurochem Res. 2009;34:1021–9.
Topol A, English JA, Flaherty E, Rajarajan P, Hartley BJ, Gupta S. et al. Increased abundance of translation machinery in stem cell-derived neural progenitor cells from four schizophrenia patients. Transl Psychiatry. 2015;5:e662. https://doi.org/10.1038/tp.2015.118.
Luppi P, Haluszczak C, Betters D, Richard CA, Trucco M, DeLoia JA. Monocytes are progressively activated in the circulation of pregnant women. J Leukoc Biol. 2002;72:874–84.
Sabunciyan S, Maher B, Bahn S, Dickerson F, Yolken RH. Association of DNA methylation with acute mania and inflammatory markers. PLoS One. 2015;10:e0132001. https://doi.org/10.1371/journal.pone.
Fond G, Godin O, Boyer L, Berna F, Andrianarisoa M, Coulon N, et al. Chronic low-grade peripheral inflammation is associated with ultra resistant schizophrenia. Results from the FACE-SZ cohort. Eur Arch Psychiatry Clin Neurosci. 2019;269:985–92.
Fiedorowicz JG, Prossin AR, Johnson CP, Christensen GE, Magnotta VA, Wemmie JA. Peripheral inflammation during abnormal mood states in bipolar I disorder. J Affect Disord. 2015;187:172–8.
Krishnadas R, Cavanagh J. Depression: an inflammatory illness? J Neurol Neurosurg Psychiatry. 2012;83:495–502.
Smith RS. The macrophage theory of depression. Med Hypotheses. 1991;35:298–306.
Kreisel T, Frank MG, Licht T, Reshef R, Ben-Menachem-Zidon O, Baratta MV, et al. Dynamic microglial alterations underlie stress-induced depressive-like behavior and suppressed neurogenesis. Mol Psychiatry. 2014;19:699–709.
Prinz M, Jung S, Priller J. Microglia biology: one century of evolving concepts. Cell. 2019;179:292–311.
Mondelli V, Vernon AC, Turkheimer F, Dazzan P, Pariante CM. Brain microglia in psychiatric disorders. Lancet Psychiatry. 2017;4:563–72.
Salter MW, Stevens B. Microglia emerge as central players in brain disease. Nat Med. 2017;23:1018–27.
Sherer ML, Posillico CK, Schwarz JM. An examination of changes in maternal neuroimmune function during pregnancy and the postpartum period. Brain Behav Immun. 2017;66:201–9.
Posillico CK, Schwarz JM. An investigation into the effects of antenatal stressors on the postpartum neuroimmune profile and depressive-like behaviors. Behavioural Brain Res. 2016;298(Pt B):218–28.
Tan X, Du X, Jiang Y, Botchway BOA, Hu Z, Fang M. Inhibition of autophagy in microglia alters depressive-like behavior via BDNF pathway in postpartum depression. Front Psychiatry. 2018;9:434.
Acknowledgements
The authors would like to thank Ms. Ou Chen for isolating EV mRNAs and constructing sequencing libraries and Ms. Treva Rowley for the CD72 PCR analysis. We also thank Dr. Stephanie Hicks for providing advice on cellular deconvolution analysis and Ms. Samantha Meilman for assistance with randomizing the initial cohort and matching controls to cases.
Funding
This study was funded by the following NIH grants NIH-NIMH R01 MH112704, NIH-NIMH 1K23 MH110607, and NIH-NIAID T32 AI007417.
Author information
Authors and Affiliations
Contributions
LMO, JLP, and SS conceived the idea and designed the initial experiments. LMO, JLP, and MLS collected the samples, characterized the patients and selected the cohorts used in the study. SS performed the laboratory assays and bioinformatic analysis. Everyone was involved in data interpretation and writing the paper.
Corresponding author
Ethics declarations
Competing interests
JLP has served as a consultant for SAGE Therapeutics, Brii Biosciences, and Pure Tech Health. JLP has received an honorarium from Karuna Therapeutics for speaking to the company. JLP owns a patent entitled “Epigenetic Biomarkers of Postpartum Depression.” LMO, MLS, and SS have no biomedical financial interests or potential conflicts of interest to report.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Osborne, L.M., Payne, J.L., Sherer, M.L. et al. Altered extracellular mRNA communication in postpartum depression is associated with decreased autophagy. Mol Psychiatry 27, 4526–4535 (2022). https://doi.org/10.1038/s41380-022-01794-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-022-01794-2