Abstract
Astrocytes are involved in various processes in the central nervous system (CNS). As the most abundant cell type in the CNS, astrocytes play an essential role in neuronal maintenance and support, synaptic activity, neuronal metabolism, and amyloid-beta (Aβ) clearance. Alzheimer’s disease (AD) is a neurodegenerative disorder associated with cognitive and behavioral impairment. The transformation of astrocytes is involved in various neurodegenerative diseases, such as AD. Since astrocytes have functional diversity and morphological and physiological heterogeneity in the CNS, AD-related astrocytes might show various pathological phenotypes during AD. Astrocytes developing pathological phenotypes could contribute to AD progression. In this review, we provide an overview of the pathological phenotypes of astrocytes in the context of AD, highlighting recent findings in human and mouse AD.
Similar content being viewed by others
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disease and the most common type of dementia that is characterized by memory loss and cognitive dysfunction1,2,3. The pathological hallmark of AD is the deposition of amyloid-beta (Aβ) plaques and the formation of neurofibrillary tangles (NFTs) that are composed of hyperphosphorylated tau protein4,5. Despite numerous studies, the pathophysiological mechanisms of AD are still not fully understood. In contrast, neuronal cell death is a known prominent pathological feature of AD4,5. There is a limited understanding of the changes in astrocytes that promote AD pathogenesis.
The diversity of astrocyte populations has been described in different brain regions, and these populations are classified based on morphological and functional features6,7,8,9,10,11,12,13. As two large morphological groups, fibrous and protoplasmic astrocytes are located in the white and gray matter of the brain, respectively. Furthermore, astrocytes are classified based on distinct morphological and functional features, such as synapse association11,12, membrane properties, Ca2+ signaling12, and neuronal maturation13.
Recent studies have shown that the alteration of astrocytes is involved in the initiation and progression of AD14,15. As astrocytes play various physiological roles in synapse formation and function, neurotransmitter release and uptake, the production of trophic factors, and neuronal survival by energetic supports6,16,17,18,19, the morphological and functional dysregulation of astrocytes is linked to neuronal cell death in AD20,21. Understanding AD-related astrocyte subtypes could help identify new pathophysiological mechanisms of AD. In this review, we explore the features of AD-related astrocytes and discuss the pathological phenotypes of astrocytes in the context of humans with AD and mouse AD models.
The reactive phenotype of astrocytes in AD
Proinflammatory, neurotoxic A1 reactive astrocytes
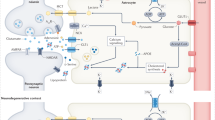
Astrocytes can be changed to reactive astrocytes via morphological, molecular, and functional alterations in various pathological conditions22,23. Reactive astrocytes induce neuropathology and neurodegeneration in neurodegenerative diseases and neurotoxic conditions24. As part of astrocyte polarization, reactive astrocytes can switch to either the pro-inflammatory, neurotoxic A1 phenotype (A1 astrocytes) or the anti-inflammatory, neuroprotective A2 phenotype (A2 astrocytes)25,26.
In a study of reactive astrocytes in the brains of AD patients, both A1 and A2 reactive astrocytes showed upregulated expression of genes such as chitinase 3 like 1 (CHI3L1), ferritin light chain (FTL), integral membrane protein 2 C (ITM2C), aquaporin 4 (AQP4), hepatocellular carcinoma down-regulated 1 (HEPN1), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), angiopoietin-like 4 (ANGPTL4), pleiotrophin (PTN), integral membrane protein 2B (ITM2B), store-operated calcium entry associated regulatory factor (SARAF), interferon-induced transmembrane protein 3 (IFITM3), HtrA serine peptidase 1 (HTRA1), vimentin (VIM), CD63, tweety family member 1 (TTYH1), gap junction protein beta 6 (GJB6), heat shock protein family A (HSP70) member 5 (HSPA5), beta-2 microglobulin (B2M), transmembrane protein 59 (TMEM59), and RAS, dexamethasone-induced 1 (RASD1)27. Additionally, reactive astrocytes showed downregulated expression of homeostatic genes such as neurexin 1 (NRXN1), neuregulin 3 (NRG3), glypican 5 (GPC5), and erb-b2 receptor tyrosine kinase 4 (ERBB4)27.
As a significant phenotype of reactive astrocytes in AD, A1 reactive astrocytes were identified in the brains of AD patients28. A1 reactive astrocytes have high gene levels of glial fibrillary acidic protein (GFAP), S100 calcium-binding protein B (S100B), and complement C3 (C3) in the brains of AD patients28.
In studies of reactive astrocytes in mouse AD models, A1 and A2 reactive astrocytes were characterized in the brains of model AD mice24,29,30,31. A1- and A2-reactive astrocytes showed upregulated expression of genes such as lipocalin 2 (Lcn2), six-transmembrane epithelial antigen of prostate 4 (Steap4), sphingosine-1-phosphate receptor 3 (S1pr3), TIMP metallopeptidase inhibitor 1 (Timp1), heat shock protein family B (Small) member 1 (Hspb1), C-X-C motif chemokine ligand 10 (Cxcl10), Cd44, oncostatin m receptor (Osmr), ceruloplasmin (Cp), serine (or cysteine) peptidase inhibitor, clade A, member 3 N (Serpina3n), asparaginase (Aspg), vimentin (Vim), and glial fibrillary acidic protein (Gfap).
A1 reactive astrocytes showed upregulated expression of classical complement cascade genes related to the destruction of synapses24,30,31. In the brains of model AD mice, A1 reactive astrocytes had upregulated expression of genes including histocompatibility 2, T region locus 23 (H2-T23), serpin family G member 1 (Serping1), histocompatibility 2, D region locus 1 (H2-D1), glycoprotein alpha-galactosyltransferase 1 (Ggta1), interferon inducible GTPase 1 (Iigp1), guanylate binding protein 2 (Gbp2), fibulin 5 (Fbln5), UDP glucuronosyltransferase family one member A1 (Ugt1a1), FK506 binding protein 5 (Fkbp5), proteasome 20 S subunit beta 8 (Psmb8), serglycin (Srgn), and adhesion molecule with Ig like domain 2 (Amigo2). Additionally, A1 reactive astrocytes produced proinflammatory cytokines such as TNF-α, interleukin (IL)-6, IL-1β, and IL-1α in mouse AD models31.
A2 reactive astrocytes showed upregulated expression of several neurotrophic factors that promote neuronal survival and growth. A2 reactive astrocytes showed upregulated expression of genes including cardiotrophin like cytokine factor 1 (Clcf1), transglutaminase 1 (Tgm1), pentraxin 3 (Ptx3), S100 calcium binding protein a10 (S100a10), sphingosine kinase 1 (Sphk1), Cd109, prostaglandin-endoperoxide synthase 2 (Ptgs2), epithelial membrane protein 1 (Emp1), solute carrier family 10 member 6 (Slc10a6), transmembrane 4 L six family member 1 (Tm4sf1), UDP-GlcNAc:betaGal beta-1,3-N-acetylglucosaminyltransferase 5 (B3gnt5), and Cd1424. Figure 1 summarizes the phenotypes of A1 reactive astrocytes in human and mouse AD.
The pathological phenotypes of astrocytes include the reactive phenotype, the death phenotype, the senescence phenotype and functional impairment phenotypes in human and mouse AD. GFAP glial fibrillary acidic protein, S100B S100 calcium binding protein B, C3 complement C3, H2-T23 histocompatibility 2, T region locus 23, H2-D1 histocompatibility 2, D region locus 1, Serping1 serpin family G member 1, Ggta1 glycoprotein alpha-galactosyltransferase 1, Iigp1 interferon inducible GTPase 1, Gbp2 guanylate binding protein 2, Fbln5 fibulin 5, Ugt1a1 UDP glucuronosyltransferase family 1 member A1, Fkbp5 FK506 binding protein 5, Psmb8 proteasome 20 S subunit beta 8, Srgn serglycin, Amigo2 adhesion molecule with Ig like domain 2, TNF-α tumor necrosis factor-alpha, IL-6 interleukin-6, IL-1β interleukin-1 beta, IL-1α interleukin-1 alpha, DNA deoxyribonucleic acid, 4-HNE 4-hydroxynonenal, MDA malondialdehyde, Ireb2, iron response element binding protein 2, Cs citrate synthase, Rpl8 ribosomal protein L8, Ptgs2 prostaglandin-endoperoxide synthase 2, CDKN2A cyclin-dependent kinase inhibitor 2A, γH2AX a phosphorylated form of H2A. X variant histone (H2AX), ITPR2 inositol 1,4,5-trisphosphate receptor type 2, CN calcineurin, EAAT2 glutamate uptake transporter, excitatory amino acid transporters 2, Slc1a2 solute carrier family 1 (glial high-affinity glutamate transporter), member 2.
The death phenotypes of astrocytes in AD
Apoptotic phenotype of astrocytes
Apoptosis is involved in various diseases of the nervous system32. The presence of numerous apoptotic cells is a pathological feature of the brains of patients with AD33,34. The levels of the active form of caspase-3 protein, an executioner caspase in apoptosis, were increased in the brains of AD patients33. The apoptosis of astrocytes may contribute to the pathogenesis of AD.
In a study on the apoptotic phenotype of astrocytes in the brains of AD patients, the number of DNA fragmentation-positive astrocytes was increased in the temporal lobe of the brain34. The number of terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-positive apoptotic astrocytes was increased in the brains of AD patients35. The density of TUNEL-positive astrocytes was correlated with the density of uncored and cored senile plaques, which are polymorphous Aβ protein deposits35. TUNEL-positive apoptotic astrocytes showed regressive changes with fragmented processes and cytoplasmic vacuoles in the brains of AD patients36. Figure 1 summarizes the apoptotic phenotype of astrocytes in human AD.
Ferroptosis phenotype of astrocytes
Ferroptosis is a nonapoptotic form of cell death dependent upon intracellular iron that is morphologically, biochemically, and genetically distinct from other forms of cell death, including apoptosis, necrosis, and autophagy37. Ferroptosis is involved in neuronal death during AD pathogenesis38,39. Ferroptosis of astrocytes has been identified in AD38,39.
In a study on the ferroptosis of astrocytes in AD patients, ferroptosis-related oxidative stress markers, including 4-hydroxynonenal (4-HNE) and malondialdehyde (MDA), were elevated in astrocytes of the cerebral cortex of brains of AD patients38. The number of ferroptotic astrocytes was increased in the brains of AD patients38. As an upstream molecule involved in ferroptosis in astrocytes, the levels of NADPH oxidase 4 (NOX4) were increased in the 4-HNE-positive astrocytes in the cerebral cortex of brains of AD patients38.
In a study of ferroptosis in AD model mice, the levels of the ferroptosis-related proteins 4-HNE and MDA was elevated astrocytes in the cortex of APP/PS1 AD model mice38. Furthermore, the number of ferroptotic astrocytes was increased in the cortex of APP/PS1 AD model mice38. The expression of ferroptosis-related genes, such as iron response element binding protein 2 (Ireb2), citrate synthase (Cs), ribosomal protein L8 (Rpl8), and prostaglandin-endoperoxide synthase 2 (Ptgs2), was upregulated in hippocampal tissues of APP/PS1 AD model mice39. Figure 1 summarizes the phenotypes of ferroptotic astrocytes in human and mouse AD.
The senescence phenotype of astrocytes in AD
Aging is a primary risk factor in the pathogenesis of AD40. Cellular senescence is the hallmark of aging41,42. Cellular senescence in the brain may link the aging process to AD progression. The senescence of astrocytes is related to AD pathogenesis43.
In a study on the senescence of astrocytes in AD patients, senescent astrocytes showed upregulated expression of cyclin-dependent kinase inhibitor 2 A (CDKN2A) (also known as p16INK4a), which is a marker of senescence, in the frontal cortex of AD patients44. The number of senescent astrocytes was increased in AD patients44. As a marker of aging-related DNA damage, the expression of γH2AX, a phosphorylated form of H2A. X variant histone (H2AX), which is a part of the nucleosome structure, was increased in the hippocampal regions and cerebral cortex of AD patients45. γH2AX is produced through phosphorylation in response to the formation of double-stranded breaks in chromosomal DNA46,47,48. Furthermore, the phosphorylation of H2AX induces the translocation of phosphatidylserine to the outer cell membrane and the internucleosomal DNA fragments during apoptosis49.
In a study on the senescence of astrocytes in mouse AD, the expression of senescence-associated genes such as Cdkn2a was increased in the astrocytes of tau MAPT P301S PS19 transgenic mice44. Pharmacological elimination of senescent astrocytes by the senolytic agent ABT263 prevented the upregulation of senescence-associated gene expression and attenuated tau phosphorylation in the brains of tau MAPT P301S PS19 transgenic mice50,51,52. Figure 1 summarizes the phenotypes of senescent astrocytes in human and mouse AD.
The functional impairment of astrocytes in AD
Impaired Ca2+ signaling function in astrocytes during AD
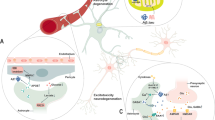
Astrocytes play a role in maintaining the homeostasis of neuronal circuits and regulating neuronal activity via intracellular calcium signals6,53,54. Dysfunction of astrocyte calcium signaling leads to network hyperactivity in the early stage of AD55. Dysregulation of calcium signaling in astrocytes might contribute to the pathological progression of AD.
In the study of astrocyte calcium signaling in AD patients, the expression of inositol 1,4,5-trisphosphate receptor type 2 (ITPR2) (also known as IP3 receptor type 2 (IP3R2)), an intracellular calcium release channel, was decreased in the astrocytes in brains of AD patients55,56. Furthermore, the levels and nuclear localization of nuclear factor of activated T cells 3 (NFAT3), which regulates calcineurin (CN), a Ca2+/calmodulin-dependent protein phosphatase, were elevated in the brains of AD patients57. Elevation of CN in astrocytes triggered the induction of genes associated with inflammatory responses in early-stage AD patients58.
In a study on calcium signaling in astrocytes in mouse AD, decreased calcium signaling was caused by a reduction in Itpr2 expression in astrocytes before the accumulation of Aβ plaques in the early stage of AD in AppNL-F model mice55,56. Figure 1 summarizes the phenotypes of Ca2+ signaling-impaired astrocytes in human and mouse AD.
Impaired glutamate buffering in astrocytes during AD
Glutamate is an excitatory neurotransmitter in the brain. Glutamate receptor activation by interaction with glutamate contributes to various neurologic functions, such as cognition, memory, movement, and sensation59,60. Astrocytes play a significant role in glutamate uptake by clearing 80 ~ 90% of synaptic glutamate in the synaptic cleft;61,62,63,64,65 glutamate uptake transporters, excitatory amino acid transporters 1 (EAAT1) and 2 (EAAT2) (known in mouse as Slc1a3 solute carrier family 1 (glial high-affinity glutamate transporter), member 3 (Slc1a3) (also known as GLAST) and solute carrier family 1 (glial high-affinity glutamate transporter), member 2 (Slc1a2) (also known as GLT-1), respectively) were expressed in astrocytes of AD patients66,67,68,69. EAAT-1 and EAAT-2 are localized in perisynaptic astrocytes and are in contact with active synapses of glutamatergic neurons70. Slc1a3 and Slc1a2 are localized in astrocytic soma68,69. Glutamate excitotoxicity has implications for neurodegeneration in AD71.
In a study on glutamate uptake in astrocytes in AD patients, glutamate transport activity was reduced in the cortex of brains of AD patients72. The expression of the EAAT2 protein and glutamate transport activity were decreased in the frontal cortex of AD patients73. Additionally, a reduction in glutamate transport activity was associated with enhanced Aβ accumulation in AD patients73.
In a study on glutamate uptake in the astrocytes of AD mice, the loss of Slc1a2 exacerbated cognitive impairment in the AD model mice74. Additionally, restoration of Slc1a2 function improved cognitive functions, restored synaptic integrity and reduced amyloid plaques in a AD model mice75. Figure 1 summarizes the phenotypes of impaired glutamate buffering in astrocytes in human and mouse AD.
Conclusions
We reviewed the pathological phenotypes of astrocytes in AD and discussed the transcriptomic and proteomic features of the pathological phenotypes of astrocytes in the brains of AD patients and AD model mice. We described the reactive phenotype, death phenotype, senescence phenotype, and functional impairment phenotypes of astrocytes in human and mouse AD. The development of pathological phenotypes by astrocytes may be an essential event in the pathogenesis of AD. Understanding the pathological phenotypes of astrocytes may help maintain normal brain function and prevent neurodegeneration during AD. Along with current therapies for AD that target Aβ and tau pathology, the proper control of astrocyte pathology could be an alternative therapeutic approach for AD treatment.
References
Selkoe, D. J. Alzheimer’s disease: genes, proteins, and therapy. Physiol. Rev. 81, 741–766 (2001).
Holtzman, D. M., Morris, J. C. & Goate, A. M. Alzheimer’s disease: the challenge of the second century. Sci. Transl. Med. 3, 77sr1 (2011).
Gustavsson, A. et al. Global estimates on the number of persons across the Alzheimer’s disease continuum. Alzheimers Dement. 19, 658–670 (2023).
Buckwalter, M. S. & Wyss-Coray, T. Modelling neuroinflammatory phenotypes in vivo. J. Neuroinflammation 1, 10 (2004).
Zhang, B. et al. Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer’s disease. Cell 153, 707–720 (2013).
Khakh, B. S. & Deneen, B. The emerging nature of astrocyte diversity. Annu. Rev. Neurosci. 42, 187–207 (2019).
Saunders, A. et al. Molecular diversity and specializations among the cells of the adult mouse brain. Cell 174, 1015–1030.e16 (2018).
Khakh, B. S. & Sofroniew, M. V. Diversity of astrocyte functions and phenotypes in neural circuits. Nat. Neurosci. 18, 942–952 (2015).
Ben Haim, L. & Rowitch, D. H. Functional diversity of astrocytes in neural circuit regulation. Nat. Rev. Neurosci. 18, 31–41 (2017).
Cahoy, J. D. et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J. Neurosci. 28, 264–278 (2008).
Lanjakornsiripan, D. et al. Layer-specific morphological and molecular differences in neocortical astrocytes and their dependence on neuronal layers. Nat. Commun. 9, 1623 (2018).
Chai, H. et al. Neural circuit-specialized astrocytes: transcriptomic, proteomic, morphological, and functional evidence. Neuron 95, 531–549 e9 (2017).
Morel, L. et al. Molecular and functional properties of regional astrocytes in the adult brain. J. Neurosci. 37, 8706–8717 (2017).
Acosta, C., Anderson, H. D. & Anderson, C. M. Astrocyte dysfunction in Alzheimer. Dis. J. Neurosci. Res. 95, 2430–2447 (2017).
Gonzalez-Reyes, R. E., Nava-Mesa, M. O., Vargas-Sanchez, K., Ariza-Salamanca, D. & Mora-Munoz, L. Involvement of astrocytes in alzheimer’s disease from a neuroinflammatory and oxidative stress perspective. Front. Mol. Neurosci. 10, 427 (2017).
Ricci, G., Volpi, L., Pasquali, L., Petrozzi, L. & Siciliano, G. Astrocyte-neuron interactions in neurological disorders. J. Biol. Phys. 35, 317–336 (2009).
Rocchi, A. et al. NMR metabolomic investigation of astrocytes interacted with Abeta(4)(2) or its complexes with either copper(II) or zinc(II.). J. Inorg. Biochem. 117, 326–333 (2012).
Figley, C. R. & Stroman, P. W. The role(s) of astrocytes and astrocyte activity in neurometabolism, neurovascular coupling, and the production of functional neuroimaging signals. Eur. J. Neurosci. 33, 577–588 (2011).
Ishibashi, T. et al. Astrocytes promote myelination in response to electrical impulses. Neuron 49, 823–832 (2006).
Phatnani, H. & Maniatis, T. Astrocytes in neurodegenerative disease. Cold Spring Harb. Perspect. Biol. 7, a020628 (2015).
Habib, N. et al. Disease-associated astrocytes in Alzheimer’s disease and aging. Nat. Neurosci. 23, 701–706 (2020).
Ben Haim, L., Carrillo-de Sauvage, M. A., Ceyzeriat, K. & Escartin, C. Elusive roles for reactive astrocytes in neurodegenerative diseases. Front. Cell. Neurosci. 9, 278 (2015).
Escartin, C., Guillemaud, O. & Carrillo-de Sauvage, M. A. Questions and (some) answers on reactive astrocytes. Glia 67, 2221–2247 (2019).
Liddelow, S. A. & Barres, B. A. Reactive astrocytes: production, function, and therapeutic potential. Immunity 46, 957–967 (2017).
Hinkle, J. T., Dawson, V. L. & Dawson, T. M. The A1 astrocyte paradigm: new avenues for pharmacological intervention in neurodegeneration. Mov. Disord. 34, 959–969 (2019).
Neal, M. et al. Prokineticin-2 promotes chemotaxis and alternative A2 reactivity of astrocytes. Glia 66, 2137–2157 (2018).
Dai, D. L., Li, M. & Lee, E. B. Human Alzheimer’s disease reactive astrocytes exhibit a loss of homeostastic gene expression. Acta Neuropathol. Commun. 11, 127 (2023).
Liddelow, S. A. et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487 (2017).
Zamanian, J. L. et al. Genomic analysis of reactive astrogliosis. J. Neurosci. 32, 6391–6410 (2012).
Li, C. et al. Astrocytes: implications for neuroinflammatory pathogenesis of Alzheimer’s disease. Curr. Alzheimer Res. 8, 67–80 (2011).
Patel, N. S. et al. Inflammatory cytokine levels correlate with amyloid load in transgenic mouse models of Alzheimer’s disease. J. Neuroinflammation. 2, 9 (2005).
Thompson, C. B. Apoptosis in the pathogenesis and treatment of disease. Science 267, 1456–1462 (1995).
Shimohama, S., Tanino, H. & Fujimoto, S. Changes in caspase expression in Alzheimer’s disease: comparison with development and aging. Biochem. Biophys. Res. Commun. 256, 381–384 (1999).
Lassmann, H. et al. Cell death in Alzheimer’s disease evaluated by DNA fragmentation in situ. Acta Neuropathol. 89, 35–41 (1995).
Kobayashi, K. et al. Correlation between astrocyte apoptosis and Alzheimer changes in gray matter lesions in Alzheimer’s disease. J. Alzheimers Dis. 6, 623–632 (2004). discussion 673-681.
Kobayashi, K. et al. Apoptosis of astrocytes with enhanced lysosomal activity and oligodendrocytes in white matter lesions in Alzheimer’s disease. Neuropathol. Appl. Neurobiol. 28, 238–251 (2002).
Dixon, S. J. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072 (2012).
Park, M. W. et al. NOX4 promotes ferroptosis of astrocytes by oxidative stress-induced lipid peroxidation via the impairment of mitochondrial metabolism in Alzheimer’s diseases. Redox Biol. 41, 101947 (2021).
Bao, W. D. et al. Loss of ferroportin induces memory impairment by promoting ferroptosis in Alzheimer’s disease. Cell Death Differ. 28, 1548–1562 (2021).
Hou, Y. et al. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 15, 565–581 (2019).
Guerrero, A., De Strooper, B. & Arancibia-Carcamo, I. L. Cellular senescence at the crossroads of inflammation and Alzheimer’s disease. Trends Neurosci. 44, 714–727 (2021).
Lopez-Otin, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013).
Han, X., Zhang, T., Liu, H., Mi, Y. & Gou, X. Astrocyte senescence and Alzheimer’s disease: a review. Front. Aging Neurosci. 12, 148 (2020).
Bhat, R. et al. Astrocyte senescence as a component of Alzheimer’s disease. PLoS ONE 7, e45069 (2012).
Myung, N. H. et al. Evidence of DNA damage in Alzheimer disease: phosphorylation of histone H2AX in astrocytes. Age (Dordr.) 30, 209–215 (2008).
Burma, S., Chen, B. P., Murphy, M., Kurimasa, A. & Chen, D. J. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J. Biol. Chem. 276, 42462–42467 (2001).
Rogakou, E. P., Pilch, D. R., Orr, A. H., Ivanova, V. S. & Bonner, W. M. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 273, 5858–5868 (1998).
Sedelnikova, O. A., Pilch, D. R., Redon, C. & Bonner, W. M. Histone H2AX in DNA damage and repair. Cancer Biol. Ther. 2, 233–235 (2003).
Rogakou, E. P., Nieves-Neira, W., Boon, C., Pommier, Y. & Bonner, W. M. Initiation of DNA fragmentation during apoptosis induces phosphorylation of H2AX histone at serine 139. J. Biol. Chem. 275, 9390–9395 (2000).
Bussian, T. J. et al. Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature 562, 578–582 (2018).
Chang, J. et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med. 22, 78–83 (2016).
Zhu, Y. et al. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell 15, 428–435 (2016).
Lines, J., Martin, E. D., Kofuji, P., Aguilar, J. & Araque, A. Astrocytes modulate sensory-evoked neuronal network activity. Nat. Commun. 11, 3689 (2020).
Guerra-Gomes, S., Sousa, N., Pinto, L. & Oliveira, J. F. Functional roles of astrocyte calcium elevations: from synapses to behavior. Front. Cell. Neurosci. 11, 427 (2017).
Shah, D. et al. Astrocyte calcium dysfunction causes early network hyperactivity in Alzheimer’s disease. Cell Rep. 40, 111280 (2022).
Saito, T. et al. Single App knock-in mouse models of Alzheimer’s disease. Nat. Neurosci. 17, 661–663 (2014).
Abdul, H. M. et al. Cognitive decline in Alzheimer’s disease is associated with selective changes in calcineurin/NFAT signaling. J. Neurosci. 29, 12957–12969 (2009).
Blalock, E. M. et al. Incipient Alzheimer’s disease: microarray correlation analyses reveal major transcriptional and tumor suppressor responses. Proc. Natl Acad. Sci. USA 101, 2173–2178 (2004).
Gasic, G. P. & Hollmann, M. Molecular neurobiology of glutamate receptors. Annu. Rev. Physiol. 54, 507–536 (1992).
Lipton, S. A. & Rosenberg, P. A. Excitatory amino acids as a final common pathway for neurologic disorders. N. Engl. J. Med. 330, 613–622 (1994).
Anderson, C. M. & Swanson, R. A. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia 32, 1–14 (2000).
Bergles, D. E. & Jahr, C. E. Synaptic activation of glutamate transporters in hippocampal astrocytes. Neuron 19, 1297–1308 (1997).
Otis, T. S., Kavanaugh, M. P. & Jahr, C. E. Postsynaptic glutamate transport at the climbing fiber-Purkinje cell synapse. Science 277, 1515–1518 (1997).
Bergles, D. E. & Jahr, C. E. Glial contribution to glutamate uptake at Schaffer collateral-commissural synapses in the hippocampus. J. Neurosci. 18, 7709–7716 (1998).
Melone, M., Bellesi, M. & Conti, F. Synaptic localization of GLT-1a in the rat somatic sensory cortex. Glia 57, 108–117 (2009).
Rothstein, J. D. et al. Localization of neuronal and glial glutamate transporters. Neuron 13, 713–725 (1994).
Chaudhry, F. A. et al. Glutamate transporters in glial plasma membranes: highly differentiated localizations revealed by quantitative ultrastructural immunocytochemistry. Neuron 15, 711–720 (1995).
Rimmele, T. S. & Rosenberg, P. A. GLT-1: The elusive presynaptic glutamate transporter. Neurochem. Int. 98, 19–28 (2016).
Danbolt, N. C., Furness, D. N. & Zhou, Y. Neuronal vs glial glutamate uptake: resolving the conundrum. Neurochem. Int. 98, 29–45 (2016).
Zhou, J. & Sutherland, M. L. Glutamate transporter cluster formation in astrocytic processes regulates glutamate uptake activity. J. Neurosci. 24, 6301–6306 (2004).
Hynd, M. R., Scott, H. L. & Dodd, P. R. Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer’s disease. Neurochem. Int. 45, 583–595 (2004).
Masliah, E., Alford, M., DeTeresa, R., Mallory, M. & Hansen, L. Deficient glutamate transport is associated with neurodegeneration in Alzheimer’s disease. Ann. Neurol. 40, 759–766 (1996).
Li, S., Mallory, M., Alford, M., Tanaka, S. & Masliah, E. Glutamate transporter alterations in Alzheimer disease are possibly associated with abnormal APP expression. J. Neuropathol. Exp. Neurol. 56, 901–911 (1997).
Mookherjee, P. et al. GLT-1 loss accelerates cognitive deficit onset in an Alzheimer’s disease animal model. J. Alzheimers Dis. 26, 447–455 (2011).
Takahashi, K. et al. Restored glial glutamate transporter EAAT2 function as a potential therapeutic approach for Alzheimer’s disease. J. Exp. Med. 212, 319–332 (2015).
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grants (NRF- 2021R1F1A1059133 to I.D.Y., NRF-2021R1F1A1057801 to J.L. and NRF-2021R1C1C1007810 to J.-S.M.) and Soonchunhyang University Research Fund (20210137 to J.-S.M.).
Author information
Authors and Affiliations
Contributions
J.K., J.L., and J.-S.M. conceived the study. J.K. and I.D.Y. performed the investigation. J.L. and J.-S.M. performed visualization. J.K., I.D.Y., J.L., and J.-S.M. wrote the paper. J.-S.M. supervised the entire project. All the authors have read, revised, and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, J., Yoo, I.D., Lim, J. et al. Pathological phenotypes of astrocytes in Alzheimer’s disease. Exp Mol Med 56, 95–99 (2024). https://doi.org/10.1038/s12276-023-01148-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s12276-023-01148-0