Abstract
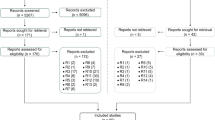
The γ-aminobutyric acid (GABA)ergic system is the primary inhibitory neurotransmission system in the mammalian brain. Its dysregulation has been shown in multiple brain conditions, but in Alzheimer’s disease (AD) studies have provided contradictory results. Here, we conducted a systematic review with meta-analysis to investigate whether the GABAergic system is altered in AD patients compared to healthy controls (HC), following the PRISMA 2020 Statement. We searched PubMed and Web of Science from database inception to March 18th, 2023 for studies reporting GABA, glutamate decarboxylase (GAD) 65/67, GABAA, GABAB, and GABAC receptors, GABA transporters (GAT) 1–3 and vesicular GAT in the brain, and GABA levels in the cerebrospinal fluid (CSF) and blood. Heterogeneity was estimated using the I2 index, and the risk of bias was assessed with an adapted questionnaire from the Joanna Briggs Institute Critical Appraisal Tools. The search identified 3631 articles, and 48 met the final inclusion criteria (518 HC, mean age 72.2, and 603 AD patients, mean age 75.6). Random-effects meta-analysis [standardized mean difference (SMD)] revealed that AD patients presented lower GABA levels in the brain (SMD = −0.48 [95% CI = −0.7, −0.27], adjusted p value (adj. p) < 0.001) and in the CSF (−0.41 [−0.72, −0.09], adj. p = 0.042), but not in the blood (−0.63 [−1.35, 0.1], adj. p = 0.176). In addition, GAD65/67 (−0.67 [−1.15, −0.2], adj. p = 0.006), GABAA receptor (−0.51 [−0.7, −0.33], adj. p < 0.001), and GABA transporters (−0.51 [−0.92, −0.09], adj. p = 0.016) were lower in the AD brain. Here, we showed a global reduction of GABAergic system components in the brain and lower GABA levels in the CSF of AD patients. Our findings suggest the GABAergic system is vulnerable to AD pathology and should be considered a potential target for developing pharmacological strategies and novel AD biomarkers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data are available on GitHub (bit.ly/3o5nqcn). Supplementary information is available at Molecular Psychiatry’s website.
Code availability
R codes generated in this work are available on GitHub (bit.ly/3o5nqcn).
References
Alzheimer’s Association. 2023 Alzheimer’s disease Facts and Figures. Alzheimer’s Dement. 2023;19:1598–695.
Stelzmann RA, Norman SH, Reed MF. An English translation of Alzheimer’s 1907 paper, “Uber eine eigenartige Erkankung der Hirnrinde”. Clin Anat. 1995;8:429–31.
Liu PP, Xie Y, Meng XY, Kang JS. History and progress of hypotheses and clinical trials for Alzheimer’s disease. Signal Transduct Target Ther. 2019;4:37.
Bartus RT, Dean RL, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–17.
Greenamyre JT, Maragos WF, Albin RL, Penney JB, Young AB. Glutamate transmission and toxicity in Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 1988;12:421–IN4.
Bear MF, Connors BW, Paradiso, MA. Neuroscience: Exploring the Brain. 4th ed. Wolters Kluwer Health; 2015.
Davies P. Neurotransmitter-related enzymes in senile dementia of the Alzheimer type. Brain Res. 1979;171:319–27.
Davies P, Maloney AJF. Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet. 1976;2:1403.
Rossor MN, Garrett NJ, Johnson AL, Mountjoy CQ, Roth M, Iversen LL. A post-mortem study of the cholinergic and GABA systems in senile dementia. Brain. 1982;105:313–30.
Tarbit I, Perry EK, Perry RH, Blessed G, Tomlinson BE. Hippocampal free amino acids in Alzheimer’s disease. J Neurochem. 1980;35:1246–9.
Perry EK, Atack JR, Perry RH, Hardy JA, Dodd PR, Edwardson JA, et al. Intralaminar neurochemical distributions in human midtemporal cortex: comparison between Alzheimer’s disease and the normal. J Neurochem. 1984;42:1402–10.
Perry EK, Blessed G, Tomlinson BE, Perry RH, Crow TJ, Cross AJ, et al. Neurochemical activities in human temporal lobe related to aging and Alzheimer-type changes. Neurobiol Aging. 1981;2:251–6.
Cross AJ, Crow TJ, Johnson JA, Perry EK, Perry RH, Blessed G, et al. Studies on neurotransmitter receptor systems in neocortex and hippocampus in senile dementia of the Alzheimer-type. J Neurol Sci. 1984;64:109–17.
Hardy J, Cowburn R, Barton A, Reynolds G, Dodd P, Wester P, et al. A disorder of cortical GABAergic innervation in Alzheimer’s disease. Neurosci Lett. 1987;73:192–6.
Govindpani K, Guzmán BCF, Vinnakota C, Waldvogel HJ, Faull RL, Kwakowsky A. Towards a Better Understanding of GABAergic Remodeling in Alzheimer’s Disease. Int J Mol Sci. 2017;18:1813.
Marczynski TJ. GABAergic deafferentation hypothesis of brain aging and Alzheimer’s disease: pharmacologic profile of the benzodiazepine antagonist, flumazenil. Rev Neurosci. 1995;6:221–58.
Bi D, Wen L, Wu Z, Shen Y. GABAergic dysfunction in excitatory and inhibitory (E/I) imbalance drives the pathogenesis of Alzheimer’s disease. Alzheimer’s Dement. 2020;16:1312–29.
Jiménez-Balado J, Eich TS. GABAergic dysfunction, neural network hyperactivity and memory impairments in human aging and Alzheimer’s disease. Semin Cell Dev Biol. 2021;116:146–59.
PROSPERO - International Prospective Register of Systematic Reviews. https://www.crd.york.ac.uk/prospero/
Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:160.
Ambrad GE, Fuhrmann M. Unsupervised excitation: GABAergic dysfunctions in Alzheimer’s disease. Brain Res. 2019;1707:216–26.
Calvo-Flores GB, Vinnakota C, Govindpani K, Waldvogel HJ, Faull RLM, Kwakowsky A. The GABAergic system as a therapeutic target for Alzheimer’s disease. J Neurochem. 2018;146:649–69.
Bellaver B, Ferrari-Souza JP, da Ros LU, Carter SF, Rodriguez-Vieitez E, Nordberg A, et al. Astrocyte Biomarkers in Alzheimer Disease: A Systematic Review and Meta-analysis. Neurology. 2021;96:E2944–55.
Olsson B, Lautner R, Andreasson U, Öhrfelt A, Portelius E, Bjerke M, et al. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. Lancet Neurol. 2016;15:673–84.
Yoon S, Kim SE, Ko Y, Jeong GH, Lee KH, Lee J, et al. Differential expression of MicroRNAs in Alzheimer’s disease: a systematic review and meta-analysis. Mol Psychiatry. 2022;27:2405–13.
Gao S, Burney HN, Callahan CM, Purnell CE, Hendrie HC. Incidence of Dementia and Alzheimer Disease Over Time: A Meta-Analysis. J Am Geriatr Soc. 2019;67:1361–9.
Bramer WM, Rethlefsen ML, Kleijnen J, Franco OH. Optimal database combinations for literature searches in systematic reviews: a prospective exploratory study. Syst Rev. 2017;6:245.
Hirt J, Bergmann J, Karrer M. Overlaps of multiple database retrieval and citation tracking in dementia care research: a methodological study. J Med Libr Assoc. 2021;109:275–85.
Gusenbauer M. Search where you will find most: Comparing the disciplinary coverage of 56 bibliographic databases. Scientometrics. 2022;127:2683–745.
Eybye MN, Madsen SD, Schultz ANØ, Nim CG. Database coverage and their use in systematic reviews regarding spinal manipulative therapy: an exploratory study. Chiropr Man Therap. 2022;30:57.
Rayyan – Intelligent Systematic Reviews. https://www.rayyan.ai/
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–44.
Jack CR, Albert MS, Knopman DS, McKhann GM, Sperling RA, Carrillo MC, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011;7:257–62.
Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 2014;13:614–29.
WebPlotDigitizer - Extract data from plots, images, and maps. https://automeris.io/WebPlotDigitizer/
Viechtbauer W. Conducting Meta-Analyses in R with the metafor Package. J Stat Softw. 2010;36:1–48.
Forest Plots, Funnel Plots, and Visual Funnel Plot Inference for Meta-Analysis [R package metaviz version 0.3.1]. 2020 Apr 9; https://CRAN.R-project.org/package=metaviz
R: The R Project for Statistical Computing. https://www.r-project.org/
Gryglewski G, Lanzenberger R, Silberbauer LR, Pacher D, Kasper S, Rupprecht R, et al. Meta-analysis of brain structural changes after electroconvulsive therapy in depression. Brain Stimul. 2021;14:927–37.
Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Psychology Press; 1988.
Veroniki AA, Jackson D, Viechtbauer W, Bender R, Bowden J, Knapp G, et al. Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res Synth Methods. 2016;7:55–79.
Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J Royal Statis Soc: Series B (Methodological). 1995;57:289–300.
Critical Appraisal Tools | JBI. https://jbi.global/critical-appraisal-tools
Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Miller RG. The Jackknife – A Review. Biometrika. 1974;61:15.
Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58.
Chu DCM, Penney JB, Young AB. Cortical GABAB and GABAA receptors in Alzheimer’s disease: a quantitative autoradiographic study. Neurology. 1987;37:1454–9.
Cruz-Aguilar MA, Ramírez-Salado I, Hernández-González M, Guevara MA, Rivera-García AP. EEG coherence and power spectra during REM sleep related to melatonin intake in mild-to-moderate Alzheimer’s disease: a pilot study. Int J Neurosci. 2021;133:441–9.
Kuroda H. Gamma-aminobutyric acid (GABA) in cerebrospinal fluid. Acta Med Okayama. 1983;37:167–77.
Sedvall G. Neurotransmitter receptor imaging in Alzheimer’s disease. J Neural Transm. 1987;24:43–8.
Zilles K, Qu M, Schleicher A, Schroeter M, Kraemer M, Witte OW. Plasticity and neurotransmitter receptor changes in Alzheimer’s disease and experimental cortical infarcts. Arzneimittelforschung. 1995;45:361–6.
Corrigan F, Reynolds G, Ward N. Reductions of zinc and selenium in brain in Alzheimer’s disease. Trace Elem Med. 1991;8:1–5.
Pascual B, Prieto E, Marti-Climent J, Lostao J, Quincoces G, Penuelas I, et al. Decreased Medial Temporal GABAA Binding on C-11-Flumazenil PET in Early Alzheimer Disease. Neurology. 2011;76:177.
Iwakiri M, Mizukami K, Ikonomovic MD, Ishikawa M, Hidaka S, Abrahamson EE, et al. Changes in hippocampal GABABR1 subunit expression in Alzheimer’s patients: association with Braak staging. Acta Neuropathol. 2005;109:467–74.
Nägga K, Bogdanovic N, Marcusson J. GABA transporters (GAT-1) in Alzheimer’s disease. J Neural Transm (Vienna). 1999;106:1141–9.
Sasaki H, Muramoto O, Kanazawa I, Arai H, Kosaka K, Iizuka R. Regional distribution of amino acid transmitters in postmortem brains of presenile and senile dementia of Alzheimer type. Ann Neurol. 1986;19:263–9.
Schwab C, Yu S, Wong W, McGeer EG, McGeer PL. GAD65, GAD67, and GABAT immunostaining in human brain and apparent GAD65 loss in Alzheimer’s disease. J Alzheimers Dis. 2013;33:1073–88.
Tohgi H, Abe T, Takahashi S, Kimura M. A selective reduction of excitatory amino acids in cerebrospinal fluid of patients with Alzheimer type dementia compared with vascular dementia of the Binswanger type. Neurosci Lett. 1992;141:5–8.
Griffiths PD, Perry RH, Crossman AR. A detailed anatomical analysis of neurotransmitter receptors in the putamen and caudate in Parkinson’s disease and Alzheimer’s disease. Neurosci Lett. 1994;169:68–72.
Liu P, Fleete MS, Jing Y, Collie ND, Curtis MA, Waldvogel HJ, et al. Altered arginine metabolism in Alzheimer’s disease brains. Neurobiol Aging. 2014;35:1992–2003.
Aisa B, Gil-Bea FJ, Solas M, García-Alloza M, Chen CP, Lai MK, et al. Altered NCAM expression associated with the cholinergic system in Alzheimer’s disease. J Alzheimers Dis. 2010;20:659–68.
Gueli MC, Taibi G. Alzheimer’s disease: amino acid levels and brain metabolic status. Neurol Sci. 2013;34:1575–9.
Jansen KLR, Faull RLM, Dragunow M, Synek BL. Alzheimer’s disease: changes in hippocampal N-methyl-D-aspartate, quisqualate, neurotensin, adenosine, benzodiazepine, serotonin and opioid receptors-an autoradiographic study. Neuroscience. 1990;39:613–27.
Smith CCT, Bowen DM, Sims NR, Neary D, Davison AN. Amino acid release from biopsy samples of temporal neocortex from patients with Alzheimer’s disease. Brain Res. 1983;264:138–41.
Mohanakrishnan P, Fowler AH, Vonsattel JP, Husain MM, Jolles PR, Liem P, et al. An in vitro 1H nuclear magnetic resonance study of the temporoparietal cortex of Alzheimer brains. Exp Brain Res. 1995;102:503–10.
Weiner MF, Speciale SG, Risser RC, Kramer GL, Petty F. Cerebrospinal fluid and plasma gamma-aminobutyric acid in Alzheimer’s disease. Biol Psychiatry. 1996;40:933–4.
Shimohama S, Taniguchi T, Fujiwara M, Kameyama M. Changes in benzodiazepine receptors in Alzheimer-type dementia. Ann Neurol. 1988;23:404–6.
Pomara N, Deptula D, Galloway MP, LeWitt PA, Stanley M. CSF GABA in caregiver spouses of Alzheimer patients. Am J Psychiatry. 1989;146:787–8.
Howell O, Atack JR, Dewar D, McKernan RM, Sur C. Density and pharmacology of α5 subunit-containing GABA(A) receptors are preserved in hippocampus of Alzheimer’s disease patients. Neuroscience. 2000;98:669–75.
Seidl R, Cairns N, Singewald N, Kaehler ST, Lubec G. Differences between GABA levels in Alzheimer’s disease and Down syndrome with Alzheimer-like neuropathology. Naunyn Schmiedebergs Arch Pharmacol. 2001;363:139–45.
Kwakowsky A, Calvo-Flores Guzmán B, Pandya M, Turner C, Waldvogel HJ, Faull RL. GABAA receptor subunit expression changes in the human Alzheimer’s disease hippocampus, subiculum, entorhinal cortex and superior temporal gyrus. J Neurochem. 2018;145:374–92.
Burbaeva GS, Boksha IS, Tereshkina EB, Savushkina OK, Prokhorova TA, Vorobyeva EA. Glutamate and GABA-metabolizing enzymes in post-mortem cerebellum in Alzheimer’s disease: phosphate-activated glutaminase and glutamic acid decarboxylase. Cerebellum. 2014;13:607–15.
Huang D, Liu D, Yin J, Qian T, Shrestha S, Ni H. Glutamate-glutamine and GABA in brain of normal aged and patients with cognitive impairment. Eur Radiol. 2017;27:2698–705.
Garcia-Alloza M, Tsang SW, Gil-Bea FJ, Francis PT, Lai MK, Marcos B, et al. Involvement of the GABAergic system in depressive symptoms of Alzheimer’s disease. Neurobiol Aging. 2006;27:1110–7.
Oishi M, Mochizuki Y, Yoshihashi H, Takasu T, Nakano E. Laboratory examinations correlated with severity of dementia. Ann Clin Lab Sci. 1996;26:340–5.
Simpson MDC, Cross AJ, Slater P, Deakin JFW. Loss of cortical GABA uptake sites in Alzheimer’s disease. J Neural Transm. 1988;71:219–26.
Zhang Y, Liu Z, Ji B, Liu L, Wu S, Liu X, et al. Metabolite Profile of Alzheimer’s Disease in the Frontal Cortex as Analyzed by HRMAS 1H NMR. Front Aging Neurosci. 2019;10:424.
Poirel O, Mella S, Videau C, Ramet L, Davoli MA, Herzog E, et al. Moderate decline in select synaptic markers in the prefrontal cortex (BA9) of patients with Alzheimer’s disease at various cognitive stages. Sci Rep. 2018;8:938.
Yew DT, Li WP, Webb SE, Lai HWL, Zhang L. Neurotransmitters, peptides, and neural cell adhesion molecules in the cortices of normal elderly humans and Alzheimer patients: a comparison. Exp Gerontol. 1999;34:117–33.
Jiménez-Jiménez FJ, Molina JA, Gómez P, Vargas C, De Bustos F, Benito-León J, et al. Neurotransmitter amino acids in cerebrospinal fluid of patients with Alzheimer’s disease. J Neural Transm (Vienna). 1998;105:269–77.
Carlson MD, Penney JB, Young AB. NMDA, AMPA, and benzodiazepine binding site changes in Alzheimer’s disease visual cortex. Neurobiol Aging. 1993;14:343–52.
Meyer M, Koeppe RA, Frey KA, Kuhl DE, Foster NL. Positron emission tomography measures of benzodiazepine binding in Alzheimer’s disease. Arch Neurol. 1995;52:314–7.
Ohyama M, Senda M, Ishiwata K, Kitamura S, Mishina M, Ishii K, et al. Preserved benzodiazepine receptors in Alzheimer’s disease measured with C-11 flumazenil PET and I-123 iomazenil SPECT in comparison with CBF. Ann Nucl Med. 1999;13:309–15.
Soricelli A, Postiglione A, Grivet-Fojaja MR, Mainenti PP, Discepolo A, Varrone A, et al. Reduced cortical distribution volume of iodine-123 iomazenil in Alzheimer’s disease as a measure of loss of synapses. Eur J Nucl Med. 1996;23:1323–8.
Mohanakrishnan P, Fowler AH, Vonsattel JP, Jolles PR, Husain MM, Liem P, et al. Regional metabolic alterations in Alzheimer’s disease: an in vitro 1H NMR study of the hippocampus and cerebellum. J Gerontol A Biol Sci Med Sci. 1997;52:111–7.
Cross AJ, Crow TJ, Ferrier IN, Johnson JA, Markakis D. Striatal dopamine receptors in Alzheimer-type dementia. Neurosci Lett. 1984;52:1–6.
Boissière F, Faucheux B, Duyckaerts C, Hauw JJ, Agid Y, Hirsch EC. Striatal expression of glutamic acid decarboxylase gene in Alzheimer’s disease. J Neurochem. 1998;71:767–74.
Rissman RA, Bennett DA, Armstrong DM. Subregional analysis of GABAA receptor subunit mRNAs in the hippocampus of older persons with and without cognitive impairment. J Chem Neuroanat. 2004;28:17–25.
Cross AJ, Crow TJ, Ferrier IN, Johnson JA. The selectivity of the reduction of serotonin S2 receptors in Alzheimer-type dementia. Neurobiol Aging. 1986;7:3–7.
Bai X, Edden RAE, Gao F, Wang G, Wu L, Zhao B, et al. Decreased γ-aminobutyric acid levels in the parietal region of patients with Alzheimer’s disease. J Magn Reson Imaging. 2015;41:1326–31.
Li X, Wang Z, Tan L, Wang Y, Lu C, Chen R, et al. Correcting miR92a-vGAT-Mediated GABAergic Dysfunctions Rescues Human Tau-Induced Anxiety in Mice. Mol Ther. 2017;25:140–52.
Govindpani K, Turner C, Waldvogel HJ, Faull RLM, Kwakowsky A. Impaired Expression of GABA Signaling Components in the Alzheimer’s Disease Middle Temporal Gyrus. Int J Mol Sci. 2020;21:1–19.
Lauterborn JC, Scaduto P, Cox CD, Schulmann A, Lynch G, Gall CM, et al. Increased excitatory to inhibitory synaptic ratio in parietal cortex samples from individuals with Alzheimer’s disease. Nat Commun. 2021;12:2603.
Gleichmann M, Zhang Y, Wood WH, Becker KG, Mughal MR, Pazin MJ, et al. Molecular changes in brain aging and Alzheimer’s disease are mirrored in experimentally silenced cortical neuron networks. Neurobiol Aging. 2012;33:205.e1–205.e18.
Owen F, Poulter M, Waddington JL, Mashal RD, Crow TJ. [3H] R05-4864 and [3H] flunitrazepam binding in kainate-lesioned rat striatum and in temporal cortex of brains from patients with senile dementia of the Alzheimer type. Brain Res. 1983;278:373–5.
Zhang H, Zhang L, Zhou D, He X, Wang D, Pan H, et al. Ablating ErbB4 in PV neurons attenuates synaptic and cognitive deficits in an animal model of Alzheimer’s disease. Neurobiol Dis. 2017;106:171–80.
Zhang H, Zhang L, Zhou D, Li H, Xu Y. ErbB4 mediates amyloid β-induced neurotoxicity through JNK/tau pathway activation: Implications for Alzheimer’s disease. J Comp Neurol. 2021;529:3497–512.
Martinez-Losa M, Tracy TE, Ma K, Verret L, Clemente-Perez A, Khan AS, et al. Nav1.1-Overexpressing Interneuron Transplants Restore Brain Rhythms and Cognition in a Mouse Model of Alzheimer’s Disease. Neuron. 2018;98:75–89.e5.
Zheng J, Li HL, Tian N, Liu F, Wang L, Yin Y, et al. Interneuron Accumulation of Phosphorylated tau Impairs Adult Hippocampal Neurogenesis by Suppressing GABAergic Transmission. Cell Stem Cell. 2020;26:331–345.e6.
Levenga J, Krishnamurthy P, Rajamohamedsait H, Wong H, Franke TF, Cain P, et al. Tau pathology induces loss of GABAergic interneurons leading to altered synaptic plasticity and behavioral impairments. Acta Neuropathol Commun. 2013;1:34.
Murari G, Liang DRS, Ali A, Chan F, Mulder-Heijstra M, Verhoeff NPLG, et al. Prefrontal GABA Levels Correlate with Memory in Older Adults at High Risk for Alzheimer’s Disease. Cereb Cortex Commun. 2020;1:1–8.
Wang Y, Wu Z, Bai YT, Wu GY, Chen G. Gad67 haploinsufficiency reduces amyloid pathology and rescues olfactory memory deficits in a mouse model of Alzheimer’s disease. Mol Neurodegener. 2017;12:73.
Thal DR, Rüb U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–800.
Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59.
Brewer AA, Barton B. Visual cortex in aging and Alzheimer’s disease: changes in visual field maps and population receptive fields. Front Psychol. 2014;5:74.
Madsen SK, Ho AJ, Hua X, Saharan PS, Toga AW, Jack CR, et al. 3D maps localize caudate nucleus atrophy in 400 Alzheimer’s disease, mild cognitive impairment, and healthy elderly subjects. Neurobiol Aging. 2010;31:1312–25.
Gellersen HM, Guell X, Sami S. Differential vulnerability of the cerebellum in healthy ageing and Alzheimer’s disease. Neuroimage Clin. 2021;30:102605.
Gellersen HM, Guo CC, O’callaghan C, Tan RH, Sami S, Hornberger M. Cerebellar atrophy in neurodegeneration-a meta-analysis. J Neurol Neurosurg Psychiatry. 2017;88:780–8.
Fukutani Y, Cairns NJ, Rossor MN, Lantos PL. Purkinje cell loss and astrocytosis in the cerebellum in familial and sporadic Alzheimer’s disease. Neurosci Lett. 1996;214:33–6.
Mavroudis I, Petridis F, Kazis D, Njau SN, Costa V, Baloyannis SJ. Purkinje Cells Pathology in Alzheimer’s Disease. Am J Alzheimer’s Dis Other Demen. 2019;34:439–49.
Fukutani Y, Cairns NJ, Rossor MN, Lantos PL. Cerebellar pathology in sporadic and familial Alzheimer’s disease including APP 717 (Val->Ile) mutation cases: a morphometric investigation. J Neurol Sci. 1997;149:177–84.
Manyevitch R, Protas M, Scarpiello S, Deliso M, Bass B, Nanajian A, et al. Evaluation of Metabolic and Synaptic Dysfunction Hypotheses of Alzheimer’s Disease (AD): A Meta-Analysis of CSF Markers. Curr Alzheimer Res. 2018;15:164–81.
Teunissen CE, Verberk IMW, Thijssen EH, Vermunt L, Hansson O, Zetterberg H, et al. Blood-based biomarkers for Alzheimer’s disease: towards clinical implementation. Lancet Neurol. 2022;21:66–77.
Hansson O, Edelmayer RM, Boxer AL, Carrillo MC, Mielke MM, Rabinovici GD, et al. The Alzheimer’s Association appropriate use recommendations for blood biomarkers in Alzheimer’s disease. Alzheimer’s Dement. 2022;18:2669–86.
Blennow K, Zetterberg H. Biomarkers for Alzheimer’s disease: current status and prospects for the future. J Intern Med. 2018;284:643–63.
Acknowledgements
We thank Dr Gregor Gryglewski, who kindly provided the original meta-analysis R code, Dr Gabriel Pires, who helped with methodological questions during the planning and conduction of this work, Dr Victorio Bambini-Junior, who read the first drafts of the paper and provided thoughtful insights, and all the corresponding authors who clarified information on request. We thank BioRender.com for images used in Figs. 5 and 6. Any entity or organization has not directly funded this systematic review with meta-analysis.
Funding
GC-C has received funding from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) [88887.687008/2022-00]. BB receives financial support from CAPES [88887.336490/2019-00] and the Alzheimer’s Association (AA) [AARFD-22-974627]. PCLF has received funding from AA [AARFD-22-923814]. JPF-S receives financial support from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) [200691/2021-0]. JT is funded by the Canadian Institutes of Health Research (CIHR) doctoral award. CT is funded by the Fonds de Recherche du Québec – Santé (FRQS) doctoral award. MAB has received funding from CNPq PDJ [25/2021]. CS has received funding from CAPES [88887.696202/2022-00] and CNPq [141357/2020-7]. TAP is supported by AA [AACSF-20-648075] and the National Institute of Aging [R01AG075336; R01AG073267]. PR-N receives funding from CIHR [MOP-11-51-31 and RFN 152985; 159815; 162303], the Canadian Consortium of Neurodegeneration and Aging (CCNA) [MOP-11-51-31], the Weston Brain Institute, AA [NIRG-12-92090; NIRP-12-259245], the Brain Canada Foundation [34874 and 33397], FRQS [2020-VICO-279314], and Colin J. Adair Charitable Foundation. DOS is supported by CNPQ/INCT [465671/2014-4], CNPQ/FAPERGS/PRONEX [16/2551- 0000475-7], and FAPERGS [19/2551-0000700-0]. ERZ receives financial support from CNPq [312410/2018-2; 435642/2018-9; 312306/2021-0; 409066/2022-2], ARD/FAPERGS [21/2551-0000673-0], AA [AARGD-21-850670], CNPQ/FAPERGS/PRONEX [16/2551-0000475-7], the Brazilian National Institute of Science and Technology in Excitotoxicity and Neuroprotection [465671/2014-4], Instituto Serrapilheira [Serra-1912-31365], and AA and National Academy of Neuropsychology [ALZ-NAN-22-928381]. The funders had no role in the elaboration of the protocol, data collection and analysis, and manuscript preparation.
Author information
Authors and Affiliations
Contributions
Study conception and design: GC-C, BB, CS, and ERZ. Paper screening and data extraction: GC-C, PCLF, JPF-S, and VGR. Data preparation: GC-C and BB. Meta-analysis: GC-C, BB, and MAB. Data interpretation: GC-C, BB, and ERZ. Manuscript preparation and figure elaboration: GC-C. Intellectual content: GC-C, BB, PCLF, JPF-S, VGR, JT, CT, MAB, CS, TAP, PR-N, DOS, ERZ. All authors revised and approved the final version of the paper.
Corresponding author
Ethics declarations
Competing interests
ERZ serves on the scientific advisory board of Next Innovative Therapeutics (Nintx). The other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Carello-Collar, G., Bellaver, B., Ferreira, P.C.L. et al. The GABAergic system in Alzheimer’s disease: a systematic review with meta-analysis. Mol Psychiatry 28, 5025–5036 (2023). https://doi.org/10.1038/s41380-023-02140-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-023-02140-w
This article is cited by
-
Impairments of GABAergic transmission in hippocampus mediate increased susceptibility of epilepsy in the early stage of Alzheimer’s disease
Cell Communication and Signaling (2024)
-
Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases
Signal Transduction and Targeted Therapy (2024)