Abstract
Trauma elicits various adaptive and maladaptive responses among all exposed people. There may be distinctively different patterns of adaptation/maladaptation or types according to neurobiological predisposition. The present study aims to dissect the heterogeneity of posttraumatic conditions in order to identify clinically meaningful subtypes in recently traumatized individuals and evaluate their neurobiological correlates and long-term prognosis. We implemented a data-driven classification approach in both discovery (n = 480) and replication (n = 220) datasets of trauma-exposed and trauma-unexposed individuals based on the clinical data across a wide range of assessments. Subtype-specific patterns of functional connectivity in higher-order cortical networks, longitudinal clinical outcomes, and changes in functional connectivity were also evaluated. We identified four distinct and replicable subtypes for trauma-exposed individuals according to posttraumatic stress symptoms. Each subtype was distinct in clinical characteristics, brain functional organization, and long-term trajectories for posttraumatic symptoms. These findings help enhance current understanding of mechanisms underlying the human-specific heterogeneous responses to trauma. Furthermore, this study contributes data towards the development of improved interventions, including targeting of subtype-specific characteristics, for trauma-exposed individuals and those with PTSD.
Similar content being viewed by others
Introduction
The neurobiology of human responses to trauma extends beyond the fear response found in animals, due to higher-order cortical interactions that are far more advanced [1]. In this context, a substantial proportion of trauma-exposed individuals experiences a range of emotional, behavioral, and cognitive distress or dysfunction, where the extent to which symptom categories or clusters occur vary greatly among individuals [2].
There have been several important studies to characterize the heterogeneous responses after trauma exposure based on clinical data [3,4,5,6] as well as neuroimaging data [7]. These studies, particularly on individuals in the immediate aftermath of trauma, may enable to identify reliable predictors for developing posttraumatic stress disorder (PTSD) among at-risk individuals.
In the current study, we aimed to identify discrete subtypes of posttraumatic stress conditions based on an unsupervised, data-driven cluster analysis of posttraumatic symptoms in recently traumatized individuals using discovery and replication samples. Given that heterogeneity in posttraumatic stress conditions is associated with highly variable outcomes and treatment responses [8, 9], this type of approach for a refined classification of trauma-exposed populations may enable the development of more customized and subtype-specific intervention strategies for trauma-exposed individuals and those with PTSD.
For this classification, we have taken the comprehensive approach of selecting posttraumatic symptoms as candidate features. Specifically, in addition to the typical symptoms related to PTSD such as reexperience and avoidance, a range of behavioral symptoms including impulsivity, anger, and substance abuse have been considered, as each can occur independently or in relation to PTSD as a result of trauma exposure [10, 11]. Also, symptoms of cognitive dysfunction including attention deficits and altered emotion recognition have been taken into account as part of a dimension of posttraumatic stress symptom, since they have been frequently reported in trauma-exposed individuals [12, 13]. Furthermore, we examined the neurobiological distinctiveness of the identified subtypes for trauma-exposed individuals using resting-state functional neuroimaging. Finally, using longitudinal follow-up data, we characterized the trajectories of posttraumatic symptoms as well as functional brain network organization in each subtype of recently traumatized individuals. In these analyses, we identified the specific subtypes among the trauma-exposed individuals that may be at higher risk for PTSD and comorbid conditions. A flowchart of the study process is presented in Supplementary Fig. 1.
Methods
STEP 1: Study participants
Subject recruitment
The current study used the discovery and replication datasets both of which were recruited from the Ewha Brain Institute of Ewha W. University, Seoul, South Korea. The discovery dataset included 240 recently traumatized individuals who reported posttraumatic stress symptoms in relation to the index trauma regardless of PTSD diagnosis (the trauma group). In addition, 240 demographically matched individuals who had not been exposed to any type of traumatic event were recruited and assigned as the control group at a one-to-one ratio (Step 1 of Supplementary Fig. 1). The criteria for demographical matching were sex and age difference of less than 5 years. Similarly, the replication dataset consisted of 110 trauma-exposed and 110 demographically matched trauma-unexposed individuals.
Characteristics of both datasets are summarized in Table 1. A detailed description on the inclusion and exclusion criteria is provided in Supplementary Methods. The study was approved by the Institutional Review Board of Ewha W. University. Written informed consent was obtained from each subject prior to enrollment.
Assessment schedule
The assessment of study participants consisted of two parts: (1) clinical assessments of posttraumatic symptoms and (2) neurobiological assessments using functional magnetic resonance imaging (fMRI) data. Trauma-exposed individuals of the discovery and replication datasets were assessed approximately 7 and 9 months after exposure to index trauma, respectively (time 1 assessment). Among 480 subjects of the discovery dataset, 198 (41.3%, 111 and 87 for the trauma and control groups, respectively) participated in one or more follow-up assessments between 3 and 6 months (time 2 assessment) as well as between 12 and 24 months (time 3 assessment) after time 1 assessment. In order to examine posttraumatic trajectories under the naturalistic conditions, study participants were recommended to make one or more visits during the period of each assessment (3-month and/or 6-month visits after baseline for time 2 assessment; 12-month and/or 24-month visits after baseline for time 3 assessment). There were no significant differences in demographic and clinical characteristics between subjects who only participated in the baseline assessment and those who participated in the follow-up assessments other than educational level (Supplementary Table 1). Clinical and neuroimaging measures from a total of 475 and 415 data points of trauma and control groups, respectively, were used in the longitudinal analysis. Data points of study participants are presented in Supplementary Fig. 2.
STEP 2: Subtype identification and validation
Clinical measurements
In order to identify symptom-based subtypes for recently trauma-exposed individuals in the discovery dataset, scale scores from the Clinician Administered PTSD Scale for DSM-5 (CAPS) [14], Hamilton Depression Rating Scale (HDRS) [15], Alcohol Use Disorder Identification Test (AUDIT) [16], Barratt Impulsiveness Scale (BIS) [17], State-Trait Anger Expression Inventory (STAXI) [18], Digit Span [19], Spatial Span [19], and Emotion Recognition Task (ERT) from the Cambridge Neuropsychological Test Automated Battery Eclipse (CANTAB®, Cambridge Cognition Ltd., Cambridge, UK) [20] were used as measures of posttraumatic symptoms including reexperience, avoidance, depression, alcohol use, impulsivity, anger, auditory attention, spatial attention, and emotional recognition, respectively. For the replication dataset, Trail Making Test (TMT)-A [21], TMT-B [21], and ERT from the CANTAB Connect (Cambridge Cognition Ltd., Cambridge, UK) were used to assess visual attention, focused attention, and emotional recognition, respectively. All other scales were identical to those used in the discovery dataset. A detailed description on scale measurements is provided in Supplementary Methods.
The principal component analysis (PCA) was performed to decompose nine scale measures for posttraumatic symptoms into meaningful constructs using a correlation matrix and a varimax rotation. A scree plot, which is a plot of eigenvalue ordered from largest to smallest, was produced to determine the number of principal components. The number of components retained was selected at the elbow point where the slope of the curve clearly levels off [22]. Meaningful components in both datasets should also meet the eigenvalue criteria of having eigenvalues greater than 1.0 [23]. The extracted principal components were then used (1) to characterize distinct symptom profiles of identified subtypes and (2) as the relevant features for sensitivity analysis of clustering solutions.
Cluster analysis and validation
Prior to clustering analysis, cluster forming tendency was calculated by the Hopkins statistic using Euclidean distance. The Hopkins statistic values of discovery and replication datasets were 0.66 and 0.67, respectively. This value between 0.5 and 1 is generally considered to indicate that the dataset contains a meaningful cluster structure [24, 25].
The scores of the scales mentioned above were converted to standardized z scores. For subtype identification of trauma-exposed individuals, an agglomerative hierarchical cluster analysis was performed using nine individual scale z scores [26]. To examine the stability of cluster solution, we also repeated cluster analysis based on the extracted components from the PCS analysis as a sensitivity analysis. Using Ward’s minimum variance method while considering the squared Euclidean distance, a dendrogram was constructed that suggest the appropriate cluster solution. This cluster solution was considered most optimal for discriminating the subjects into clusters that are maximally dissimilar from each other and could be theoretically interpreted.
The stability of cluster solution was examined using the Jaccard coefficient [27]. In addition, replicability of the cluster solution in the discovery dataset was assessed using a train and test methodology [28]. A k-nearest-neighbors (kNN) model was trained in the discovery dataset based on the cluster labels derived from the hierarchical clustering analysis. This trained kNN model was tested in the replication dataset to obtain new cluster labels. The new cluster labels from the kNN model were compared to those from the hierarchical cluster analysis using kappa statistics.
Cluster analysis was conducted using R version 4.1.1 (https://cran.r-project.org/bin/windows/base/).
STEP 3: Subtype characterization
Control group assignment
For examining subtype-specific alterations in functional connectivity by the comparison with demographically matched trauma-unexposed healthy individuals, trauma-unexposed individuals were assigned to the corresponding control group for each subtype (Step 3 of Supplementary Fig. 1) according to the labels of their previously matched trauma-exposed subjects (refer to Step 1 of Supplementary Fig. 3).
Neurobiological assessments
High-resolution structural and resting-state fMRI scans were obtained for the discovery and replication datasets using a 3.0 Tesla Philips Achieva MRI scanner and 3.0 Tesla Philips dStream MRI scanner (Philips Medical System, Netherlands), respectively, both of which were equipped with a 32-channel head coil.
Detailed information regarding the parameters to obtain MRI data, data preprocessing, and the construction of the functional connectivity matrix is described in Supplementary Methods.
In the current study, we have focused on the functional connectivity alteration in higher-order cognitive networks including the salience/cingulo-opercular (SAL), frontoparietal (FPT), default mode (DM), and orbitofrontolimbic (LIMB) networks, all of which have been known to be involved in the modulation of emotional responses [29, 30]. Specifically, alterations in these functional networks may underlie unique clinical features of PTSD [31,32,33] as well as identify the differences and similarities of PTSD and other anxiety disorders [34]. The four networks were selected as network-of-interests (NOIs) for subsequent analyses. As such, mean functional connectivity values of the NOIs, which represent network connectivity, were also calculated for each subject by averaging all functional connectivity values of every edge constituting the respective NOIs.
Between-group differences in functional connectivity
Group differences in mean functional connectivity values of NOIs at baseline were assessed for each subtype using linear regression analyses after adjusting for age and sex. Mixed-effects linear regression analysis with the group membership as a fixed effect (trauma vs. control) and within-subject dependence as a random-effect was used to examine between-group differences in mean functional connectivity values of NOIs at each time 2 and 3 assessment. Age and sex were included as covariates. An empirical two-tailed P value was defined as the proportion of group-membership resampled data (n = 5000) in which the permuted null distribution is greater than the observed values from the actual data [35]. The Benjamini-Hochberg procedure at a false discovery rate (FDR) of 0.05 was applied to account for multiple comparisons [36]. Statical analyses were performed using STATA version 16.1 (Stata Corp, College Station, Texas).
Longitudinal trajectories of the posttraumatic symptoms or functional connectivity
For the examination of posttraumatic trajectories of PTSD diagnosis in the discovery dataset, mixed-effects logistic regression analysis was applied to examine the time effects on the prevalence of PTSD diagnosis in each subtype. Age and sex were included into the model as covariates. Using mixed-effects regression analysis, time effects on CAPS subscale scores, composite scores of the three posttraumatic symptom domains were examined in each subtype. Time effects on mean functional connectivities of NOIs were also assessed in each subtype using mixed-effects regression analysis after adjusting for age and sex.
All tests were two-tailed and performed using STATA version 16.1 (Stata Corp, College Station, Texas).
Results
Proposed subtypes for recently traumatized individuals
In order to characterize dimensional symptom profiles of the identified subtypes, nine individual posttraumatic symptoms were categorized into meaningful constructs (principal component) by the PCA. Three distinct symptom domains were extracted as to represent classic PTSD symptoms and depression (principal component 1, reexperience, avoidance, and depression; hereafter referred to as “posttraumatic symptom domain A”), externalizing symptoms and behavioral problems (principal component 3, alcohol use, impulsivity, and anger; hereafter referred to as “posttraumatic symptom domain B”), and attention and cognitive problems (principal component 2, auditory attention, spatial attention, and emotional recognition; hereafter referred to as “posttraumatic symptom domain C”) in both discovery and replication datasets (Supplementary Fig. 3 and Supplementary Table 2). In the discovery dataset, three principal components explained for 67.7% of the total variance while 70.7% of the total variance was explained by three principal components retained in the replication dataset (Supplementary Table 2). A composite score for each symptom domain was calculated by averaging the standardized scale scores that constitute the respective symptom domain and used to characterize subtype-specific clinical features.
A data-driven clustering approach was implemented to classify the 240 trauma-exposed individuals into discrete subtypes based on 9 individual scale scores for posttraumatic symptoms including reexperience, avoidance, depression, alcohol use, impulsivity, anger, auditory attention, spatial attention, and emotional recognition.
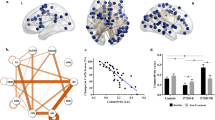
In the discovery dataset, agglomerative hierarchical cluster analysis identified four subtypes of trauma-exposed individuals, each with a significantly different posttraumatic symptom pattern (Fig. 1a–b).
a Dendrogram resulting from agglomerative hierarchical cluster analysis on trauma-exposed individuals of the discovery dataset. b Distinct patterns of symptom profiles (radar charts) and composite scores for each symptom domain (bar graphs) across all four subtypes of the discovery dataset. c, d Similar results that were obtained in trauma-exposed individuals of the replication dataset. Error bars in the bar graphs represent the standard errors of mean. A-sym, posttraumatic symptom domain A, B-sym, posttraumatic symptom domain B; C-sym, posttraumatic symptom domain C.
Subtype 1 consisted of 75 (31.3%) of the 240 trauma-exposed individuals and was characterized by relatively lower scale scores for all three symptom domains as compared with other subtypes. Subtype 2, which included 72 trauma-exposed individuals (30.0%) showed higher scores on the posttraumatic symptom domain A, as compared to subtype 1. Subtype 3 consisted of 39 trauma-exposed individuals (16.3%) and was similar in nature to subtype 2 with the addition of higher scores on alcohol use, impulsivity, and anger. Subtype 4, which comprised 54 individuals (22.5%), showed low performance on attention and emotional recognition. In addition to the dendrogram in Fig. 1a, these four subtype structures are also clearly represented in the 3-dimensional cluster plot and dissimilarity matrix to support the visualize the meaningfulness of the clusters (Supplementary Figs. 4a and 5a).
Agglomerative hierarchical cluster analysis on the replication dataset yielded similar results for subtype characteristics to those of the discovery dataset (Fig. 1c, d and Supplementary Figs. 4b and 5b).
Results for cluster stability, the replicability of cluster solution, and the repeated analyses for clustering with other feature options, all of which strongly supported the robustness of the clustering results are provided in Supplementary Results and Supplementary Fig. 6.
Further details on differences in demographic and clinical characteristics across the subtypes of both discovery and replication datasets are described in Supplementary Results, Supplementary Tables 3 and 4.
Subtype-specific alterations in brain functional connectivity
To examine subtype-specific patterns of functional connectivity alterations, we compared functional brain network organization between trauma-exposed individuals in each subtype and its corresponding control group (Fig. 2).
a Functional connectivity matrices constructed by averaging the edge-wise functional connectivity values of all control subjects (discovery dataset, n = 413; replication dataset, n = 110). Anatomical locations of brain regions that constitute the NOIs are projected onto an inflated cortical surface. b Mean functional connectivities of NOIs were compared between each trauma subtype and respective control group for the discovery and replication datasets. Standardized difference in functional connectivity at every edge of the NOIs between each trauma subtype and the corresponding control group presented in 2-D matrices. Yellow and blue edges indicate greater and lower functional connectivity in the trauma group relative to the control group, respectively. NOIs showing significant between-group differences in mean functional connectivity are highlighted in red boxes. Bar graphs represent mean functional connectivity of each NOI. Error bars in the bar graphs represent the standard errors of mean. NOI network-of-interest, SAL salience/cingulo-opercular, FPT frontoparietal, DM default mode, LIMB orbitofrontolimbic, SSM somatosensory-motor, PDM posterior default mode, VIS visual.
In the comparison between the trauma (n = 75) and control (n = 75) groups of subtype 1 in the discovery dataset, the significant group difference in mean functional connectivity was found in the LIMB network (β = −0.22, P = 0.006) after FDR correction, but not in other NOIs (SAL, β = −0.13, P = 0.116; FPT, β = 0.01, P = 0.922; DM, β = −0.06, P = 0.417).
Trauma-exposed individuals of subtype 2 (n = 72) had lower functional connectivity of the SAL network (β = −0.23, P = 0.008) as compared to the corresponding control group (n = 72) after FDR correction. There were no significant differences in mean functional connectivity of other NOIs between the trauma and control groups of subtype 2 (FPT, β = −0.02, P = 0.776; DM, β = −0.06, P = 0.473; LIMB, β = −0.04, P = 0.622).
On the other hand, trauma-exposed individuals of subtype 3 were characterized with higher functional connectivity of the DM network. Specifically, there was a significant difference in mean functional connectivity of the DM network between the trauma (n = 39) and control (n = 38) groups of subtype 3 (β = 0.30, P = 0.007) after FDR correction. The mean functional connectivity of other NOIs did not differ between the two groups (SAL, β = −0.02, P = 0.824; FPT, β = 0.12, P = 0.315; LIMB, β = 0.15, P = 0.205).
Functional connectivity alterations were prominent in the FPT network of trauma-exposed individuals (n = 54) of subtype 4 as compared to the corresponding control group (n = 54)(β = −0.20, P = 0.043). However, this between-group difference did not survive FDR correction. In the replication dataset, a significant difference in mean functional connectivity of FPT was found between the trauma (n = 26) and control (n = 26) groups of subtype 4 (β = −0.36, P = 0.011) after FDR correction. Between-group difference was not found in other NOIs (SAL, β = −0.15, P = 0.143; DM, β = −0.14, P = 0.142; LIMB, β = −0.07, P = 0.470).
The findings regarding subtype-specific alterations in functional connectivity of the higher-order cognitive networks were replicated in the comparisons between the trauma and control groups of the replication dataset. A similar trend for the between-group differences in mean functional connectivity of the NOIs was found in the replication dataset despite having used different acquisition parameters for resting-state fMRI data (Fig. 2b and Supplementary Table 5).
We further explored our clinical subtypes beyond the general functional connectivity circuits in existing literature, by assessing the associations of our subtypes with previously reported functional brain circuits that are specific and related to PTSD: these include circuits related with emotional regulation and executive function, threat and salience detection, contextual processing, and fear learning [37] (Supplementary Table 6). Specific brain regions constituting each of these four circuits are presented in Supplementary Table 7. We compared the mean functional connectivity within these four brain circuits between the trauma group of each subtype and the corresponding control group. A significant difference in mean functional connectivity of the circuit subserving emotional regulation and executive function was found in the trauma and control groups of subtype 4 (discovery dataset, β = −0.20, P = 0.044; replication dataset, β = −0.35, P = 0.009). There was a significant difference in mean functional connectivity of the circuit for threat and salience detection between the trauma and control groups of subtype 2 (discovery dataset, β = −0.21, P = 0.011; replication dataset, β = −0.26, P = 0.031). Between-group differences in mean functional connectivity of the circuit subserving contextual processing were found in subtype 2 (discovery dataset, β = −0.20, P = 0.017; replication dataset, β = −0.27, P = 0.021). Furthermore, mean functional connectivity of the circuit for fear learning was higher in the trauma group of subtype 3 as compared with the corresponding control group (discovery dataset, β = 0.26, P = 0.024). Detailed statistical information on the between-group comparisons is provided in Supplementary Table 6.
Subtype-specific posttraumatic stress symptom trajectories
We first assessed the changes in PTSD symptoms over time for each subtype (Fig. 3). Most trauma-exposed individuals of subtype 2 (100%), subtype 3 (100%), and subtype 4 (94.4%) were diagnosed with lifetime PTSD following the index trauma, while the prevalence of lifetime PTSD was much lower in trauma-exposed individuals of subtype 1 (69.3%). For the diagnosis of current PTSD at time 1 assessment, significantly lower prevalence of PTSD was found in trauma-exposed individuals of subtype 1 (24.0%), but not in other trauma groups (97.2% for subtype 2; 89.7% for subtype 3; 75.9% for subtype 4).
We examined whether the status of PTSD diagnosis (stacked bar graphs in the left column), PTSD symptom severity (line graphs in the center column), and scale scores of the four symptom domains of PTSD (bar graph in the right 4 columns) changed over time in each subtype using mixed-effects logistic regression analyses and mixed-effects linear regression analyses, appropriately. Age and sex were included as relevant covariates into the models. The diagnosis and symptom severity of PTSD were measured using the CAPS. Overall results demonstrated distinct trajectories of PTSD symptoms according to subtypes. Results from the (a) subtype 1, (b) subtype 2, (c) subtype 3, and (d) subtype 4 are presented. Error bars in the bar graphs represent the standard errors of mean. Abbreviations: PTSD, posttraumatic stress disorder; CAPS, Clinician-Administered Posttraumatic Stress Disorder Scale for DSM-5; T0, at least 1 month after trauma; T1, time 1 assessment; T2, time 2 assessment; T3, time 3 assessment.
Results from the follow-up assessments indicated that the prevalence of being diagnosed with current PTSD significantly reduced over time in subtype 2 (z = −3.21, P = 0.001) and subtype 4 (z = −2.34, P = 0.019). However, in subtype 3, a high prevalence of current PTSD persisted throughout follow-up (z = −0.48, P = 0.628).
A similar trend was observed in the trajectories of typical PTSD symptom domains. A significant improvement in PTSD symptom severity of all four domains as measured by the CAPS was observed in trauma-exposed individuals of subtypes 1, 2, and 4. In contrast, there were no changes in severity of each symptom domain in trauma-exposed individuals of subtype 3. Detailed information on statistical values of these longitudinal analyses is provided in Supplementary Table 8.
Next, we assessed the subtype-specific changes in the composite scores of the posttraumatic symptom domains throughout follow-up assessments (Fig. 4). In subtype 1, a significant improvement in composite scores was found in the posttraumatic symptom domains A and C (domain A, z = −4.93, P < 0.001; domain C, z = −4.39, P < 0.001). A lower composite score of the posttraumatic symptom domain B at baseline assessment persisted and did not change throughout follow-up assessments (z = −0.43, P = 0.670).
Trajectories of three posttraumatic stress symptom domains were examined in subtype 1 (a), subtype 2 (c), subtype 3 (e), and subtype 4 (g). Radar charts (left) represent changes in each dimensional symptom and bar graphs (center) indicate changes in composite scores for posttraumatic symptom domains A, B, and C. P values in the bar graphs indicate time effects on domain symptom severity. Time effects on the functional connectivity of NOIs were examined in subtype 1 (b), subtype 2 (d), subtype 3 (f), and subtype 4 (h) and respective control. P values in the bar graphs indicate time effects on the mean functional connectivity of NOIs. Error bars in the bar graphs represent the standard errors of mean.
Trauma-exposed individuals of subtype 2 exhibited a significant decline in composite scores of both posttraumatic symptom domains A and C (domain A, z = −8.19, P < 0.001; domain C, z = −3.76, P < 0.001). In contrast, composite scores of the posttraumatic symptom domain B increased over time in subtype 2 (z = 3.71, P < 0.001).
In subtype 3, although a decreasing trend of composite scores for the posttraumatic symptom domain A was observed (z = −2.37, P = 0.018), there were no significant changes in the severity of symptoms for the posttraumatic symptom domains B and C (domain B, z = −1.70, P = 0.089; domain C, z = −1.46, P = 0.144).
Trauma-exposed individuals of subtype 4 demonstrated a significant improvement in severity of the posttraumatic symptom domains A and C (domain A, z = −4.08, P < 0.001; domain C, z = −7.26, P < 0.001). In addition, a low severity in terms of the posttraumatic symptom domain B as demonstrated at time 1 assessment persisted throughout follow-up (z = −1.31, P = 0.192).
Changes in individual scale scores over time were also analyzed in each subtype and the results are presented in Supplementary Table 9.
Subtype-specific trajectories of functional connectivity
We examined whether subtype-specific functional connectivity alterations at time 1 assessment (approximately 7 months since the index trauma) changed over time in the discovery dataset (Fig. 4 and Supplementary Fig. 7). We first examined time effects on the mean functional connectivity of the NOIs in each trauma group and respective control group (Fig. 4). Then, the mean functional connectivity of the NOIs, which showed significant group differences at time 1 assessment, was compared between the trauma subtype group and its respective control group at each assessment (Supplementary Fig. 7).
In subtype 1, functional connectivity alterations in the LIMB network as observed at the initial assessment were normalized over time (Fig. 4b). Specifically, between-group differences in the mean functional connectivity of the LIMB network were no longer significant at time 2 (z = −0.82, P = 0.438) and 3 (z = 0.19, P = 0.866) assessments. In addition, time effects on the mean functional connectivity of the LIMB network were significant in the trauma group (z = 2.23, P = 0.026), but not in the control group (z = −0.76, P = 0.450) of subtype 1.
For trauma-exposed individuals of subtype 2, lower functional connectivity of the SAL network, as observed in time 1 assessment, was normalized over time (Fig. 4d). Specifically, the mean functional connectivity of the SAL network no longer differed between the trauma and control groups at time 2 (z = 0.05, P = 0.949) and 3 (z = 0.53, P = 0.506) assessments. Furthermore, mean functional connectivity of the SAL network significantly increased over time in the trauma group (z = 2.09, P = 0.036), while no significant changes were observed in the control group of subtype 2 (z = −0.96, P = 0.339). A similar pattern of normalization was observed in the functional connectivity of the FPT network in the trauma group of subtype 4 as compared with the corresponding control group (Fig. 4h), where group differences in mean functional connectivity of the FPT network were no longer present at time 2 (z = −0.77, P = 0.428) and 3 (z = −0.49, P = 0.650) assessments. Moreover, a significant increment in mean functional connectivity of the FPT network was observed in the trauma group (z = 4.57, P < 0.001), while these changes were not observed in the control group of subtype 4 (z = 0.34, P = 0.736).
In contrast, functional connectivity alterations in the DM network at the baseline assessment persisted over time in trauma-exposed individuals of subtype 3 (Fig. 4f). Group differences in mean functional connectivity of the DM network between the trauma and control groups of subtype 3 were significant across all assessments including time 1, time 2 (z = 2.08, P = 0.042), and time 3 (z = 3.33, P = 0.024) assessments. In addition, there was no significant time effect on the mean functional connectivity of the DM network in both the trauma (z = 1.19, P = 0.233) and control (z = 0.45, P = 0.655) groups of subtype 3.
Discussion
Using a data-driven clustering of recently traumatized individuals, we identified four robust and neurobiologically distinct subtypes of trauma-exposed individuals, each with specific clinical profiles. The subtype-specific characteristics were successfully replicated in the replication dataset, demonstrating the reliability of the current subtyping approach. The characteristics of four identified subtypes were further validated and supported by subsequent fMRI analyses that suggested distinct functional brain organization, particularly of the higher-order cognitive networks, across subtypes. Each subtype showed differential clinical and neurobiological outcomes across a 2-year longitudinal follow-up analysis, demonstrating subtype-specific distinct longitudinal trajectories of brain functional organizations. This clustering of recently traumatized individuals may enhance the understanding the long-term prognosis of individuals following trauma exposure according to subtypes and may help to provide potential subtype-specific approaches for early and targeted intervention.
Compared to other subtypes, trauma-exposed individuals of subtype 1 were less likely to be diagnosed with PTSD for an index trauma and had less severe symptoms with substantial improvement over time in all PTSD symptom domains. Considering that there were no differences in trauma-related characteristics across the four subtypes including type of trauma and time elapsed since trauma (Supplementary Results), clinical features specific to the subtype 1 may be suggested as a reliable predictor of subtype-specific PTSD trajectory.
Brain regions of the LIMB network encompassing the medial prefrontal cortex and limbic structures have been suggested to be actively involved in the process of resilience [38, 39]. In particular, emotional regulation through the downregulation of medial prefrontal-limbic structures may render traumatized individuals more resilient to the development of PTSD [38]. This notion is partly supported by our finding that the extent of lower functional connectivity of the LIMB network in trauma-exposed individuals relative to their matched control subjects was greater in subtype 1 relative to other subtypes. Notably, these functional alterations in the LIMB network may be normalized over time with improved posttraumatic symptoms. This longitudinal finding supports the previous notions that the brain mechanism involved in resilience is dynamic in nature, and are changeable over time [40, 41].
Individuals who were classified as subtype 2 were characterized by notable symptoms under the posttraumatic symptom domain A such as classic PTSD symptoms and emotional problems, while the severity of other symptom domains was comparable to that observed in the corresponding control group. The functional network analysis showed connectivity of the SAL network was typically lower in the trauma group of subtype 2 as compared to the corresponding control group. Disrupted function of the SAL network in the processing of salient stimuli in the environment as well as in cognitive emotional processing may contribute to the clinical features specific for subtype 2 [29, 31, 42]. Here, it is noteworthy that such functional alterations may be state-dependent rather than persistent, as these alterations normalized over time with improved emotional symptoms. The majority of individuals under subtype 2 (97.2%) were diagnosed with current PTSD at time 1 assessment. For trajectories of typical symptoms of PTSD, substantial improvements in all four PTSD symptom domains as well as a significant reduction in incidence of PTSD diagnosis were observed over time in this subtype. Despite overall improvement, longitudinal observation of this subtype exhibited an increasing trend of symptoms under the posttraumatic symptom domain B such as alcohol use and anger. However, these behavioral symptoms were subthreshold and the overall severity of symptoms was much lower as compared to those of subtype 3. The current findings suggest the need for increased alertness to the late-onset of subthreshold behavioral distress in traumatized individuals of subtype 2, even after the improvement of traditional PTSD symptoms.
In contrast to the clinical symptom profiles of subtype 2 which are close in appearance to the traditional PTSD diagnostic criteria under the CAPS, subtype 3 represents a novel and more severe form of posttraumatic stress condition that may have been masked within the diagnosis of PTSD. Trauma-exposed individuals who were categorized into subtype 3 were relatively younger with a mean age of 34.4 years in the discovery dataset (a mean age of 32.9 years in the replication dataset) and exhibited high levels of symptoms under the posttraumatic symptom domain B, such as anger, impulsivity, and alcohol use. Individuals in this subtype were also characterized with more severe classic PTSD and emotional symptoms constituting the posttraumatic symptom domain A. The current findings suggest that externalizing symptoms combined with emotional distress may be associated with poorer outcomes in trauma-exposed individuals [43, 44]. For instance, while the overall scale scores for PTSD symptoms demonstrated a slight improvement over time, the prevalence of PTSD diagnosis did not change significantly throughout follow-up. Therefore, subtype 3 may be at greater risk with respect to the development of PTSD and comorbid conditions associated with externalizing symptoms and behavioral problems. As such, early and specialized interventions that target individuals under this subtype may aid in improving the prognosis of PTSD.
For the brain network mechanisms, higher functional connectivity was continually observed in the DM network of trauma-exposed individuals under subtype 3 throughout the study period, as compared to the corresponding control group. Given the critical role of suppression of the DM network in attention shift to external stimuli [42, 45], increased aberrant connectivity in the DM network may be related to behavioral distress that results from impaired concentration or attention difficulties [46]. Failure to de-activate the DM network may also be involved in behavioral problems such as impulsivity [46, 47], aggression [48, 49], and addiction [50, 51]. Given the persistent nature of altered functional connectivity of the DM network in subtype 3, compensatory mechanisms to regulate emotional and behavioral distress may also be an alternative explanation for these results.
It should be noted that the current design, which has no pre-exposure observation, could not assure that greater externalizing symptoms and behavioral problems in trauma-exposed individuals of subtype 3 were the result from trauma exposure. For instance, although the current study subjects were not diagnosed with any significant psychiatric disorder prior to trauma exposure, these characteristics may also reflect a pre-exposure disposition. This is indeed possible in that alterations in functional connectivity of the DM network are maintained throughout the follow-up period. Likewise, previous studies have reported that a high level of pre-exposure tendency for hostility, anger, or alcohol misuse may increase the vulnerability for developing PTSD and predict severe symptoms such as violent offending after trauma exposure [52,53,54]. Furthermore, although none of study participants were diagnosed with any personality disorder(s), trauma-exposed individuals of subtype 3 showed higher scores in the Personality Diagnostic Questionnaire Version 4 (PDQ-4) than those of other subtypes (Supplementary Results), potentially indicating their subthreshold personality difficulties. Given the previous prospective studies reporting the predictive role of personality traits in the development and severity of posttraumatic stress symptoms [55,56,57], pre-existing personality traits may influence the symptom prevalence and severity in trauma-exposed individuals of subtype 3. However, since the current study did not assess personality traits prior to trauma exposure, it cannot be ruled out that higher scale scores in the PDQ-4 in individuals of subtype 3 may merely reflect their posttraumatic symptom profile.
Approximately over 20% of the trauma-exposed individuals (22.5% and 23.6% in the discovery and replication datasets, respectively) were categorized into subtype 4. Along with the presentation of symptoms clustered into the posttraumatic symptom domain A, individuals under this subtype exhibited attention deficits and lower cognitive performance relative to their age- and sex-matched control group. Additionally, trauma-exposed individuals of subtype 4 were relatively older than those of the other subtypes (a mean age of 50.6 years in the discovery dataset and 55.0 years in the replication dataset). Previous studies reported that attentional deficits and impaired emotion recognition are frequently observed in relation to trauma exposure [12, 13]. Although there was gradual improvement, trauma-exposed individuals of subtype 4 continued to report domain C symptoms such as attention and cognitive problems that persisted at time 3 assessment (approximately 23.7 months after trauma). More importantly, these symptoms persisted even when with the absence of PTSD diagnosis. Consistent with previous findings [32, 58], our functional network analysis reveals that hypoconnectivity of the FPT network may contribute to diminished cognitive functions of subtype 4. This explanation is plausible as the FPT network is known to play an important role in attentional control and working memory, both of which are frequently impaired in PTSD [32, 59, 60].
Exploratory analysis on the brain circuits implicated in the pathophysiology of PTSD [37] also support the subtype-specific alterations in functional brain organization. Given the regional similarity between the SAL network vs. the circuit subserving threat and salience detection as well as the FPT network vs. the circuit subserving emotional regulation and executive function, it is expected that the results regarding subtype-specific functional alterations were comparable between these brain regions. Interestingly, functional connectivity alterations in the circuit for contextual processing were found in the trauma group of subtype 2, as compared to the respective control group, which may suggest prevalence of traditional PTSD symptoms in this subtype. This finding corroborated the important role of dysregulated hippocampal-prefrontal-thalamic circuit in the pathophysiology of PTSD by deficient contextual processing [37, 61].
It should be noted that the types of index trauma and the experience of lifetime stressors were similar across the four identified subtypes. These findings suggested that the types of trauma and the experience of lifetime stressors other than the index trauma may not determine the subtype-specific characteristics in trauma-exposed individuals.
The present study found that posttraumatic stress symptom profiles specific for each subtype are maintained longitudinally despite changes in symptom severity (the radar charts of Fig. 4). These findings suggest that the four identified subtypes are stable and empirically distinct, rather than represent the different stages within the same posttraumatic stress condition. Our notion is further supported through the subtype-specific brain functional organization observed. However, it is noteworthy that the current stage in research cannot determine whether this clustering solution also differ in etiologies or genetic backgrounds. Moreover, further research is warranted to determine whether our subtyping approach is the optimal classification method of traumatized populations.
There is a relatively high rate of attrition from time 1 assessment to time 3 assessment in the current study. This may potentially limit the value of our longitudinal trajectory findings and should be considered as a limitation of the study. A high attrition rate is common in cohort studies of trauma-exposed individuals [1, 62] and problematic especially when it appears in a non-random way [63]. In the current study, however, there were no significant differences in demographic and clinical characteristics between subjects who were retained across longitudinal assessments and those who participated only at baseline. This suggests that dropouts may have occurred randomly.
As trauma-exposed individuals were first assessed at approximately 7 months after the index trauma, the identification of trajectories of multidimensional responses in the immediate aftermath of trauma may have been precluded. Future longitudinal studies that assess trauma-exposed individuals immediately after trauma may enable the characterization of acute trauma response under naturalistic conditions.
To conclude, the present study identified four robust subtypes with clinical relevance for recently traumatized individuals. This method of classification was also supported in the neurobiological distinctiveness of each subtype as observable through brain functional network analysis. Given the widely recognized complexity of human neurobiological responses to trauma exposure, the current explorative study may provide additional knowledge to the subtype-specific trajectories after trauma in recent trauma survivors. In addition, knowledge gleaned from this study may provide foundational and evidence-based theoretical grounds for future design and development of innovative, targeted treatment strategies such as those that are subtype-specific in trauma-exposed individuals.
References
Lyoo IK, Kim JE, Yoon SJ, Hwang J, Bae S, Kim DJ. The neurobiological role of the dorsolateral prefrontal cortex in recovery from trauma. Longitudinal brain imaging study among survivors of the South Korean subway disaster. Arch Gen Psychiatry. 2011;68:701–13.
Fink DS, Lowe S, Cohen GH, Sampson LA, Ursano RJ, Gifford RK, et al. Trajectories of posttraumatic stress symptoms after civilian or deployment traumatic event experiences. Psychol Trauma. 2017;9:138–46.
Andersen SB, Karstoft KI, Bertelsen M, Madsen T. Latent trajectories of trauma symptoms and resilience: the 3-year longitudinal prospective USPER study of Danish veterans deployed in Afghanistan. J Clin Psychiatry. 2014;75:1001–8.
Bonanno GA, Mancini AD, Horton JL, Powell TM, Leardmann CA, Boyko EJ, et al. Trajectories of trauma symptoms and resilience in deployed U.S. military service members: prospective cohort study. Br J Psychiatry. 2012;200:317–23.
Schultebraucks K, Shalev AY, Michopoulos V, Grudzen CR, Shin SM, Stevens JS, et al. A validated predictive algorithm of post-traumatic stress course following emergency department admission after a traumatic stressor. Nat Med. 2020;26:1084–8.
Schultebraucks K, Qian M, Abu-Amara D, Dean K, Laska E, Siegel C, et al. Pre-deployment risk factors for PTSD in active-duty personnel deployed to Afghanistan: a machine-learning approach for analyzing multivariate predictors. Mol Psychiatry. 2020;26:5011–22.
Stevens JS, Harnett NG, Lebois LAM, van Rooij SJH, Ely TD, Roeckner A, et al. Brain-based biotypes of psychiatric vulnerability in the acute aftermath of trauma. Am J Psychiatry. 2021;178:1037–49.
DiMauro J, Carter S, Folk JB, Kashdan TB. A historical review of trauma-related diagnoses to reconsider the heterogeneity of PTSD. J Anxiety Disord. 2014;28:774–86.
Koek RJ, Schwartz HN, Scully S, Langevin JP, Spangler S, Korotinsky A, et al. Treatment-refractory posttraumatic stress disorder (TRPTSD): a review and framework for the future. Prog Neuropsychopharmacol Biol Psychiatry. 2016;70:170–218.
Jakupcak M, Conybeare D, Phelps L, Hunt S, Holmes HA, Felker B, et al. Anger, hostility, and aggression among Iraq and Afghanistan War veterans reporting PTSD and subthreshold PTSD. J Trauma Stress. 2007;20:945–54.
Orth U, Wieland E. Anger, hostility, and posttraumatic stress disorder in trauma-exposed adults: a meta-analysis. J Consult Clin Psychol. 2006;74:698–706.
McNally RJ. Cognitive abnormalities in post-traumatic stress disorder. Trends Cogn Sci. 2006;10:271–7.
Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62:273–81.
Weathers FW, Bovin MJ, Lee DJ, Sloan DM, Schnurr PP, Kaloupek DG, et al. The Clinician-Administered PTSD Scale for DSM-5 (CAPS-5): Development and initial psychometric evaluation in military veterans. Psychol Assess. 2018;30:383–95.
Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62.
Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption-II. Addiction. 1993;88:791–804.
Barratt E. Impulsiveness and aggression. In: Monahan J, Steadman H, editors. Violence and mental disorder: Developments in risk assessment. Chicago and London: The University of Chicago Press; 1994, pp 61–79.
Spielberger C. Manual for the stale-trait anger expression inventory (STAXI). Odessa: Psychological Assessment Resources; 1988.
Wechsler D. Wechsler adult intelligence scale–Fourth Edition (WAIS–IV). San Antonio: NCS Pearson; 2008.
Strauss E, Sherman EM, Spreen O. A compendium of neuropsychological tests: Administration, norms, and commentary. New York: Oxford University Press; 2006.
Reitan RM, Wolfson D. The Halstead-Reitan neuropsychological test battery: Theory and clinical interpretation. Tuscon: Neuropsychology press; 1985.
Cattell RB. The scree test for the number of factors. Multivar Behav Res. 1966;1:245–76.
Kaiser HF. The application of electronic computers to factor analysis. Educ Psychol Meas. 1960;20:141–51.
Hopkins B, Skellam JG. A new method for determining the type of distribution of plant individuals. Ann Bot. 1954;18:213–27.
Banerjee A, Dave RN. Validating clusters using the Hopkins statistic. IEEE International Conference on Fuzzy Systems. 2004:149–53.
Ward JH Jr. Hierarchical grouping to optimize an objective function. J Am Stat Assoc. 1963;58:236–44.
Hennig C. Cluster-wise assessment of cluster stability. Comput Stat Data. 2007;52:258–71.
Halkidi M, Batistakis Y, Vazirgiannis M. On clustering validation techniques. J Intell Inf Sys. 2001;17:107–45.
Xu J, Van Dam NT, Feng C, Luo Y, Ai H, Gu R, et al. Anxious brain networks: A coordinate-based activation likelihood estimation meta-analysis of resting-state functional connectivity studies in anxiety. Neurosci Biobehav Rev. 2019;96:21–30.
Langner R, Leiberg S, Hoffstaedter F, Eickhoff SB. Towards a human self-regulation system: Common and distinct neural signatures of emotional and behavioural control. Neurosci Biobehav Rev. 2018;90:400–10.
Nicholson AA, Harricharan S, Densmore M, Neufeld RWJ, Ros T, McKinnon MC, et al. Classifying heterogeneous presentations of PTSD via the default mode, central executive, and salience networks with machine learning. Neuroimage Clin. 2020;27:102262.
Patel R, Spreng RN, Shin LM, Girard TA. Neurocircuitry models of posttraumatic stress disorder and beyond: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2012;36:2130–42.
Akiki TJ, Averill CL, Abdallah CG. A network-based neurobiological model of PTSD: evidence from structural and functional neuroimaging studies. Curr Psychiatry Rep. 2017;19:81.
Peterson A, Thome J, Frewen P, Lanius RA. Resting-state neuroimaging studies: a new way of identifying differences and similarities among the anxiety disorders? Can J Psychiatry. 2014;59:294–300.
Westfall PH, Young SS. Resampling-based multiple testing: Examples and methods for p-value adjustment. New York: John Wiley & Sons; 1993.
Benjamini Y, Hochberg Y. Controlling for the false discovery rate: A practical and power approach to multiple testing. J R Stat Soc. 1995;57:289–300.
Shalev A, Liberzon I, Marmar C. Post-traumatic stress disorder. N. Engl J Med. 2017;376:2459–9.
Moreno-López L, Ioannidis K, Askelund AD, Smith AJ, Schueler K, van Harmelen AL. The resilient emotional brain: A scoping review of the medial prefrontal cortex and limbic structure and function in resilient adults with a history of childhood maltreatment. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;5:392–402.
Insana SP, Banihashemi L, Herringa RJ, Kolko DJ, Germain A. Childhood maltreatment is associated with altered frontolimbic neurobiological activity during wakefulness in adulthood. Dev Psychopathol. 2016;28:551–64.
Rutter M. Resilience as a dynamic concept. Dev Psychopathol. 2012;24:335–44.
Kalisch R, Cramer AOJ, Binder H, Fritz J, Leertouwer I, Lunansky G, et al. Deconstructing and reconstructing resilience: a dynamic network approach. Perspect Psychol Sci. 2019;14:765–77.
Fischer AS, Keller CJ, Etkin A. The clinical applicability of functional connectivity in depression: Pathways toward more targeted intervention. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1:262–70.
Blanco C, Xu Y, Brady K, Pérez-Fuentes G, Okuda M, Wang S. Comorbidity of posttraumatic stress disorder with alcohol dependence among US adults: results from National Epidemiological Survey on Alcohol and Related Conditions. Drug Alcohol Depend. 2013;132:630–8.
Elbogen EB, Wagner HR, Fuller SR, Calhoun PS, Kinneer PM, Beckham JC. Correlates of anger and hostility in Iraq and Afghanistan war veterans. Am J Psychiatry. 2010;167:1051–8.
Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–8.
McCarthy H, Skokauskas N, Mulligan A, Donohoe G, Mullins D, Kelly J, et al. Attention network hypoconnectivity with default and affective network hyperconnectivity in adults diagnosed with attention-deficit/hyperactivity disorder in childhood. JAMA Psychiatry. 2013;70:1329–37.
Fassbender C, Zhang H, Buzy WM, Cortes CR, Mizuiri D, Beckett L, et al. A lack of default network suppression is linked to increased distractibility in ADHD. Brain Res. 2009;1273:114–28.
Freeman SM, Clewett DV, Bennett CM, Kiehl KA, Gazzaniga MS, Miller MB. The posteromedial region of the default mode network shows attenuated task-induced deactivation in psychopathic prisoners. Neuropsychology. 2015;29:493–500.
Lu FM, Zhou JS, Wang XP, Xiang YT, Yuan Z. Short- and long-range functional connectivity density alterations in adolescents with pure conduct disorder at resting-state. Neuroscience. 2017;351:96–107.
Han DH, Kim SM, Bae S, Renshaw PF, Anderson JS. A failure of suppression within the default mode network in depressed adolescents with compulsive internet game play. J Affect Disord. 2016;194:57–64.
Zhu X, Cortes CR, Mathur K, Tomasi D, Momenan R. Model-free functional connectivity and impulsivity correlates of alcohol dependence: a resting-state study. Addict Biol. 2017;22:206–17.
van Zuiden M, Kavelaars A, Rademaker AR, Vermetten E, Heijnen CJ, Geuze E. A prospective study on personality and the cortisol awakening response to predict posttraumatic stress symptoms in response to military deployment. J Psychiatr Res. 2011;45:713–9.
Marshall RE, Milligan-Saville JS, Mitchell PB, Bryant RA, Harvey SB. A systematic review of the usefulness of pre-employment and pre-duty screening in predicting mental health outcomes amongst emergency workers. Psychiatry Res. 2017;253:129–37.
Macmanus D, Dean K, Jones M, Rona RJ, Greenberg N, Hull L, et al. Violent offending by UK military personnel deployed to Iraq and Afghanistan: a data linkage cohort study. Lancet. 2013;381:907–17.
Bramsen I, Dirkzwager AJ, van der Ploeg HM. Predeployment personality traits and exposure to trauma as predictors of posttraumatic stress symptoms: a prospective study of former peacekeepers. Am J Psychiatry. 2000;157:1115–9.
Schnurr PP, Friedman MJ, Rosenberg SD. Premilitary MMPI scores as predictors of combat-related PTSD symptoms. Am J Psychiatry. 1993;150:479–83.
Gil S, Caspi Y. Personality traits, coping style, and perceived threat as predictors of posttraumatic stress disorder after exposure to a terrorist attack: a prospective study. Psychosom Med. 2006;68:904–9.
Koch SB, van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, Olff M. Aberrant resting-state brain activity in posttraumatic stress disorder: a meta-analysis and systematic review. Depress Anxiety. 2016;33:592–605.
Falconer E, Bryant R, Felmingham KL, Kemp AH, Gordon E, Peduto A, et al. The neural networks of inhibitory control in posttraumatic stress disorder. J Psychiatry Neurosci. 2008;33:413–22.
Weber DL, Clark CR, McFarlane AC, Moores KA, Morris P, Egan GF. Abnormal frontal and parietal activity during working memory updating in post-traumatic stress disorder. Psychiatry Res. 2005;140:27–44.
Liberzon I, Abelson JL. Context processing and the neurobiology of post-traumatic stress disorder. Neuron. 2016;92:14–30.
Galea S, Nandi A, Vlahov D. The epidemiology of post-traumatic stress disorder after disasters. Epidemiol Rev. 2005;27:78–91.
Solomon Z, Mikulincer M. Trajectories of PTSD: a 20-year longitudinal study. Am J Psychiatry. 2006;163:659–66.
Funding
This study was partly supported by the National Research Foundation of Korea grants funded by the Ministry of Science and ICT (2020M3E5D9080555 to IKL) and funded by the Ministry of Education (2020R1A6A1A03043528 to IKL). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
SY and IKL. conceptualized and designed the study. SL, EN, TDK, HH, EH, RYK, YS, HL, and CS performed the research and participated in data acquisition. SL, SY, EN, TDK, HH, EH, RYK, YS, HL, CS, and IKL analyzed the data and interpreted the results. SL, SY, and IKL drafted the manuscript. All authors revised and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, S., Yoon, S., Namgung, E. et al. Distinctively different human neurobiological responses after trauma exposure and implications for posttraumatic stress disorder subtyping. Mol Psychiatry 28, 2964–2974 (2023). https://doi.org/10.1038/s41380-023-01995-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-023-01995-3