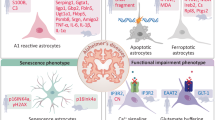
Abstract
Although Alzheimer’s disease is the most pervasive neurodegenerative disorder, the mechanism underlying its development is still not precisely understood. Available data indicate that pathophysiology of this disease may involve impaired autophagy in glial cells. The dysfunction is manifested as reduced ability of astrocytes and microglia to clear abnormal protein aggregates. Consequently, excessive accumulation of amyloid beta plaques and neurofibrillary tangles activates microglia and astrocytes leading to decreased number of mature myelinated oligodendrocytes and death of neurons. These pathologic effects of autophagy dysfunction can be rescued by pharmacological activation of autophagy. Therefore, a deeper understanding of the molecular mechanisms involved in autophagy dysfunction in glial cells in Alzheimer’s disease may lead to the development of new therapeutic strategies. However, such strategies need to take into consideration differences in regulation of autophagy in different types of neuroglia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Wong W. Economic burden of Alzheimer disease and managed care considerations. Am J Manag Care. 2020;26:S177–83.
Niikura T, Tajima H, Kita Y. Neuronal cell death in Alzheimers disease and a neuroprotective factor, humanin [Internet]. Curr Neuropharmacol. 2006;4:139–47. https://doi.org/10.2174/157015906776359577.
Canet G, Chevallier N, Zussy C, Desrumaux C, Givalois L. Central role of glucocorticoid receptors in Alzheimer’s disease and depression [Internet]. Front Neurosci. 2018;12:739. https://doi.org/10.3389/fnins.2018.00739. Available from
Gu L, Guo Z. Alzheimer’s Aβ42 and Aβ40 peptides form interlaced amyloid fibrils. J Neurochem. 2013;126:305–11.
Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221:3–12.
Fakhoury M. Microglia and astrocytes in Alzheimer’s disease: implications for therapy. Curr Neuropharmacol. 2018;16:508–18.
Lorenzini L, Fernandez M, Baldassarro VA, Bighinati A, Giuliani A, Calzà L, et al. White matter and neuroprotection in Alzheimer’s dementia [Internet]. Molecules. 2020;25:503 https://doi.org/10.3390/molecules25030503.
von Bartheld CS, Bahney J, Herculano-Houzel S. The search for true numbers of neurons and glial cells in the human brain: a review of 150 years of cell counting. J Comp Neurol. 2016;524:3865–95.
Papouin T, Dunphy J, Tolman M, Foley JC, Haydon PG. Astrocytic control of synaptic function. Philos Trans R Soc Lond B Biol Sci. 2017;372. Available from: https://doi.org/10.1098/rstb.2016.0154.
Eide PK, Hasan-Olive MM, Hansson HA, Enger R. Increased occurrence of pathological mitochondria in astrocytic perivascular endfoot processes and neurons of idiopathic intracranial hypertension. J Neurosci Res. 2021;99:467–80.
O’Leary LA, Mechawar N. Implication of cerebral astrocytes in major depression: a review of fine neuroanatomical evidence in humans. Glia 2021;69:2077–99.
Mills WA 3rd, Woo AM, Jiang S, Martin J, Surendran D, Bergstresser M, et al. Astrocyte plasticity in mice ensures continued endfoot coverage of cerebral blood vessels following injury and declines with age. Nat Commun. 2022;13:1794.
Cabezas R, Avila-Rodriguez M, Vega-Vela NE, Echeverria V, González J, Hidalgo OA, et al. Growth factors and astrocytes metabolism: possible roles for platelet derived growth factor. Med Chem. 2016;12:204–10.
Chen W, He B, Tong W, Zeng J, Zheng P. Astrocytic insulin-like growth factor-1 protects neurons against excitotoxicity. Front Cell Neurosci. 2019;13:298.
Litwiniuk A, Domańska A, Chmielowska M, Martyńska L, Bik W, Kalisz M. The effects of Alpha-Linolenic Acid on the secretory activity of astrocytes and amyloid-associated neurodegeneration in differentiated SH-SY5Y cells: Alpha-Linolenic Acid Protects the SH-SY5Y cells against Amyloid Toxicity. Oxid Med Cell Longev. 2020;2020:8908901.
Jiwaji Z, Hardingham GE. Good, bad, and neglectful: astrocyte changes in neurodegenerative disease. Free Radic Biol Med. 2022;182:93–9.
Norden DM, Fenn AM, Dugan A, Godbout JP. TGFβ produced by IL-10 redirected astrocytes attenuates microglial activation [Internet]. Glia. 2014;62:881–95. https://doi.org/10.1002/glia.22647.
Jha MK, Jo M, Kim JH, Suk K. Microglia-Astrocyte crosstalk: an intimate molecular conversation. Neuroscientist 2019;25:227–40.
Molina-Gonzalez I, Holloway RK, Jiwaji Z, Dando O, Kent SA, Emelianova K, et al. Astrocyte-oligodendrocyte interaction regulates central nervous system regeneration. Nat Commun. 2023;14:3372.
Nutma E, van Gent D, Amor S, Peferoen LAN Astrocyte and Oligodendrocyte Cross-Talk in the Central Nervous System. Cells. 2020;9. Available from: https://doi.org/10.3390/cells9030600.
Niu J, Tsai HH, Hoi KK, Huang N, Yu G, Kim K, et al. Aberrant oligodendroglial-vascular interactions disrupt the blood-brain barrier, triggering CNS inflammation. Nat Neurosci. 2019;22:709–18.
Ha S, Jeong SH, Yi K, Chu JJM, Kim S, Kim EK, et al. Autophagy mediates astrogenesis in adult hippocampal neural stem cells. Exp Neurobiol. 2019;28:229–46.
Wang S, Li B, Qiao H, Lv X, Liang Q, Shi Z, et al. Autophagy-related gene Atg5 is essential for astrocyte differentiation in the developing mouse cortex. EMBO Rep. 2014;15:1053–61.
Chandrasekaran A, Dittlau KS, Corsi GI, Haukedal H, Doncheva NT, Ramakrishna S, et al. Astrocytic reactivity triggered by defective autophagy and metabolic failure causes neurotoxicity in frontotemporal dementia type 3. Stem Cell Rep. 2021;16:2736–51.
Motori E, Puyal J, Toni N, Ghanem A, Angeloni C, Malaguti M, et al. Inflammation-induced alteration of astrocyte mitochondrial dynamics requires autophagy for mitochondrial network maintenance. Cell Metab. 2013;18:844–59.
Herdy JR, Traxler L, Agarwal RK, Karbacher L, Schlachetzki JCM, Boehnke L, et al. Increased post-mitotic senescence in aged human neurons is a pathological feature of Alzheimer’s disease. Cell Stem Cell. 2022;29:1637–52.e6.
Beltran-Lobo P, Reid MJ, Jimenez-Sanchez M, Verkhratsky A, Perez-Nievas BG, Noble W. Astrocyte adaptation in Alzheimer’s disease: a focus on astrocytic P2X7R. Essays Biochem. 2022; Available from: https://doi.org/10.1042/EBC20220079.
Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, et al. Genomic analysis of reactive astrogliosis. J Neurosci. 2012;32:6391–410.
Khodadadei F, Arshad R, Morales DM, Gluski J, Marupudi NI, McAllister JP 2nd, et al. The effect of A1 and A2 reactive astrocyte expression on hydrocephalus shunt failure. Fluids Barriers CNS. 2022;19:78.
Narayan P, Holmström KM, Kim DH, Whitcomb DJ, Wilson MR, St George-Hyslop P, et al. Rare individual amyloid-β oligomers act on astrocytes to initiate neuronal damage. Biochemistry. 2014;53:2442–53.
González-Reyes RE, Nava-Mesa MO, Vargas-Sánchez K, Ariza-Salamanca D, Mora-Muñoz L. Involvement of Astrocytes in Alzheimer’s disease from a neuroinflammatory and oxidative stress perspective. Front Mol Neurosci. 2017;10:427.
Beard E, Lengacher S, Dias S, Magistretti PJ, Finsterwald C. Astrocytes as key regulators of brain energy metabolism: new therapeutic perspectives. Front Physiol. 2021;12:825816.
Park JS, Kam TI, Lee S, Park H, Oh Y, Kwon SH, et al. Blocking microglial activation of reactive astrocytes is neuroprotective in models of Alzheimer’s disease. Acta Neuropathol Commun. 2021;9:78.
Di Benedetto G, Burgaletto C, Bellanca CM, Munafò A, Bernardini R, Cantarella G. Role of Microglia and Astrocytes in Alzheimer’s Disease: From Neuroinflammation to Ca Homeostasis Dysregulation. Cells. 2022;11. Available from: https://doi.org/10.3390/cells11172728.
Singh D. Astrocytic and microglial cells as the modulators of neuroinflammation in Alzheimer’s disease. J Neuroinflamm. 2022;19:206.
He M, Dong H, Huang Y, Lu S, Zhang S, Qian Y, et al. Astrocyte-Derived CCL2 is associated with M1 activation and recruitment of cultured microglial cells. Cell Physiol Biochem. 2016;38:859–70.
Park J, Wetzel I, Marriott I, Dréau D, D’Avanzo C, Kim DY, et al. A 3D human triculture system modeling neurodegeneration and neuroinflammation in Alzheimer’s disease. Nat Neurosci. 2018;21:941–51.
Norden DM, Trojanowski PJ, Walker FR, Godbout JP. Insensitivity of astrocytes to interleukin 10 signaling following peripheral immune challenge results in prolonged microglial activation in the aged brain. Neurobiol Aging. 2016;44:22–41.
Nixon RA, Wegiel J, Kumar A, Yu WH, Peterhoff C, Cataldo A, et al. Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J Neuropathol Exp Neurol. 2005;64:113–22.
Pickford F, Masliah E, Britschgi M, Lucin K, Narasimhan R, Jaeger PA, et al. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid beta accumulation in mice. J Clin Investig. 2008;118:2190–9.
Shin JY, Park HJ, Kim HN, Oh SH, Bae JS, Ha HJ, et al. Mesenchymal stem cells enhance autophagy and increase β-amyloid clearance in Alzheimer disease models. Autophagy. 2014;10:32–44.
Shi Y, Yamada K, Liddelow SA, Smith ST, Zhao L, Luo W, et al. ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature. 2017;549:523–7.
Simonovitch S, Schmukler E, Bespalko A, Iram T, Frenkel D, Holtzman DM, et al. Impaired Autophagy in APOE4 Astrocytes. J Alzheimers Dis. 2016;51:915–27.
Fote GM, Geller NR, Efstathiou NE, Hendricks N, Vavvas DG, Reidling JC, et al. Isoform-dependent lysosomal degradation and internalization of apolipoprotein E requires autophagy proteins. J Cell Sci. 2022;135. Available from: https://doi.org/10.1242/jcs.258687.
Yamazaki Y, Zhao N, Caulfield TR, Liu CC, Bu G. Apolipoprotein E and Alzheimer disease: pathobiology and targeting strategies. Nat Rev Neurol. 2019;15:501–18.
Zhao J, Davis MD, Martens YA, Shinohara M, Graff-Radford NR, Younkin SG, et al. APOE ε4/ε4 diminishes neurotrophic function of human iPSC-derived astrocytes. Hum Mol Genet. 2017;26:2690–700.
Ries M, Sastre M. Mechanisms of Aβ clearance and degradation by Glial cells. Front Aging Neurosci. 2016;8:160.
Gomez-Arboledas A, Davila JC, Sanchez-Mejias E, Navarro V, Nuñez-Diaz C, Sanchez-Varo R, et al. Phagocytic clearance of presynaptic dystrophies by reactive astrocytes in Alzheimer’s disease. Glia. 2018;66:637–53.
Mahan TE, Wang C, Bao X, Choudhury A, Ulrich JD, Holtzman DM. Selective reduction of astrocyte apoE3 and apoE4 strongly reduces Aβ accumulation and plaque-related pathology in a mouse model of amyloidosis. Mol Neurodegener. 2022;17:13.
Parcon PA, Balasubramaniam M, Ayyadevara S, Jones RA, Liu L, Shmookler Reis RJ, et al. Apolipoprotein E4 inhibits autophagy gene products through direct, specific binding to CLEAR motifs. Alzheimers Dement. 2018;14:230–42.
Napolitano G, Ballabio A. TFEB at a glance. J Cell Sci. 2016;129:2475–81.
Zhang YD, Zhao JJ. TFEB Participates in the Aβ-Induced pathogenesis of Alzheimer’s disease by regulating the autophagy-Lysosome pathway. DNA Cell Biol. 2015;34:661–8.
Xiao Q, Yan P, Ma X, Liu H, Perez R, Zhu A, et al. Neuronal-Targeted TFEB Accelerates Lysosomal Degradation of APP, reducing Aβ generation and amyloid plaque pathogenesis. J Neurosci. 2015;35:12137–51.
Yang C, Su C, Iyaswamy A, Krishnamoorthi SK, Zhu Z, Yang S, et al. Celastrol enhances transcription factor EB (TFEB)-mediated autophagy and mitigates Tau pathology: implications for Alzheimer’s disease therapy. Acta Pharm Sin B. 2022;12:1707–22.
Sohn HY, Kim SI, Park JY, Park SH, Koh YH, Kim J, et al. ApoE4 attenuates autophagy via FoxO3a repression in the brain. Sci Rep. 2021;11:17604.
Du S, Jin F, Maneix L, Gedam M, Xu Y, Catic A, et al. FoxO3 deficiency in cortical astrocytes leads to impaired lipid metabolism and aggravated amyloid pathology. Aging Cell. 2021;20:e13432.
Söllvander S, Nikitidou E, Brolin R, Söderberg L, Sehlin D, Lannfelt L, et al. Accumulation of amyloid-β by astrocytes result in enlarged endosomes and microvesicle-induced apoptosis of neurons. Mol Neurodegener. 2016;11:38.
Lööv C, Mitchell CH, Simonsson M, Erlandsson A. Slow degradation in phagocytic astrocytes can be enhanced by lysosomal acidification. Glia. 2015;63:1997–2009.
Zhou Z, Bai J, Zhong S, Zhang R, Kang K, Zhang X, et al. Downregulation of ATP6V1A Involved in Alzheimer’s disease via synaptic vesicle cycle, phagosome, and oxidative phosphorylation. Oxid Med Cell Longev. 2021;2021:5555634.
Yambire KF, Rostosky C, Watanabe T, Pacheu-Grau D, Torres-Odio S, Sanchez-Guerrero A, et al. Impaired lysosomal acidification triggers iron deficiency and inflammation in vivo. Elife. 2019;8. Available from: https://doi.org/10.7554/eLife.51031.
Huang YC, Hsu SM, Shie FS, Shiao YJ, Chao LJ, Chen HW, et al. Reduced mitochondria membrane potential and lysosomal acidification are associated with decreased oligomeric Aβ degradation induced by hyperglycemia: a study of mixed glia cultures. PLoS One. 2022;17:e0260966.
Kim HN, Seo BR, Kim H, Koh JY. Cilostazol restores autophagy flux in bafilomycin A1-treated, cultured cortical astrocytes through lysosomal reacidification: roles of PKA, zinc and metallothionein 3. Sci Rep. 2020;10:9175.
Lee H, Koh JY. Roles for H /K -ATPase and zinc transporter 3 in cAMP-mediated lysosomal acidification in bafilomycin A1-treated astrocytes. Glia. 2021;69:1110–25.
Raha S, Ghosh A, Dutta D, Patel DR, Pahan K. Activation of PPARα enhances astroglial uptake and degradation of β-amyloid. Sci Signal. 2021;14:eabg4747.
Abramov AY, Canevari L, Duchen MR. Beta-amyloid peptides induce mitochondrial dysfunction and oxidative stress in astrocytes and death of neurons through activation of NADPH oxidase. J Neurosci. 2004;24:565–75.
Schmukler E, Solomon S, Simonovitch S, Goldshmit Y, Wolfson E, Michaelson DM, et al. Altered mitochondrial dynamics and function in APOE4-expressing astrocytes. Cell Death Dis. 2020;11:578.
Palikaras K, Lionaki E, Tavernarakis N. Mechanisms of mitophagy in cellular homeostasis, physiology and pathology. Nat Cell Biol. 2018;20:1013–22.
Iorio R, Celenza G, Petricca S. Mitophagy: molecular mechanisms, new concepts on parkin activation and the emerging role of AMPK/ULK1 Axis. Cells. 2021;11. Available from: https://doi.org/10.3390/cells11010030.
Gkikas I, Palikaras K, Tavernarakis N. The role of mitophagy in innate immunity. Front Immunol. 2018;9:1283.
Lampinen R, Belaya I, Saveleva L, Liddell JR, Rait D, Huuskonen MT, et al. Neuron-astrocyte transmitophagy is altered in Alzheimer’s disease. Neurobiol Dis. 2022;170:105753.
Franco-Bocanegra DK, Gourari Y, McAuley C, Chatelet DS, Johnston DA, Nicoll JAR, et al. Microglial morphology in Alzheimer’s disease and after Aβ immunotherapy. Sci Rep. 2021;11:15955.
Arnold T, Betsholtz C. The importance of microglia in the development of the vasculature in the central nervous system. Vol. 5, Vasc Cell. 2013. p. 4. Available from: https://doi.org/10.1186/2045-824x-5-4.
Borst K, Dumas AA, Prinz M. Microglia: immune and non-immune functions. Immunity. 2021;54:2194–208.
Kalafatakis I, Karagogeos D. Oligodendrocytes and microglia: key players in myelin development, damage and repair. Biomolecules. 2021;11. Available from: https://doi.org/10.3390/biom11071058.
Prinz M, Jung S, Priller J. Microglia biology: one century of evolving concepts. Cell. 2019;179:292–311.
Lopes K, de P, Snijders GJL, Humphrey J, Allan A, Sneeboer MAM, et al. Genetic analysis of the human microglial transcriptome across brain regions, aging and disease pathologies. Nat Genet. 2022;54:4–17.
Réu P, Khosravi A, Bernard S, Mold JE, Salehpour M, Alkass K, et al. The lifespan and turnover of microglia in the human brain. Cell Rep. 2017;20:779–84.
Jordão MJC, Sankowski R, Brendecke SM, Sagar, Locatelli G, Tai YH, et al. Single-cell profiling identifies myeloid cell subsets with distinct fates during neuroinflammation. Science. 2019;363. Available from: https://doi.org/10.1126/science.aat7554.
Guo S, Wang H, Yin Y. Microglia polarization from M1 to M2 in neurodegenerative diseases. Front Aging Neurosci. 2022;14:815347.
Xu Y, Propson NE, Du S, Xiong W, Zheng H. Autophagy deficiency modulates microglial lipid homeostasis and aggravates tau pathology and spreading. Proc Natl Acad Sci USA [Internet]. 2021;118. Available from: https://doi.org/10.1073/pnas.2023418118.
Berglund R, Guerreiro-Cacais AO, Adzemovic MZ, Zeitelhofer M, Lund H, Ewing E, et al. Microglial autophagy-associated phagocytosis is essential for recovery from neuroinflammation. Sci Immunol. 2020;5. Available from: https://doi.org/10.1126/sciimmunol.abb5077.
Jin MM, Wang F, Qi D, Liu WW, Gu C, Mao CJ, et al. A critical role of autophagy in regulating microglia polarization in neurodegeneration. Front Aging Neurosci. 2018;10:378.
Cheng J, Liao Y, Dong Y, Hu H, Yang N, Kong X, et al. Microglial autophagy defect causes parkinson disease-like symptoms by accelerating inflammasome activation in mice. Autophagy. 2020;16:2193–205.
Alam MM, Zhao XF, Liao Y, Mathur R, McCallum SE, Mazurkiewicz JE, et al. Deficiency of microglial autophagy increases the density of oligodendrocytes and susceptibility to severe forms of Seizures. eNeuro [Internet]. 2021;8. Available from: https://doi.org/10.1523/ENEURO.0183-20.2021.
Hansen DV, Hanson JE, Sheng M. Microglia in Alzheimer’s disease. J Cell Biol. 2018;217:459–72.
Kiani Shabestari S, Morabito S, Danhash EP, McQuade A, Sanchez JR, Miyoshi E, et al. Absence of microglia promotes diverse pathologies and early lethality in Alzheimer’s disease mice. Cell Rep. 2022;39:110961.
Bolmont T, Haiss F, Eicke D, Radde R, Mathis CA, Klunk WE, et al. Dynamics of the microglial/amyloid interaction indicate a role in plaque maintenance. J Neurosci. 2008;28:4283–92.
Tajbakhsh A, Read M, Barreto GE, Ávila-Rodriguez M, Gheibi-Hayat SM, Sahebkar A. Apoptotic neurons and amyloid-beta clearance by phagocytosis in Alzheimer’s disease: pathological mechanisms and therapeutic outlooks. Eur J Pharm. 2021;895:173873.
Lee JW, Nam H, Kim LE, Jeon Y, Min H, Ha S, et al. TLR4 (toll-like receptor 4) activation suppresses autophagy through inhibition of FOXO3 and impairs phagocytic capacity of microglia. Autophagy. 2019;15:753–70.
Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, et al. A unique microglia type associated with restricting development of Alzheimer’s disease [Internet]. Cell. 2017;169:1276–90.e17. https://doi.org/10.1016/j.cell.2017.05.018.
Pomilio C, Gorojod RM, Riudavets M, Vinuesa A, Presa J, Gregosa A, et al. Microglial autophagy is impaired by prolonged exposure to β-amyloid peptides: evidence from experimental models and Alzheimer’s disease patients. Geroscience. 2020;42:613–32.
Estfanous S, Daily KP, Eltobgy M, Deems NP, Anne MNK, Krause K, et al. Elevated Expression of MiR-17 in Microglia of Alzheimer’s disease patients abrogates autophagy-mediated Amyloid-β degradation. Front Immunol. 2021;12:705581.
Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredesen D, et al. Inhibition of mTOR by Rapamycin Abolishes cognitive deficits and reduces Amyloid-β Levels in a Mouse Model of Alzheimer’s Disease [Internet]. PLoS One. 2010;5:e9979. https://doi.org/10.1371/journal.pone.0009979.
Lu J, Zhou W, Dou F, Wang C, Yu Z. TRPV1 sustains microglial metabolic reprogramming in Alzheimer’s disease. EMBO Rep. 2021;22:e52013.
Fairley LH, Wong JH, Barron AM. Mitochondrial regulation of microglial immunometabolism in Alzheimer’s disease. Front Immunol. 2021;12:624538.
Fang EF, Hou Y, Palikaras K, Adriaanse BA, Kerr JS, Yang B, et al. Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat Neurosci. 2019;22:401–12.
Fünfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature. 2012;485:517–21.
Edgar JM, McLaughlin M, Yool D, Zhang SC, Fowler JH, Montague P, et al. Oligodendroglial modulation of fast axonal transport in a mouse model of hereditary spastic paraplegia. J Cell Biol. 2004;166:121–31.
Akay LA, Effenberger AH, Tsai LH. Cell of all trades: oligodendrocyte precursor cells in synaptic, vascular, and immune function. Genes Dev. 2021;35:180–98.
Juurlink BH, Thorburne SK, Hertz L. Peroxide-scavenging deficit underlies oligodendrocyte susceptibility to oxidative stress. Glia. 1998;22:371–8.
Chavda V, Singh K, Patel V, Mishra M, Mishra AK. Neuronal glial crosstalk: specific and shared mechanisms in Alzheimer’s disease. Brain Sci. 2022;12. Available from: https://doi.org/10.3390/brainsci12010075.
Dulamea AO. Role of Oligodendrocyte dysfunction in demyelination, remyelination and neurodegeneration in multiple sclerosis. Adv Exp Med Biol. 2017;958:91–127.
Misrielal C, Mauthe M, Reggiori F, Eggen BJL. Autophagy in multiple sclerosis: two sides of the same coin. Front Cell Neurosci. 2020;14:603710.
Flygt J, Gumucio A, Ingelsson M, Skoglund K, Holm J, Alafuzoff I, et al. Human traumatic brain injury results in oligodendrocyte death and increases the number of oligodendrocyte progenitor cells. J Neuropathol Exp Neurol. 2016;75:503–15.
Zhang L, Wang H. Autophagy in traumatic brain injury: a new target for therapeutic intervention. Front Mol Neurosci. 2018;11:190.
Aber ER, Griffey CJ, Davies T, Li AM, Yang YJ, Croce KR, et al. Oligodendroglial macroautophagy is essential for myelin sheath turnover to prevent neurodegeneration and death. Cell Rep. 2022;41:111480.
Smith CM, Mayer JA, Duncan ID. Autophagy promotes oligodendrocyte survival and function following dysmyelination in a long-lived myelin mutant [Internet]. J Neurosci. 2013;33:8088–100. https://doi.org/10.1523/jneurosci.0233-13.2013.
Bankston AN, Forston MD, Howard RM, Andres KR, Smith AE, Ohri SS, et al. Autophagy is essential for oligodendrocyte differentiation, survival, and proper myelination. Glia. 2019;67:1745–59.
Papuć E, Rejdak K. The role of myelin damage in Alzheimer’s disease pathology [Internet]. Arch Med Sci. 2020;16:345–341. https://doi.org/10.5114/aoms.2018.76863.
Collins-Praino LE, Francis YI, Griffith EY, Wiegman AF, Urbach J, Lawton A, et al. Soluble amyloid beta levels are elevated in the white matter of Alzheimer’s patients, independent of cortical plaque severity [Internet]. Vol. 2, Acta Neuropathol Commun. 2014. Available from: https://doi.org/10.1186/s40478-014-0083-0.
Lee JT, Xu J, Lee JM, Ku G, Han X, Yang DI, et al. Amyloid-beta peptide induces oligodendrocyte death by activating the neutral sphingomyelinase-ceramide pathway. J Cell Biol. 2004;164:123–31.
Xu J, Chen S, Ahmed SH, Chen H, Ku G, Goldberg MP, et al. Amyloid-beta peptides are cytotoxic to oligodendrocytes. J Neurosci. 2001;21:RC118.
Kaya I, Jennische E, Lange S, Tarik Baykal A, Malmberg P, Fletcher JS. Brain region-specific amyloid plaque-associated myelin lipid loss, APOE deposition and disruption of the myelin sheath in familial Alzheimer’s disease mice. J Neurochem. 2020;154:84–98.
Pak K, Chan SL, Mattson MP. Presenilin-1 mutation sensitizes oligodendrocytes to glutamate and amyloid toxicities, and exacerbates white matter damage and memory impairment in mice. Neuromol Med. 2003;3:53–64.
Ferreira S, Pitman KA, Wang S, Summers BS, Bye N, Young KM, et al. Amyloidosis is associated with thicker myelin and increased oligodendrogenesis in the adult mouse brain. J Neurosci Res. 2020;98:1905–32.
Quintela-López T, Ortiz-Sanz C, Serrano-Regal MP, Gaminde-Blasco A, Valero J, Baleriola J, et al. Aβ oligomers promote oligodendrocyte differentiation and maturation via integrin β1 and Fyn kinase signaling. Cell Death Dis. 2019;10:445.
Ossola B, Zhao C, Compston A, Pluchino S, Franklin RJM, Spillantini MG. Neuronal expression of pathological tau accelerates oligodendrocyte progenitor cell differentiation. Glia. 2016;64:457–71.
Chacon-De-La-Rocha I, Fryatt G, Rivera AD, Verkhratsky A, Raineteau O, Gomez-Nicola D, et al. Accelerated Dystrophy and Decay of Oligodendrocyte Precursor Cells in the APP/PS1 Model of Alzheimer’s-Like Pathology. Front Cell Neurosci. 2020;14:575082.
Vanzulli I, Papanikolaou M, De-La-Rocha IC, Pieropan F, Rivera AD, Gomez-Nicola D, et al. Disruption of oligodendrocyte progenitor cells is an early sign of pathology in the triple transgenic mouse model of Alzheimer’s disease. Neurobiol Aging. 2020;94:130–9.
Zhang P, Kishimoto Y, Grammatikakis I, Gottimukkala K, Cutler RG, Zhang S, et al. Senolytic therapy alleviates Aβ-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer’s disease model [Internet]. Nat Neurosci. 2019;22:719–28. https://doi.org/10.1038/s41593-019-0372-9.
Li W, Tang Y, Fan Z, Meng Y, Yang G, Luo J, et al. Autophagy is involved in oligodendroglial precursor-mediated clearance of amyloid peptide. Mol Neurodegener. 2013;8:27.
Ktena N, Kaplanis SI, Kolotuev I, Georgilis A, Kallergi E, Stavroulaki V, et al. Autophagic degradation of CNS myelin maintains axon integrity. Cell Stress Chaperones. 2022;6:93–107.
Saraswat Ohri S, Bankston AN, Mullins SA, Liu Y, Andres KR, Beare JE, et al. Blocking autophagy in oligodendrocytes limits functional recovery after spinal cord injury. J Neurosci. 2018;38:5900–12.
Wang MR, Zhang XJ, Liu HC, Ma WD, Zhang ML, Zhang Y, et al. Matrine protects oligodendrocytes by inhibiting their apoptosis and enhancing mitochondrial autophagy. Brain Res Bull. 2019;153:30–8.
Fernandes MGF, Luo JXX, Cui QL, Perlman K, Pernin F, Yaqubi M, et al. Age-related injury responses of human oligodendrocytes to metabolic insults: link to BCL-2 and autophagy pathways. Commun Biol. 2021;4:20.
Karlsson M, Zhang C, Méar L, Zhong W, Digre A, Katona B, et al. A single-cell type transcriptomics map of human tissues. Sci Adv. 2021;7. Available from: https://doi.org/10.1126/sciadv.abh2169.
Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci. 2014;34:11929–47.
Eskelinen EL, Illert AL, Tanaka Y, Schwarzmann G, Blanz J, Von Figura K, et al. Role of LAMP-2 in lysosome biogenesis and autophagy. Mol Biol Cell. 2002;13:3355–68.
Lundquist MR, Goncalves MD, Loughran RM, Possik E, Vijayaraghavan T, Yang A, et al. Phosphatidylinositol-5-Phosphate 4-Kinases Regulate Cellular Lipid Metabolism By Facilitating Autophagy. Mol Cell. 2018;70:531–44.e9.
Noori A, Mezlini AM, Hyman BT, Serrano-Pozo A, Das S. Systematic review and meta-analysis of human transcriptomics reveals neuroinflammation, deficient energy metabolism, and proteostasis failure across neurodegeneration. Neurobiol Dis. 2021;149:105225.
Festa BP, Siddiqi FH, Jimenez-Sanchez M, Won H, Rob M, Djajadikerta A, et al. Microglial-to-neuronal CCR5 signaling regulates autophagy in neurodegeneration. Neuron. 2023;111:2021–37.e12.
Guo F, Liu X, Cai H, Le W. Autophagy in neurodegenerative diseases: pathogenesis and therapy. Brain Pathol. 2018;28:3–13.
Frake RA, Ricketts T, Menzies FM, Rubinsztein DC. Autophagy and neurodegeneration. J Clin Investig. 2015;125:65–74.
Bussi C, Peralta Ramos JM, Arroyo DS, Gallea JI, Ronchi P, Kolovou A, et al. Alpha-synuclein fibrils recruit TBK1 and OPTN to lysosomal damage sites and induce autophagy in microglial cells. J Cell Sci [Internet]. 2018;131. Available from: https://doi.org/10.1242/jcs.226241.
Fujikake N, Shin M, Shimizu S. Association between autophagy and neurodegenerative diseases. Front Neurosci. 2018;12:255.
Fernández-Pajarín G, Sesar A, Ares-Pensado B, Castro A. Parkinson’s disease secondary to 2 mutations of genes involved in lysosomal protein degradation. Neurol (Engl Ed). 2020;35:611–2.
Martin DDO, Ladha S, Ehrnhoefer DE, Hayden MR. Autophagy in Huntington disease and huntingtin in autophagy. Trends Neurosci. 2015;38:26–35.
Rudnick ND, Griffey CJ, Guarnieri P, Gerbino V, Wang X, Piersaint JA, et al. Distinct roles for motor neuron autophagy early and late in the SOD1 mouse model of ALS. Proc Natl Acad Sci USA. 2017;114:E8294–303.
Yilmaz R, Müller K, Brenner D, Volk AE, Borck G, Hermann A, et al. SQSTM1/p62 variants in 486 patients with familial ALS from Germany and Sweden. Neurobiol Aging. 2020;87:139.e9–139.e15.
Braak H, Sastre M, Del Tredici K. Development of alpha-synuclein immunoreactive astrocytes in the forebrain parallels stages of intraneuronal pathology in sporadic Parkinson’s disease. Acta Neuropathol. 2007;114:231–41.
Lindström V, Gustafsson G, Sanders LH, Howlett EH, Sigvardson J, Kasrayan A, et al. Extensive uptake of α-synuclein oligomers in astrocytes results in sustained intracellular deposits and mitochondrial damage. Mol Cell Neurosci. 2017;82:143–56.
Jewett M, Dickson E, Brolin K, Negrini M, Jimenez-Ferrer I, Swanberg M. Glutathione -Transferase Alpha 4 Prevents Dopamine Neurodegeneration in a Rat Alpha-Synuclein Model of Parkinson’s Disease. Front Neurol. 2018;9:222.
Angot E, Steiner JA, Lema Tomé CM, Ekström P, Mattsson B, Björklund A, et al. Alpha-synuclein cell-to-cell transfer and seeding in grafted dopaminergic neurons in vivo. PLoS One. 2012;7:e39465.
Chavarría C, Rodríguez-Bottero S, Quijano C, Cassina P, Souza JM. Impact of monomeric, oligomeric and fibrillar alpha-synuclein on astrocyte reactivity and toxicity to neurons. Biochem J. 2018;475:3153–69.
Lu SZ, Guo YS, Liang PZ, Zhang SZ, Yin S, Yin YQ, et al. Suppression of astrocytic autophagy by αB-crystallin contributes to α-synuclein inclusion formation. Transl Neurodegener. 2019;8:3.
Erustes AG, Stefani FY, Terashima JY, Stilhano RS, Monteforte PT, da Silva Pereira GJ, et al. Overexpression of α-synuclein in an astrocyte cell line promotes autophagy inhibition and apoptosis. J Neurosci Res. 2018;96:160–71.
Qiao C, Yin N, Gu HY, Zhu JL, Ding JH, Lu M, et al. Atp13a2 Deficiency Aggravates Astrocyte-Mediated Neuroinflammation via NLRP3 Inflammasome Activation. CNS Neurosci Ther. 2016;22:451–60.
Booth HDE, Hirst WD, Wade-Martins R. The role of astrocyte dysfunction in parkinson’s disease pathogenesis. Trends Neurosci. 2017;40:358–70.
Qin Y, Qiu J, Wang P, Liu J, Zhao Y, Jiang F, et al. Impaired autophagy in microglia aggravates dopaminergic neurodegeneration by regulating NLRP3 inflammasome activation in experimental models of Parkinson’s disease. Brain Behav Immun. 2021;91:324–38.
Tu HY, Yuan BS, Hou XO, Zhang XJ, Pei CS, Ma YT, et al. α-synuclein suppresses microglial autophagy and promotes neurodegeneration in a mouse model of Parkinson’s disease. Aging Cell. 2021;20:e13522.
Choi I, Zhang Y, Seegobin SP, Pruvost M, Wang Q, Purtell K, et al. Microglia clear neuron-released α-synuclein via selective autophagy and prevent neurodegeneration. Nat Commun. 2020;11:1386.
Rocha SM, Kirkley KS, Chatterjee D, Aboellail TA, Smeyne RJ, Tjalkens RB. Microglia-specific knock-out of NF-κB/IKK2 increases the accumulation of misfolded α-synuclein through the inhibition of p62/sequestosome-1-dependent autophagy in the rotenone model of Parkinson’s disease. Glia. 2023;71:2154–79.
Pukass K, Richter-Landsberg C. Oxidative stress promotes uptake, accumulation, and oligomerization of extracellular α-synuclein in oligodendrocytes. J Mol Neurosci. 2014;52:339–52.
Pukaß K, Goldbaum O, Richter-Landsberg C. Mitochondrial impairment and oxidative stress compromise autophagosomal degradation of α-synuclein in oligodendroglial cells. J Neurochem. 2015;135:194–205.
Kaji S, Maki T, Kinoshita H, Uemura N, Ayaki T, Kawamoto Y, et al. Pathological Endogenous α-Synuclein Accumulation in Oligodendrocyte Precursor Cells Potentially Induces Inclusions in Multiple System Atrophy. Stem Cell Rep. 2018;10:356–65.
Mavroeidi P, Arvanitaki F, Vetsi M, Becker S, Vlachakis D, Jensen PH, et al. Autophagy mediates the clearance of oligodendroglial SNCA/alpha-synuclein and TPPP/p25A in multiple system atrophy models. Autophagy. 2022;18:2104–33.
Wilton DK, Stevens B. The contribution of glial cells to Huntington’s disease pathogenesis. Neurobiol Dis. 2020;143:104963.
Rocha NP, Ribeiro FM, Furr-Stimming E, Teixeira AL. Neuroimmunology of Huntington’s disease: revisiting evidence from human studies. Mediators Inflamm. 2016;2016:8653132.
Wakida NM, Lau AL, Nguyen J, Cruz GMS, Fote GM, Steffan JS, et al. Diminished LC3-Associated Phagocytosis by Huntington’s Disease Striatal Astrocytes. J Huntingt Dis. 2022;11:25–33.
Pereira CA de S, Medaglia N de C, Ureshino RP, Bincoletto C, et al. NAADP-Evoked Ca Signaling Leads to Mutant Huntingtin Aggregation and Autophagy Impairment in Murine Astrocytes. Int J Mol Sci [Internet]. 2023;24. Available from: https://doi.org/10.3390/ijms24065593.
Crotti A, Benner C, Kerman BE, Gosselin D, Lagier-Tourenne C, Zuccato C, et al. Mutant Huntingtin promotes autonomous microglia activation via myeloid lineage-determining factors. Nat Neurosci. 2014;17:513–21.
Geloso MC, Corvino V, Marchese E, Serrano A, Michetti F, D’Ambrosi N. The dual role of microglia in ALS: mechanisms and therapeutic approaches. Front Aging Neurosci. 2017;9:242.
Massenzio F, Peña-Altamira E, Petralla S, Virgili M, Zuccheri G, Miti A, et al. Microglial overexpression of fALS-linked mutant SOD1 induces SOD1 processing impairment, activation and neurotoxicity and is counteracted by the autophagy inducer trehalose. Biochim Biophys Acta Mol Basis Dis. 2018;1864:3771–85.
Granatiero V, Sayles NM, Savino AM, Konrad C, Kharas MG, Kawamata H, et al. Modulation of the IGF1R-MTOR pathway attenuates motor neuron toxicity of human ALS SOD1 astrocytes. Autophagy. 2021;17:4029–42.
BaofengFeng, Amponsah AE, Guo R, Liu X, Zhang J, Du X, et al. Autophagy-Mediated Inflammatory Cytokine Secretion in Sporadic ALS Patient iPSC-Derived Astrocytes. Oxid Med Cell Longev. 2022;2022:6483582.
Noh MY, Kwon MS, Oh KW, Nahm M, Park J, Kim YE, et al. Role of NCKAP1 in the Defective Phagocytic function of microglia-like cells derived from rapidly progressing sporadic ALS. Mol Neurobiol. 2023;60:4761–77.
Banerjee P, Mehta AR, Nirujogi RS, Cooper J, James OG, Nanda J, et al. Cell-autonomous immune dysfunction driven by disrupted autophagy in -ALS iPSC-derived microglia contributes to neurodegeneration. Sci Adv. 2023;9:eabq0651.
Lall D, Lorenzini I, Mota TA, Bell S, Mahan TE, Ulrich JD, et al. C9orf72 deficiency promotes microglial-mediated synaptic loss in aging and amyloid accumulation. Neuron. 2021;109:2275–91.e8.
Sako W, Ito H, Yoshida M, Koizumi H, Kamada M, Fujita K, et al. Nuclear factor κ B expression in patients with sporadic amyotrophic lateral sclerosis and hereditary amyotrophic lateral sclerosis with optineurin mutations. Clin Neuropathol. 2012;31:418–23.
Prtenjaca N, Rob M, Alam MS, Markovinovic A, Stuani C, Buratti E, et al. Optineurin Deficiency and Insufficiency Lead to Higher Microglial TDP-43 Protein Levels. Int J Mol Sci [Internet]. 2022;23. Available from: https://doi.org/10.3390/ijms23126829.
Berdyński M, Miszta P, Safranow K, Andersen PM, Morita M, Filipek S, et al. SOD1 mutations associated with amyotrophic lateral sclerosis analysis of variant severity. Sci Rep. 2022;12:103.
Bunton-Stasyshyn RKA, Saccon RA, Fratta P, Fisher EMC. SOD1 function and its implications for amyotrophic lateral sclerosis pathology: new and renascent themes. Neuroscientist. 2015;21:519–29.
Marzella L, Ahlberg J, Glaumann H. Autophagy, heterophagy, microautophagy and crinophagy as the means for intracellular degradation. Virchows Arch B Cell Pathol Incl Mol Pathol. 1981;36:219–34.
Kaushik S, Cuervo AM. Chaperone-mediated autophagy: a unique way to enter the lysosome world. Trends Cell Biol. 2012;22:407–17.
Settembre C, Fraldi A, Medina DL, Ballabio A. Signals from the lysosome: a control centre for cellular clearance and energy metabolism. Nat Rev Mol Cell Biol. 2013;14:283–96.
Author information
Authors and Affiliations
Contributions
AL, AMS and KU have written the first draft of the manuscript. AL, GRJ, AMS and KU revised and improved the first draft. All authors have seen and agreed on the finally submitted version of the manuscript. Figures were created with BioRender.com, with agreement numbers in order: JV24V4W7U0, DJ24V547S8, RX24V4XBLY, GY24V4XKDT, EO24V4XXGY, LS25T4EGT9.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Litwiniuk, A., Juszczak, G.R., Stankiewicz, A.M. et al. The role of glial autophagy in Alzheimer’s disease. Mol Psychiatry 28, 4528–4539 (2023). https://doi.org/10.1038/s41380-023-02242-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-023-02242-5